The Laboratory Opossum (Monodelphis domestica) Is a Unique Model for Research on Zika Virus: Robust Immune Response, Widespread Dissemination, and Long-Term Persistence
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antigens and Preparation of Virus
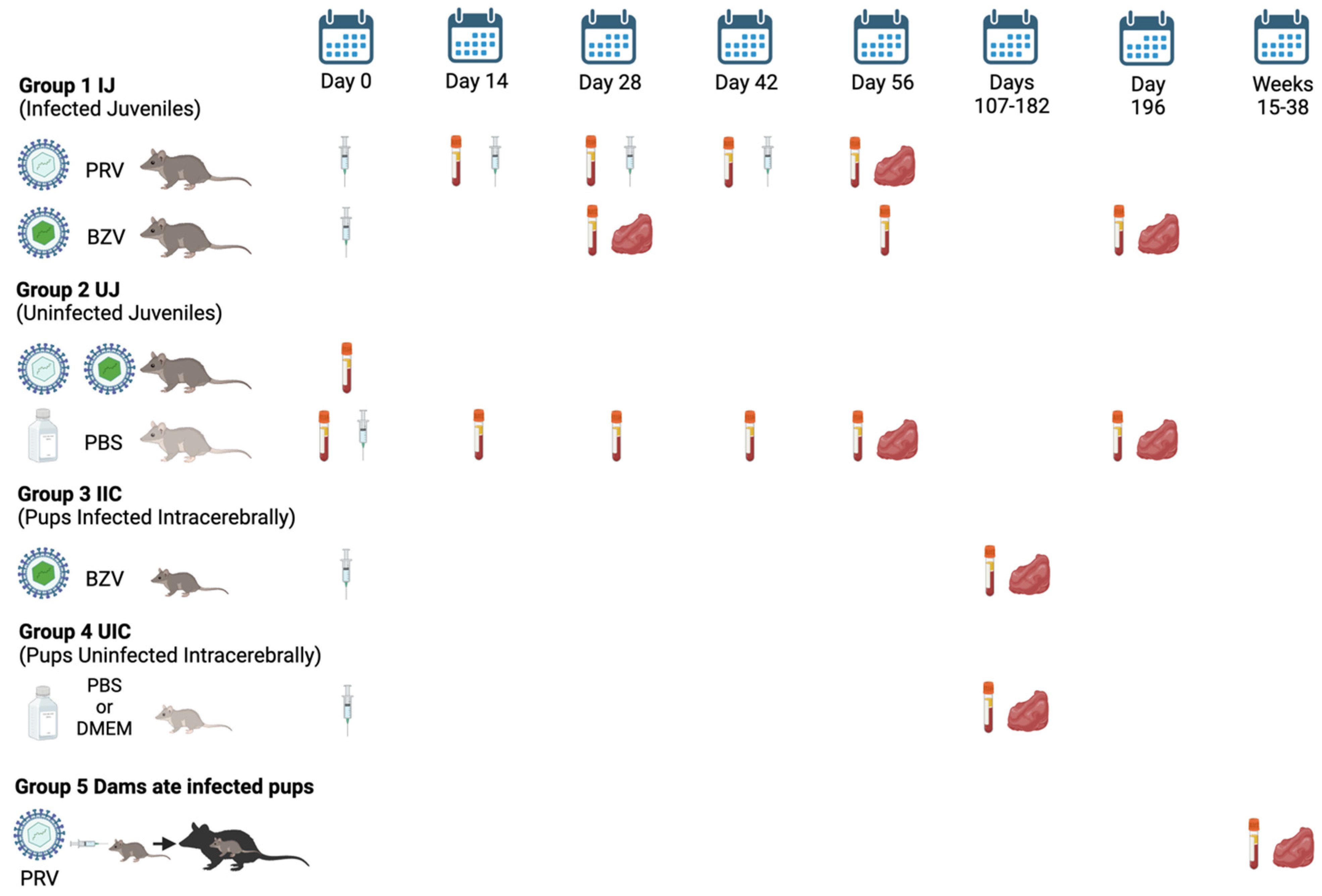
2.3. Inoculations and Sample Collection
2.4. Serum Samples
2.5. ELISA Optimization
2.6. Indirect ELISA Anti-ZIKV
2.7. Serum Pools and Quality Control
2.8. Immunohistochemistry (IHC)
2.9. Statistical Analysis
3. Results
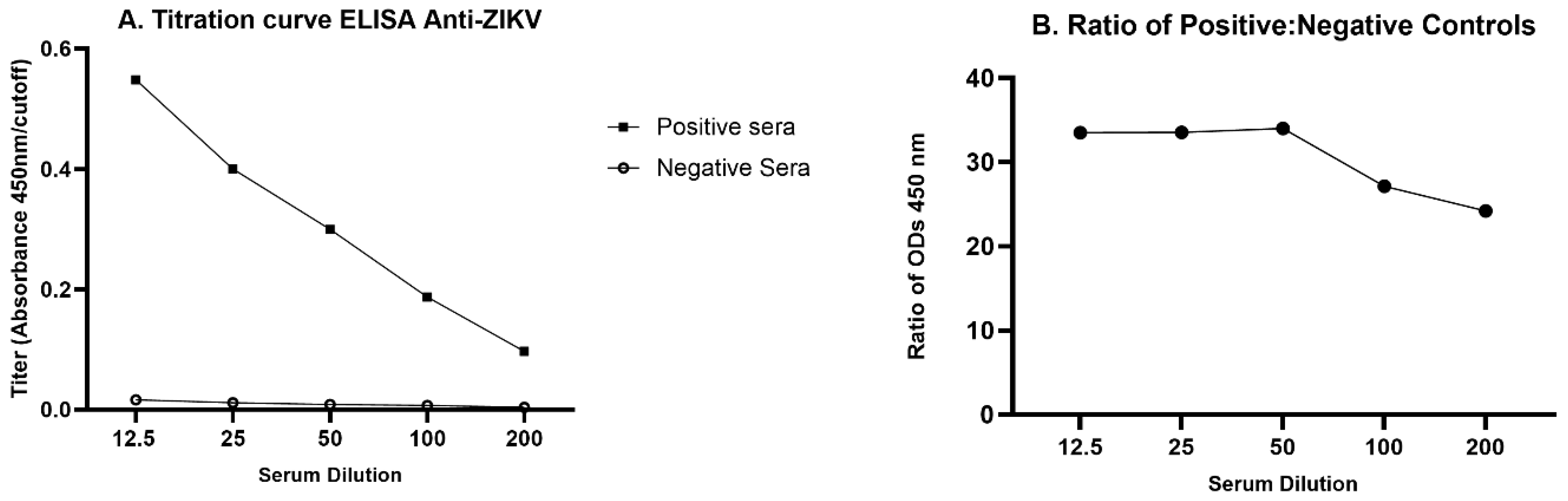
3.1. ELISA Optimization
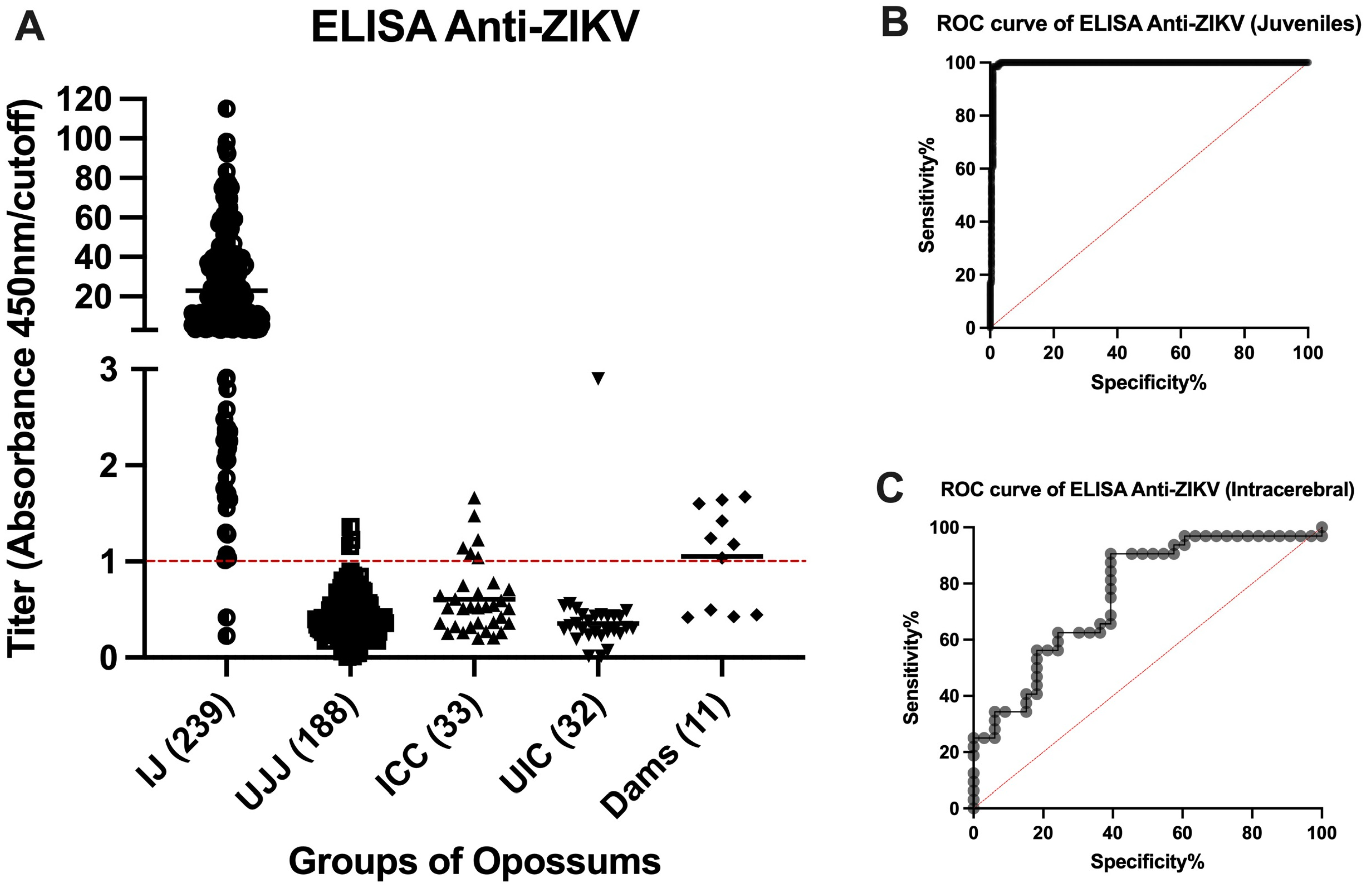
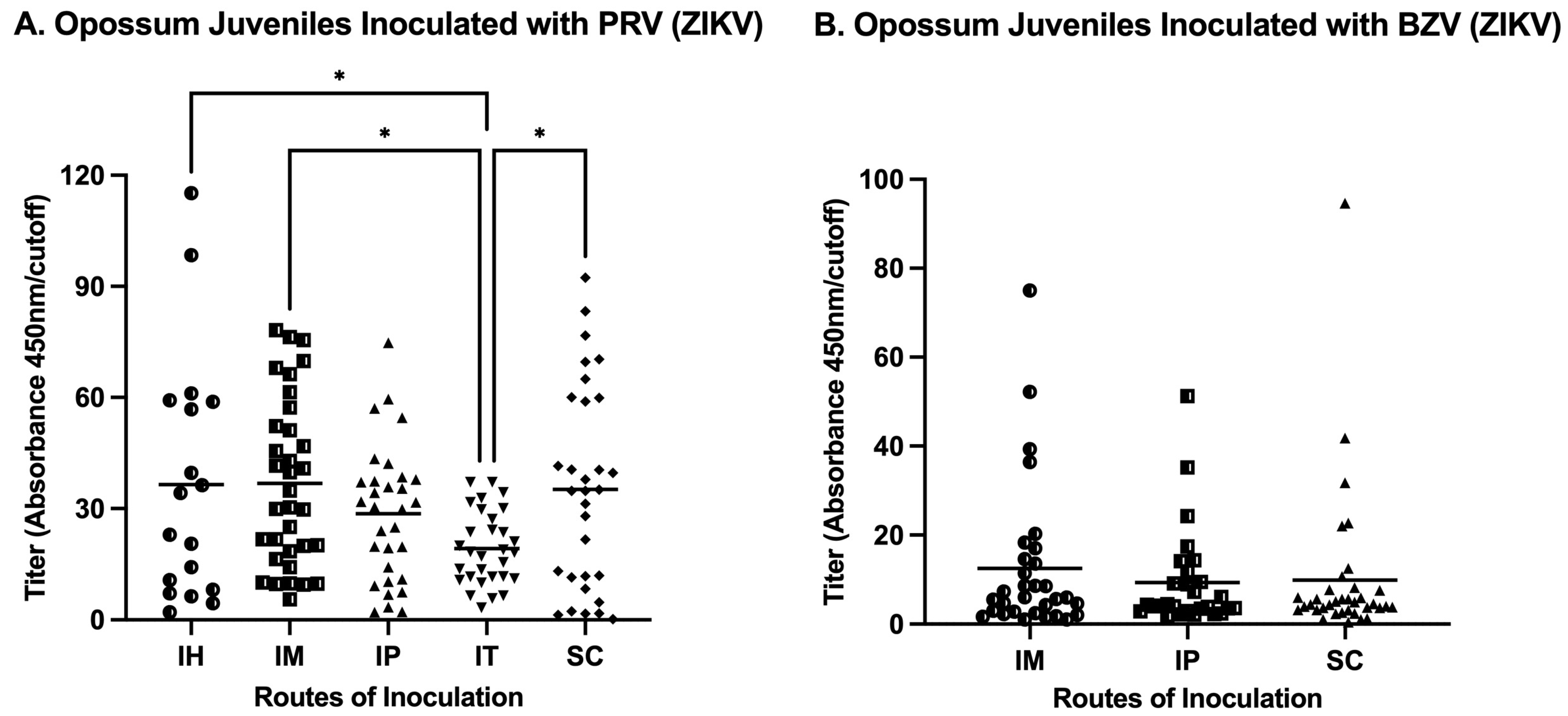
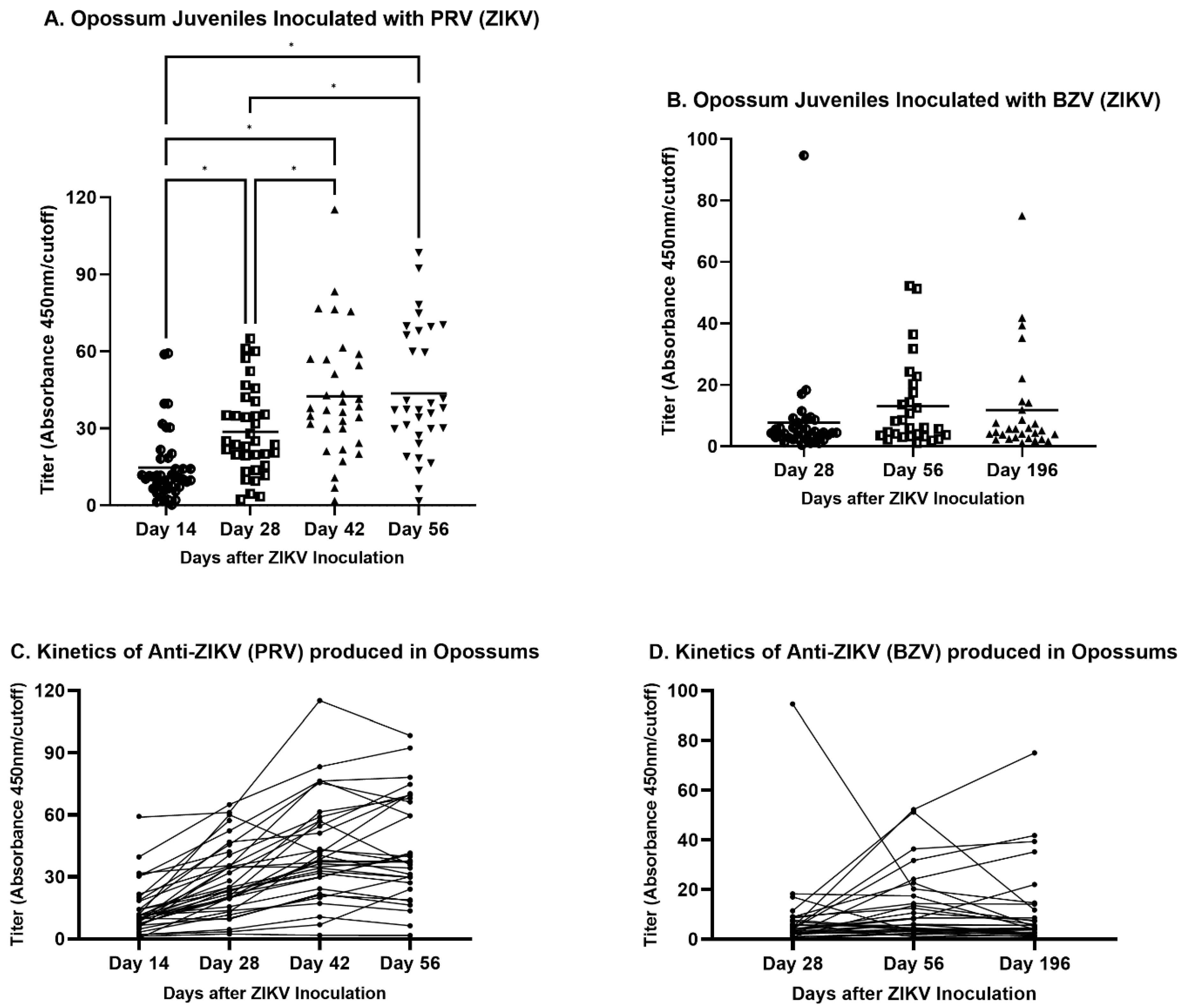
3.2. Indirect Anti-ZIKV ELISA
3.3. Quality Control
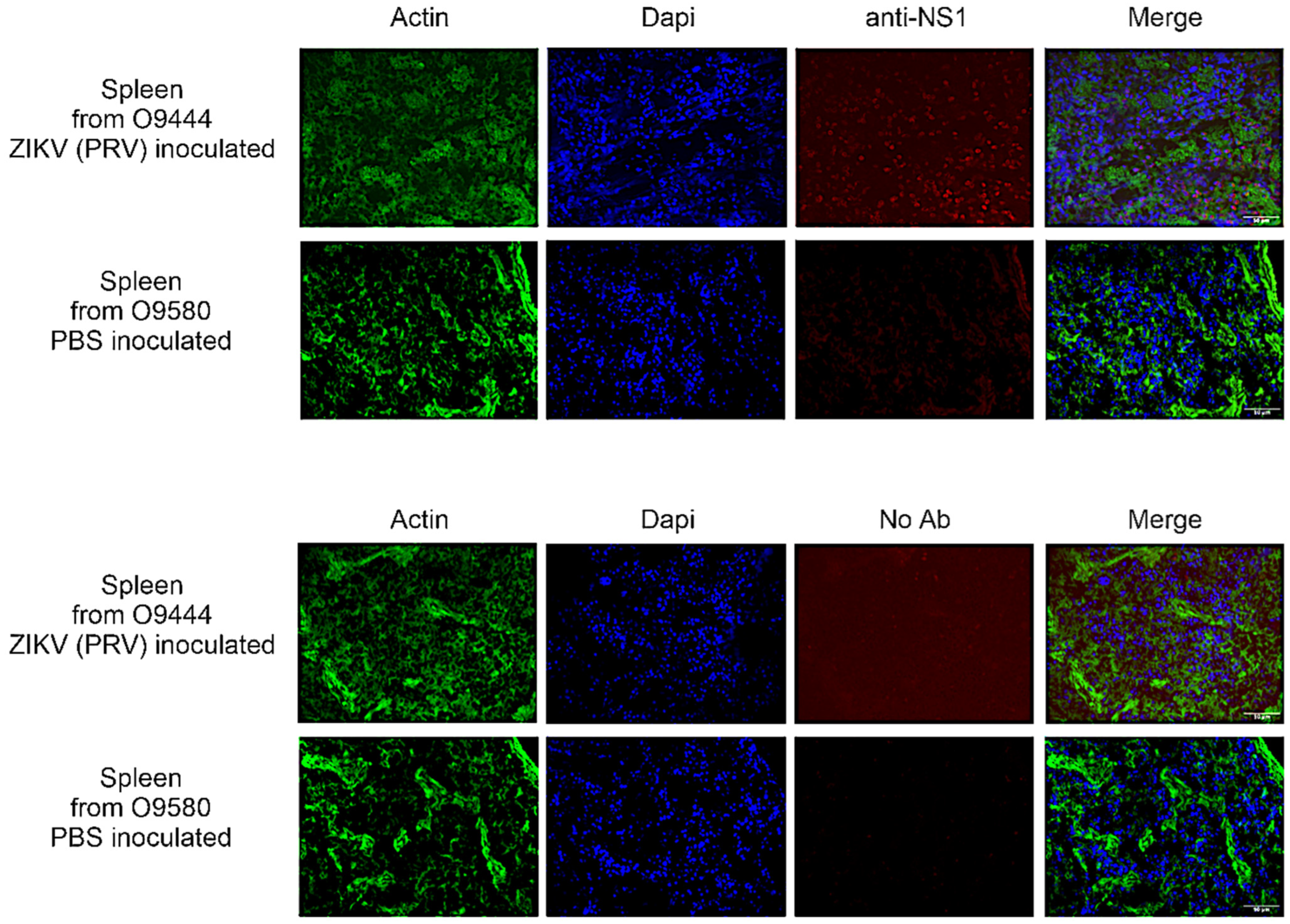
3.4. IHC
3.4.1. IJ and UJ Groups
3.4.2. Suckling Pups Inoculated Intracerebrally with BZV (IIC Group) or PBS or DMEM (UIC Group)
3.4.3. Dams
