Biological Characterization and Evaluation of the Therapeutic Value of Vibrio Phages 4141 and MJW Isolated from Clinical and Sewage Water Samples of Kolkata
Abstract
1. Introduction
2. Methods
2.1. Bacteria Isolation
2.2. Bacteriophage Isolation
2.3. Phage Purification
2.4. Determination of the Host Range and Efficiency of Plating (EOP)
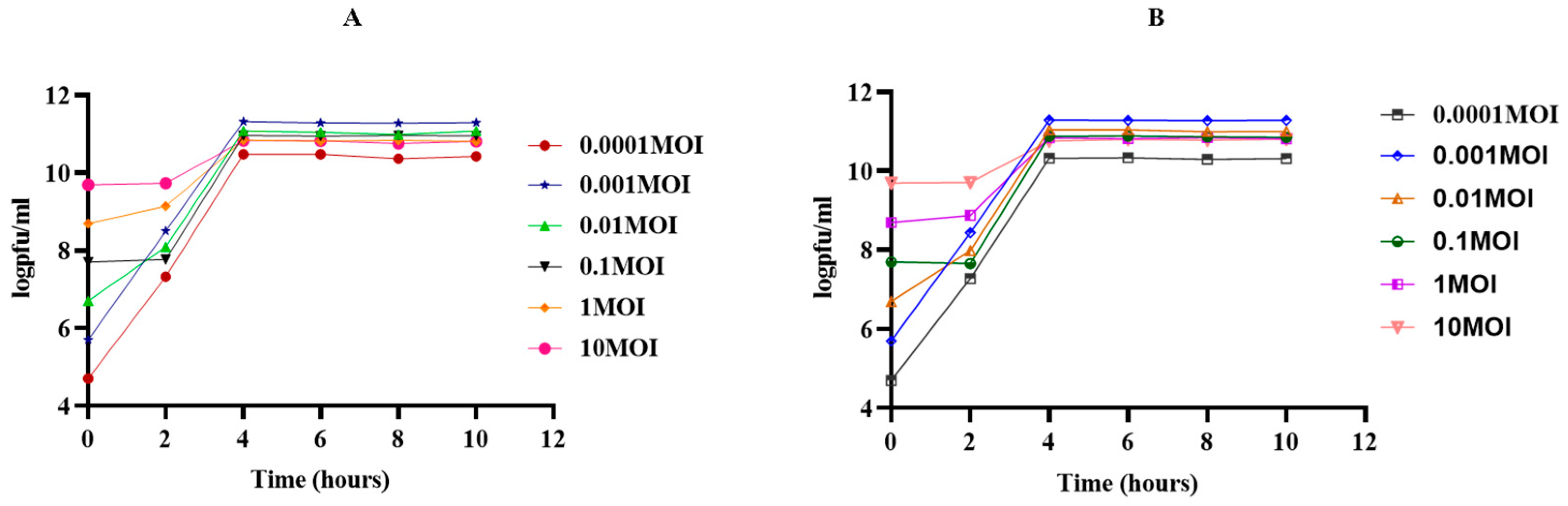
2.5. Determination of Suitable Multiplicity of Infection (MOI) and One-Step Growth Curve