3.5. Pathologies
4. Discussion
4.1. Humoral Response to ZIKV
4.2. Persistence of ZIKV Infection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dick, G.W. Epidemiological Notes on Some Viruses Isolated in Uganda; Yellow Fever, Rift Valley Fever, Bwamba Fever, West Nile, Mengo, Semliki Forest, Bunyamwera, Ntaya, Uganda S and Zika Viruses. Trans. R. Soc. Trop. Med. Hyg. 1953, 47, 13–48. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika Virus. I. Isolations and Serological Specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Turchi Martelli, C.M.; Albuquerque, M.D.F.P.; Araújo, T.V.; Barkokebas, A.; Bezerra, L.C.A.; Braga, C.; Brandão-Filho, S.P.; Brito, C.A.A.; Cabral, R.G.; Carneiro, A.R.; et al. Microcephaly in Infants, Pernambuco State, Brazil, 2015. Emerg. Infect. Dis. 2016, 22, 1090. [Google Scholar] [CrossRef]
- Barbi, L.; Coelho, A.V.C.; de Alencar, L.C.A.; Crovella, S. Prevalence of Guillain-Barré Syndrome among Zika Virus Infected Cases: A Systematic Review and Meta-Analysis. Braz. J. Infect. Dis. 2018, 22, 137–141. [Google Scholar] [CrossRef] [PubMed]
- del Campo, M.; Feitosa, I.M.L.; Ribeiro, E.M.; Horovitz, D.D.G.; Pessoa, A.L.S.; França, G.V.A.; García-Alix, A.; Doriqui, M.J.R.; Wanderley, H.Y.C.; Sanseverino, M.V.T.; et al. The Phenotypic Spectrum of Congenital Zika Syndrome. Am. J. Med. Genet. A 2017, 173, 841–857. [Google Scholar] [CrossRef]
- Pielnaa, P.; Al-Saadawe, M.; Saro, A.; Dama, M.F.; Zhou, M.; Huang, Y.; Huang, J.; Xia, Z. Zika Virus-Spread, Epidemiology, Genome, Transmission Cycle, Clinical Manifestation, Associated Challenges, Vaccine and Antiviral Drug Development. Virology 2020, 543, 34–42. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.-A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- França, G.V.A.; Schuler-Faccini, L.; Oliveira, W.K.; Henriques, C.M.P.; Carmo, E.H.; Pedi, V.D.; Nunes, M.L.; Castro, M.C.; Serruya, S.; Silveira, M.F.; et al. Congenital Zika Virus Syndrome in Brazil: A Case Series of the First 1501 Livebirths with Complete Investigation. Lancet 2016, 388, 891–897. [Google Scholar] [CrossRef]
- Oladapo, O.T.; Souza, J.P.; De Mucio, B.; de León, R.G.P.; Perea, W.; Gülmezoglu, A.M. WHO Interim Guidance on Pregnancy Management in the Context of Zika Virus Infection. Lancet Glob. Health 2016, 4, e510–e511. [Google Scholar] [CrossRef]
- World Health Organization. WHO Statement on the First Meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika Virus and Observed Increase in Neurological Disorders and Neonatal Malformations. Available online: https://www.who.int/news/item/01-02-2016-who-statement-on-the-first-meeting-of-the-international-health-regulations-(2005)-(ihr-2005)-emergency-committee-on-zika-virus-and-observed-increase-in-neurological-disorders-and-neonatal-malformations (accessed on 8 July 2021).