2.6. Phage Stability (pH and Temperature)
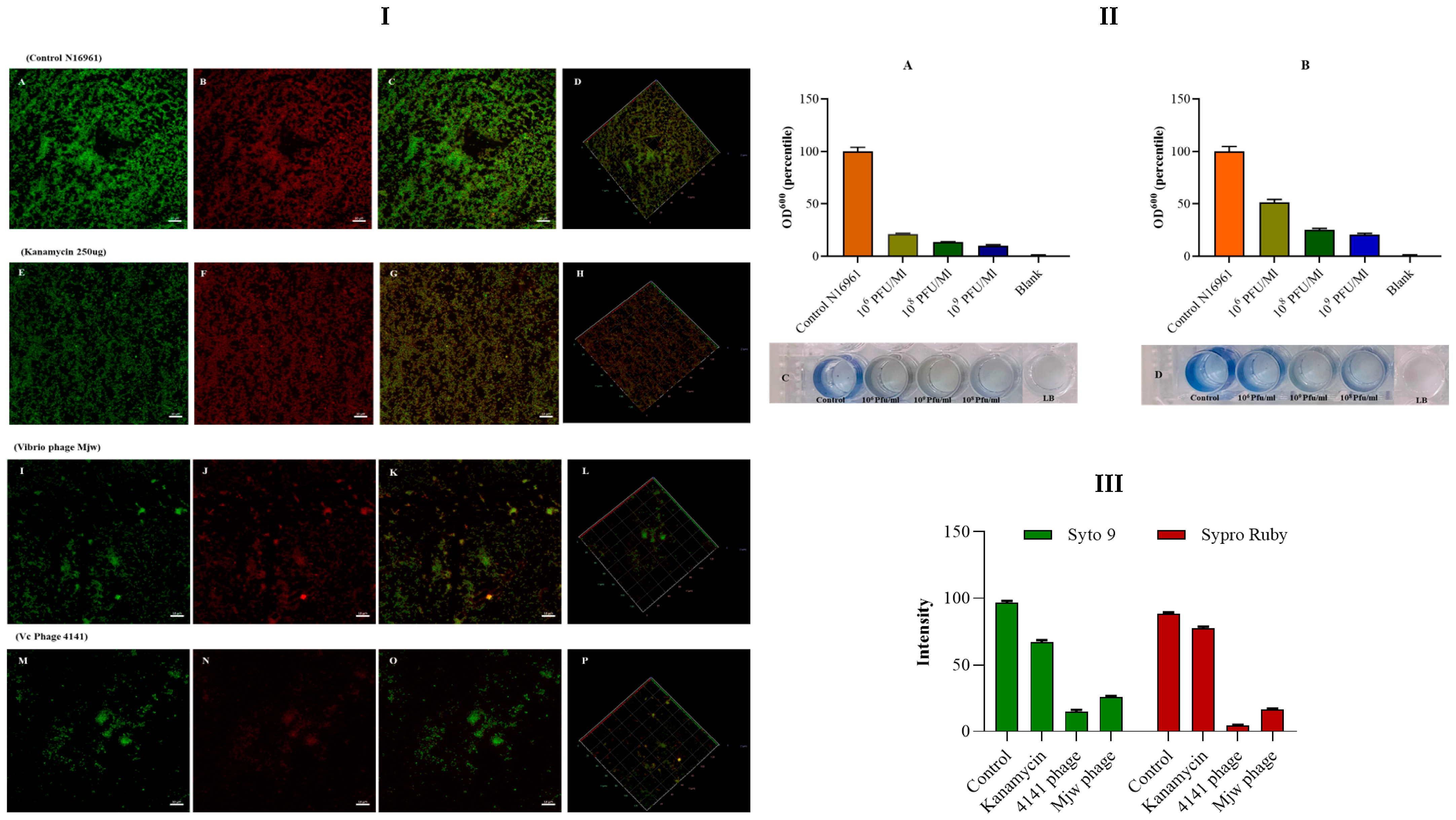
2.7. Biofilm Degradation Assay by Microtiter Plate Assay and Confocal Microscopy
2.8. Electron Microscopy
2.9. Genomic DNA Extraction and Restriction Pattern Analysis
2.10. SDS-PAGE Analysis of Phage Proteins
2.11. Whole-Genome Sequencing
2.12. Bioinformatic Analysis
2.13. Phylogenetic Analysis
2.14. Genome Sequences Accession Number