- BRASIL Monitoramento Integrado de Alterações No Crescimento e Desenvolvimento Relacionadas à Infecção Pelo Vírus Zika e Outras Etiologias Infecciosas, Até a Semana Epidemiológica 45 de 2018. Bol. Epidemiológico Secr. Vigilância Saúde Ministério Saúde 2018, 49, 8.
- Bradley, M.P.; Nagamine, C.M. Animal Models of Zika Virus. Comp. Med. 2017, 67, 242–252. [Google Scholar]
- Duggal, N.; Ritter, J.; Pestorius, S.; Zaki, S.; Davis, B.; Chang, G.; Bowen, R.; Brault, A. Frequent Zika Virus Sexual Transmission and Prolonged Viral RNA Shedding in an Immunodeficient Mouse Model. Cell Rep. 2017, 18, 1751–1760. [Google Scholar] [CrossRef]
- Krause, K.K.; Azouz, F.; Shin, O.S.; Kumar, M. Understanding the Pathogenesis of Zika Virus Infection Using Animal Models. Immune Netw. 2017, 17, 287. [Google Scholar] [CrossRef]
- Narasimhan, H.; Chudnovets, A.; Burd, I.; Pekosz, A.; Klein, S.L. Animal Models of Congenital Zika Syndrome Provide Mechanistic Insight into Viral Pathogenesis during Pregnancy. PLoS Negl. Trop. Dis. 2020, 14, e0008707. [Google Scholar] [CrossRef]
- Nazerai, L.; Schøller, A.S.; Rasmussen, P.O.S.; Buus, S.; Stryhn, A.; Christensen, J.P.; Thomsen, A.R. A New in Vivo Model to Study Protective Immunity to Zika Virus Infection in Mice with Intact Type I Interferon Signaling. Front. Immunol. 2018, 8, 593. [Google Scholar] [CrossRef]
- Pawitwar, S.S.; Dhar, S.; Tiwari, S.; Ojha, C.R.; Lapierre, J.; Martins, K.; Rodzinski, A.; Parira, T.; Paudel, I.; Li, J.; et al. Overview on the Current Status of Zika Virus Pathogenesis and Animal Related Research. J. Neuroimmune Pharmacol. 2017, 12, 371–388. [Google Scholar] [CrossRef]
- Steinbach, R.J.; Haese, N.N.; Smith, J.L.; Colgin, L.M.A.; MacAllister, R.P.; Greene, J.M.; Parkins, C.J.; Kempton, J.B.; Porsov, E.; Wang, X.; et al. A Neonatal Nonhuman Primate Model of Gestational Zika Virus Infection with Evidence of Microencephaly, Seizures and Cardiomyopathy. PLoS ONE 2020, 15, e0227676. [Google Scholar] [CrossRef]
- Dudley, D.M.; Aliota, M.; Mohr, E.; Weile, A.; Lehrer-Brey, G.; Weisgrau, K.; Mohns, M.; Breitbach, M.; Rasheed, M.; Newman, C.; et al. A Rhesus Macaque Model of Asian-Lineage Zika Virus Infection. Nat. Commun. 2016, 7, 12204. [Google Scholar] [CrossRef]
- Manangeeswaran, M.; Ireland, D.D.C.; Verthelyi, D. Zika (PRVABC59) Infection Is Associated with T Cell Infiltration and Neurodegeneration in CNS of Immunocompetent Neonatal C57Bl/6 Mice. PLoS Pathog. 2016, 12, e1006004. [Google Scholar] [CrossRef]
- Morrison, T.E.; Diamond, M.S. Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Nazerai, L.; Christensen, J.P.; Thomsen, A.R. A ‘Furry-Tale’ of Zika Virus Infection: What Have We Learned from Animal Models? Viruses 2019, 11, 29. [Google Scholar] [CrossRef]
- Goodfellow, F.T.; Tesla, B.; Simchick, G.; Zhao, Q.; Hodge, T.; Brindley, M.A.; Stice, S.L. Zika Virus Induced Mortality and Microcephaly in Chicken Embryos. Stem Cells Dev. 2016, 25, 1691–1697. [Google Scholar] [CrossRef]
- Kumar, M.; Krause, K.K.; Azouz, F.; Nakano, E.; Nerurkar, V.R. A Guinea Pig Model of Zika Virus Infection. Virol. J. 2017, 14, 75. [Google Scholar] [CrossRef]
- Saver, A.E.; Crawford, S.A.; Joyce, J.D.; Bertke, A.S. Route of Infection Influences Zika Virus Shedding in a Guinea Pig Model. Cells 2019, 8, 1437. [Google Scholar] [CrossRef]
- VandeBerg, J.L.; Williams-Blangero, S. The Laboratory Opossum. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals; Golledge, H., Richardson, C., Eds.; Wiley: West Sussex, UK, 2024; pp. 301–323. [Google Scholar]
- Thomas, J.; Garcia, J.; Terry, M.; Mahaney, S.; Quintanilla, O.; Silva, D.C.; Morales, M.; VandeBerg, J.L. Monodelphis Domestica as a Fetal Intra-Cerebral Inoculation Model for Zika Virus Pathogenesis. Pathogens 2023, 12, 733. [Google Scholar] [CrossRef]
- Cardoso-Moreira, M.; Halbert, J.; Valloton, D.; Velten, B.; Chen, C.; Shao, Y.; Liechti, A.; Ascenção, K.; Rummel, C.; Ovchinnikova, S.; et al. Gene Expression across Mammalian Organ Development. Nature 2019, 571, 505–509. [Google Scholar] [CrossRef]
- Almeida, I.C.; Covas, D.T.; Soussumi, L.M.T.; Travassos, L.R. A Highly Sensitive and Specific Chemiluminescent Enzyme-Linked Immunosorbent Assay for Diagnosis of Active Trypanosoma Cruzi Infection. Transfusion 1997, 37, 850–857. [Google Scholar] [CrossRef]
- Jacobson, R.H. Validation of Serological Assays for Diagnosis of Infectious Diseases. OIE Rev. Sci. Tech. 1998, 17, 469–486. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Zhang, N.N.; Li, X.F.; Wang, Y.Q.; Tian, M.; Qiu, Y.F.; Fan, J.W.; Hao, J.N.; Huang, X.Y.; Dong, H.L.; et al. Intranasal Infection and Contact Transmission of Zika Virus in Guinea Pigs. Nat. Commun. 2017, 8, 1648. [Google Scholar] [CrossRef]
- Nguyen, S.M.; Antony, K.M.; Dudley, D.M.; Kohn, S.; Simmons, H.A.; Wolfe, B.; Salamat, M.S.; Teixeira, L.B.C.; Wiepz, G.J.; Thoong, T.H.; et al. Highly Efficient Maternal-Fetal Zika Virus Transmission in Pregnant Rhesus Macaques. PLoS Pathog. 2017, 13, e1006378. [Google Scholar] [CrossRef]
- Julander, J.G.; Siddharthan, V. Small-Animal Models of Zika Virus. J. Infect. Dis. 2017, 216, S919–S927. [Google Scholar] [CrossRef]
- Winkler, C.W.; Myers, L.M.; Woods, T.A.; Messer, R.J.; Carmody, A.B.; McNally, K.L.; Scott, D.P.; Hasenkrug, K.J.; Best, S.M.; Peterson, K.E. Adaptive Immune Responses to Zika Virus Are Important For Controlling Virus Infection And Preventing Infection In Brain And Testes. J. Immunol. 2017, 198, 3526–3535. [Google Scholar] [CrossRef]
- Yang, M.; Lai, H.; Sun, H.; Chen, Q. Virus-like Particles That Display Zika Virus Envelope Protein Domain III Induce Potent Neutralizing Immune Responses in Mice. Sci. Rep. 2017, 7, 7679. [Google Scholar] [CrossRef]
- Espinosa, D.; Mendy, J.; Manayani, D.; Vang, L.; Wang, C.; Richard, T.; Guenther, B.; Aruri, J.; Avanzini, J.; Garduno, F.; et al. Passive Transfer of Immune Sera Induced by a Zika Virus-Like Particle Vaccine Protects AG129 Mice Against Lethal Zika Virus Challenge. eBioMedicine 2018, 27, 61–70. [Google Scholar] [CrossRef]
- Miller, M.R.; Fagre, A.C.; Clarkson, T.C.; Markle, E.D.; Foy, B.D. Three Immunocompetent Small Animal Models That Do Not Support Zika Virus Infection. Pathogens 2021, 10, 971. [Google Scholar] [CrossRef]
- Aliota, M.T.; Dudley, D.M.; Newman, C.M.; Mohr, E.L.; Gellerup, D.D.; Breitbach, M.E.; Buechler, C.R.; Rasheed, M.N.; Mohns, M.S.; Weiler, A.M.; et al. Heterologous Protection against Asian Zika Virus Challenge in Rhesus Macaques. PLoS Negl. Trop. Dis. 2016, 10, e0005168. [Google Scholar] [CrossRef]
- Ambagala, A.; Truong, T.; Cottam-Birt, C.; Berhane, Y.; Gerdts, V.; Karniychuk, U.; Safronetz, D.; Babiuk, S. Susceptibility of Chicken Embryos, Sheep, Cattle, Pigs, and Chickens to Zika Virus Infection. Front. Vet. Sci. 2020, 7, 23. [Google Scholar] [CrossRef]
- Li, C.; Xu, D.; Ye, Q.; Shi, L.; Qin, C.F.; Xu, Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 2016, 19, 120–126. [Google Scholar] [CrossRef]
- Shao, Q.; Herrlinger, S.; Yang, S.-L.; Lai, F.; Moore, J.M.; Brindley, M.A.; Chen, J.-F. Zikus Virus Infection Disrupts Neurovascular Development and Results in Postnatal Microcephaly with Brain Damage. Stem Cells Regen. 2016, 143, 4127–4136. [Google Scholar]
- Bujan, L.; Mansuy, J.M.; Hamdi, S.; Pasquier, C.; Joguet, G. 1 Year after Acute Zika Virus Infection in Men. Lancet Infect. Dis. 2020, 20, 25–26. [Google Scholar] [CrossRef]
- Fernandes, N.C.C.A.; Nogueira, J.S.; Réssio, R.A.; Cirqueira, C.S.; Kimura, L.M.; Fernandes, K.R.; Cunha, M.S.; Souza, R.P.; Guerra, J.M. Experimental Zika Virus Infection Induces Spinal Cord Injury and Encephalitis in Newborn Swiss Mice. Exp. Toxicol. Pathol. 2017, 69, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Zuo, G.L.; Li, X.F.; Ye, Q.; Deng, Y.Q.; Huang, X.Y.; Cao, W.C.; Qin, C.F.; Luo, Z.G. Vertical Transmission of Zika Virus Targeting the Radial Glial Cells Affects Cortex Development of Offspring Mice. Cell Res. 2016, 26, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.D.C.; Manangeeswaran, M.; Lewkowicz, A.P.; Engel, K.; Clark, S.M.; Laniyan, A.; Sykes, J.; Lee, H.N.; McWilliams, I.L.; Kelley-Baker, L.; et al. Long-Term Persistence of Infectious Zika Virus: Inflammation and Behavioral Sequela in Mice. PLoS Pathog. 2020, 16, e1008689. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, L.T.M. Human Urban Arboviruses Can Infect Wild Animals and Jump to Sylvatic Maintenance Cycles in South America. Front. Cell Infect. Microbiol. 2019, 9, 259. [Google Scholar] [CrossRef] [PubMed]
| Juveniles (IJ and UJ Groups) | n | Pups (IIC and UIC Groups) | n |
|---|---|---|---|
| IJ positives (true positives) | 237 | IIC positives (true positives) | 6 |
| UJ positives (false positives) | 3 | UIC positives (false positives) | 1 |
| IJ negatives (false negatives) | 2 | IIC negatives (false negative) | 27 |
| UJ negatives (true negatives) | 184 | UIC negatives (true negatives) | 31 |
| Statistical analysis | % | Statistical analysis | % |
| Sensitivity | 99.16 | Sensitivity | 18.18 |
| Specificity | 98.40 | Specificity | 96.85 |
| PPV | 98.75 | PPV | 85.71 |
| NPV | 98.93 | NPV | 53.45 |
| Accuracy | 99.45 | Accuracy | 78.61 |
| Intra-Assay CV% | Inter-Assay CV% | |||
|---|---|---|---|---|
| Mean (%) | SD/Mean OD (%) | Mean OD | SD (σ) | |
| Positive | ||||
| High | 8.5 | 2.7 | 0.970 | 0.026 |
| Medium | 11.0 | 6.8 | 0.831 | 0.056 |
| Low | 9.9 | 22.3 | 0.296 | 0.066 |
| Negative | 37.6 | 32.7 | 0.007 | 0.002 |
| Mean | 16.7 | 16.1 |
| ID Number | Sex | Group | Route | Treatment | Age of Animal at Necropsy (Weeks) | Experimental Study Day | ELISA | IHC | |
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||||
| P1366 | F | IJ | IM | BZV | 22 | 28 | + | R, S | |
| P1349 | F | IJ | IM | BZV | 46 | 196 | + | S | R |
| P1350 | F | IJ | IM | BZV | 46 | 196 | + | R | |
| P1351 | F | IJ | IM | BZV | 46 | 196 | + | R, S | |
| P1424 | F | IJ | IM | BZV | 46 | 196 | + | R, S | |
| P1465 | F | IJ | IM | BZV | 22 | 28 | + | R | |
| P1341 | F | IJ | IP | BZV | 46 | 196 | + | S | R |
| P1475 | F | IJ | IP | BZV | 22 | 28 | + | R | |
| P1477 | F | IJ | IP | BZV | 46 | 196 | + | R | |
| P1397 | F | IJ | SC | BZV | 22 | 28 | − | R | |
| P1426 | F | IJ | SC | BZV | 46 | 196 | + | R | |
| P1493 | F | IJ | SC | BZV | 22 | 28 | + | R | |
| O9343 | F | IJ | IM | PRV | 26 | 56 | + | H, R, S | B, E |
| O9347 | F | IJ | IM | PRV | 22 | 28 | + | B, R | E, H, S |
| O9374 | M | IJ | IM | PRV | 26 | 56 | + | B, E, H, R, S | |
| O9344 | F | IJ | IP | PRV | 22 | 28 | + | B, H, R, S | E |
| O9348 | F | IJ | IP | PRV | 26 | 56 | + | B, R, S | E, H |
| O9375 | M | IJ | IP | PRV | 26 | 56 | + | E, R | B, H, S |
| O9342 | F | IJ | SC | PRV | 26 | 56 | + | B, E, H, R, S | |
| O9346 | F | IJ | SC | PRV | 26 | 56 | + | E | B, H, R, S |
| O9373 | M | IJ | SC | PRV | 26 | 56 | + | E, R | B, H, S |
| O9523 | F | IJ | IH | PRV | 26 | 56 | + | S | |
| O9530 | M | IJ | IH | PRV | 26 | 56 | + | S | |
| O9534 | F | IJ | IH | PRV | 26 | 56 | + | S | |
| O9455 | F | IJ | IM | PRV | 26 | 56 | + | S | |
| O9461 | M | IJ | IM | PRV | 26 | 56 | + | S | |
| O9525 | F | IJ | IM | PRV | 26 | 56 | + | S | |
| O9526 | F | IJ | IP | PRV | 26 | 56 | + | S | |
| O9443 | M | IJ | IP | PRV | 26 | 56 | + | S | |
| O9353 | M | IJ | IT | PRV | 26 | 56 | + | S | |
| O9354 | M | IJ | IT | PRV | 26 | 56 | + | S | |
| O9376 | M | IJ | IT | PRV | 26 | 56 | + | S | |
| O9444 | M | IJ | IT | PRV | 26 | 56 | + | S | |
| O9463 | M | IJ | IT | PRV | 26 | 56 | + | S | |
| O9524 | F | IJ | SC | PRV | 26 | 56 | + | S | |
| O9460 | M | IJ | SC | PRV | 26 | 56 | + | S | |
| O9524 | F | IJ | SC | PRV | 26 | 56 | + | S | |
| O9539 | M | IJ | SC | PRV | 26 | 56 | + | S | |
| O9580 | M | UJ | IH | PBS | 26 | 56 | − | S | |
| O9572 | F | UJ | IM | PBS | 26 | 56 | − | B, E, H, R, S | |
| P1542 | F | UJ | IM | PBS | 46 | 196 | − | R, S | |
| P1330 | F | UJ | IP | PBS | 22 | 28 | − | R, S | |
| P1521 | F | UJ | IP | PBS | 46 | 196 | − | R, S | |
| P1540 | F | UJ | IP | PBS | 46 | 196 | − | R, S | |
| P1541 | F | UJ | IP | PBS | 46 | 196 | − | S | |
| O9575 | F | UJ | SC | PBS | 26 | 56 | + | B, E, H, R, S | |
| P1937 | M | IIC | IC | BZV | 26 | 177 | − | R | |
| P1965 | F | IIC | IC | BZV | 26 | 177 | − | B, R, S | |
| P1967 | M | IIC | IC | BZV | 19 | 129 | − | B, R, S | |
| P1968 | M | IIC | IC | BZV | 21 | 148 | − | R | B, S |
| P2087 | F | IIC | IC | BZV | 24 | 163 | − | B, R, S | |
| P2090 | M | IIC | IC | BZV | 24 | 163 | − | B, R, S | |
| P2133 | F | IIC | IC | BZV | 22 | 149 | − | B, R, S | |
| P2138 | M | IIC | IC | BZV | 22 | 149 | − | B, R, S | |
| P2141 | F | IIC | IC | BZV | 22 | 149 | + | B, R | S |
| P2142 | F | IIC | IC | BZV | 22 | 149 | + | B, R | S |
| P2146 | M | IIC | IC | BZV | 22 | 149 | − | S | |
| P2241 | F | IIC | IC | BZV | 22 | 146 | + | B, R, S | |
| P2246 | M | IIC | IC | BZV | 22 | 146 | − | S | |
| P2275 | F | IIC | IC | BZV | 26 | 177 | − | B, R, S | |
| P2276 | F | IIC | IC | BZV | 26 | 176 | − | B, R, S | |
| P2279 | M | IIC | IC | BZV | 26 | 176 | − | B, R, S | |
| P1945 | F | UIC | IC | PBS | 26 | 175 | − | B, R, S | |
| P2296 | M | UIC | IC | PBS | 26 | 176 | − | B, R, S | |
| P2300 | F | UIC | IC | PBS | 22 | 149 | − | B, R, S | |
| P2306 | M | UIC | IC | PBS | 22 | 149 | − | B, R, S | |
| P1456 | F | DAM | ate 1 pup | PRV | 87 | NA | + | S | |
| P1571 | F | DAM | ate 8 pups | PRV | 84 | NA | + | S | |
| P1624 | F | DAM | ate 2 pups | PRV | 83 | NA | + | S | |
| P2217 | F | DAM | ate 4 pups | PRV | 70 | NA | + | S | |
| P2344 | F | DAM | ate 2 pups | PRV | 68 | NA | − | S | |
| P2355 | F | DAM | ate 10 pups | PRV | 67 | NA | + | S | |
| P2375 | F | DAM | ate 2 pups | PRV | 67 | NA | − | S | |
| P2451 | F | DAM | ate 10 pups | PRV | 67 | NA | − | S | |
| P2452 | F | DAM | ate 6 pups | PRV | 67 | NA | − | S | |
| P3132 | F | DAM | ate 4 pups | PRV | 57 | NA | + | S | |
| Litter Inoculation Date | Dam ID | ELISA Results | Route | Age of Pups (Days) at Time of Inoculation | Number of Pups Eaten | Number of Pups and Days Between Inoculation and Being Eaten | Number of Weeks Prior to Dam’s Necropsy |
|---|---|---|---|---|---|---|---|
| 9 May 2018 | P1110 | +(1.674) | IC | 1 | 9 | 1 at 1 day, 1 at 2 days, 1 at 5 days, and 6 at 8 days | 14–15 |
| 6 November 2018 | P1456 | +(1.036) | IC | 5 | 1 | 1 pup at 20 days | 34 |
| 19 October 2018 | P1571 | +(1.241) | IC | 1 | 8 | 2 pups at 6 days, 3 at 17 days, 3 at 32 days | 34–38 |
| 1 February 2019 | P1624 | +(1.421) | IC | 3 | 2 | 1 pup at 18 days, 1 at 33 days | 19–21 |
| 22 October 2018 | P2217 | +(1.640) | IM | 3 | 4 | 2 pups at 23 days, 2 at 29 days | 34–36 |
| 26 November 2018 | P2344 | −(0.425) | SC | 4 | 2 | 1 pup at 17 days, 1 at 45 days | 27–31 |
| 3 December 2018 | P2355 | +(1.601) | IM | 3 | 10 | 5 pups at 14 days, 5 at 18 days | 30–31 |
| 3 December 2018 | P2375 | −(0.446) | IM | 5 | 2 | 1 pup at 11 days, 1 at 38 days | 27–31 |
| 6 November 2018 | P2451 | −(0.419) | IM | 4 | 10 | 10 pups at 14 days | 34 |
| 4 February 2019 | P2452 | −(0.496) | SC | 4 | 6 | 2 pups at 15 days, 3 at 25 days, 1 at 30 days | 19–21 |
| 11 February 2019 | P3132 | +(1.180) | IC | 4 | 4 | 2 pups at 12 days, 1 at 17 days, 1 at 23 days | 18–21 |
| Opossums | Mice | Humans | Life History Event |
|---|---|---|---|
| E13.5 d | E10.5 d | E4-5 w | |
| 0 d | E11.5 d | E5-6 w | Birth of opossum |
| 2 d | E13 d | E7 w | |
| 4 d | E15 d | E8-11 w | |
| 6 d | E16.5 d | E12 w | |
| 14 d | 0 d | E16 w | Detachment of opossums from nipples; the birth of a mouse |
| 21 d | 3 d | E20 w | Opossum fur growth well-initiated |
| 30 d | 6 d | 0 d | Birth of human |
| 8 w | 3.5 w | 3 y | Natural weaning, toddler stage |
| 12 w | 4 w | 6 y | |
| 18 w | 5 w | 9 y | |
| 22 w | 6 w | 12 y | Onset of puberty |
| 26 w | 8 w | 15 y | Adolescence |
| 52 w | 17 w | 25 y | Physically and reproductively prime |
| 2 y | 1 y | 50 y | Loss of female fertility |
| 3 y | 23 m | 75 y | Elderly |
| 4 y | 3 y | 100 y | Near maximum lifespan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor, A.F.; Mahaney, S.M.; Garcia, J., Jr.; Morales, M.; Quintanilla, O.; Arriaga, M.A.; Thomas, J.M., III; VandeBerg, J.L. The Laboratory Opossum (Monodelphis domestica) Is a Unique Model for Research on Zika Virus: Robust Immune Response, Widespread Dissemination, and Long-Term Persistence. Viruses 2024, 16, 1847. https://doi.org/10.3390/v16121847
Pastor AF, Mahaney SM, Garcia J Jr., Morales M, Quintanilla O, Arriaga MA, Thomas JM III, VandeBerg JL. The Laboratory Opossum (Monodelphis domestica) Is a Unique Model for Research on Zika Virus: Robust Immune Response, Widespread Dissemination, and Long-Term Persistence. Viruses. 2024; 16(12):1847. https://doi.org/10.3390/v16121847
Chicago/Turabian StylePastor, André Filipe, Susan M. Mahaney, Juan Garcia, Jr., Marisol Morales, Oscar Quintanilla, Marco A. Arriaga, John M. Thomas, III, and John L. VandeBerg. 2024. "The Laboratory Opossum (Monodelphis domestica) Is a Unique Model for Research on Zika Virus: Robust Immune Response, Widespread Dissemination, and Long-Term Persistence" Viruses 16, no. 12: 1847. https://doi.org/10.3390/v16121847
APA StylePastor, A. F., Mahaney, S. M., Garcia, J., Jr., Morales, M., Quintanilla, O., Arriaga, M. A., Thomas, J. M., III, & VandeBerg, J. L. (2024). The Laboratory Opossum (Monodelphis domestica) Is a Unique Model for Research on Zika Virus: Robust Immune Response, Widespread Dissemination, and Long-Term Persistence. Viruses, 16(12), 1847. https://doi.org/10.3390/v16121847