3. Results
3.1. Determination of the Morphology of Vibrio Phage 4141 and Vibrio Phage MJW
3.2. Host Range Determination and Efficiency of Plating (EOP)
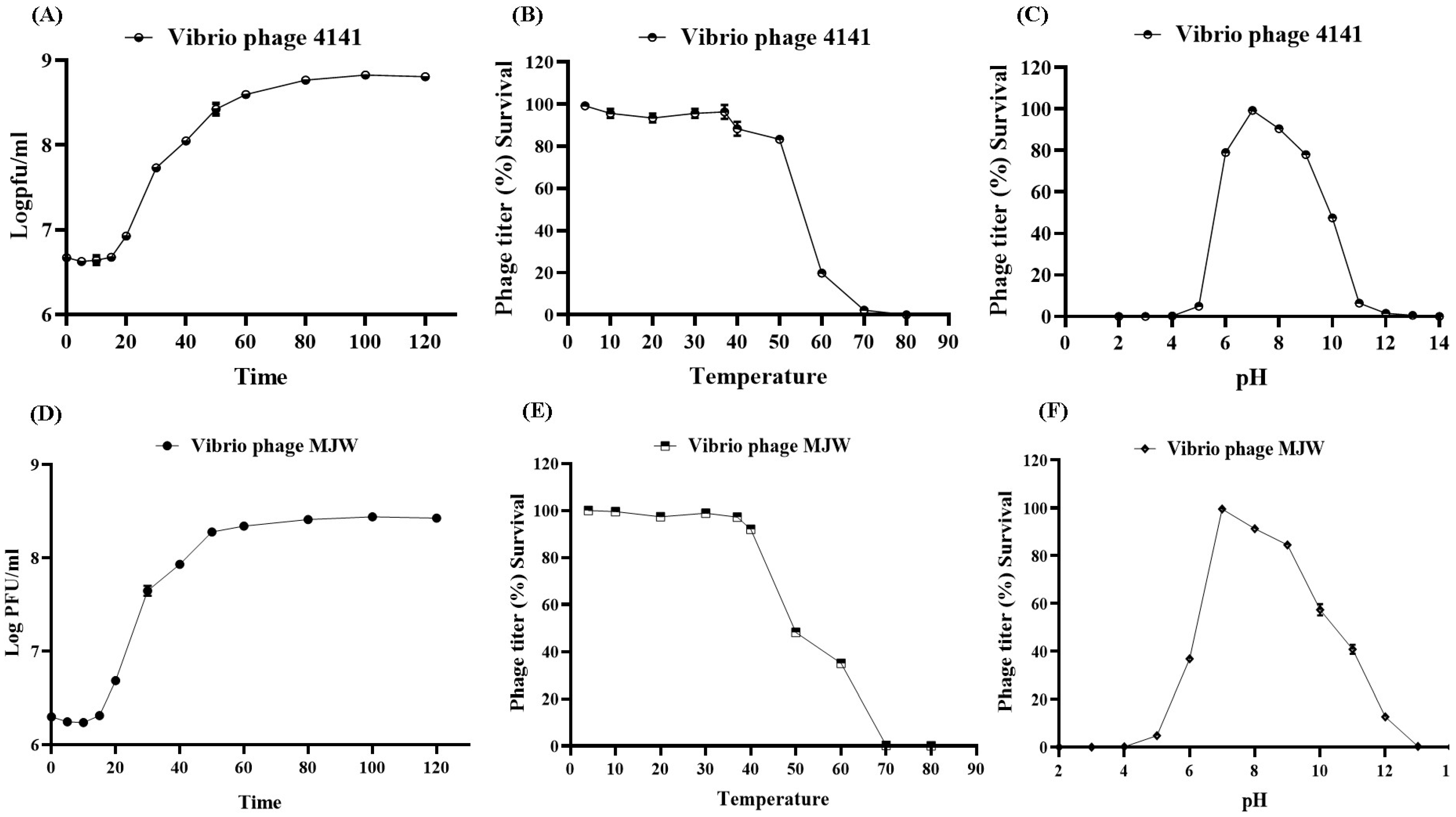
3.3. One-Step Growth Curve and Optimal MOI
3.4. Phage Stability
3.5. Antibiofilm Activity of the Vibrio Phage 4141 and the Vibrio Phage MJW
3.6. Restriction Endonuclease Digestion Pattern of Phage DNA
3.7. SDS-PAGE Analysis
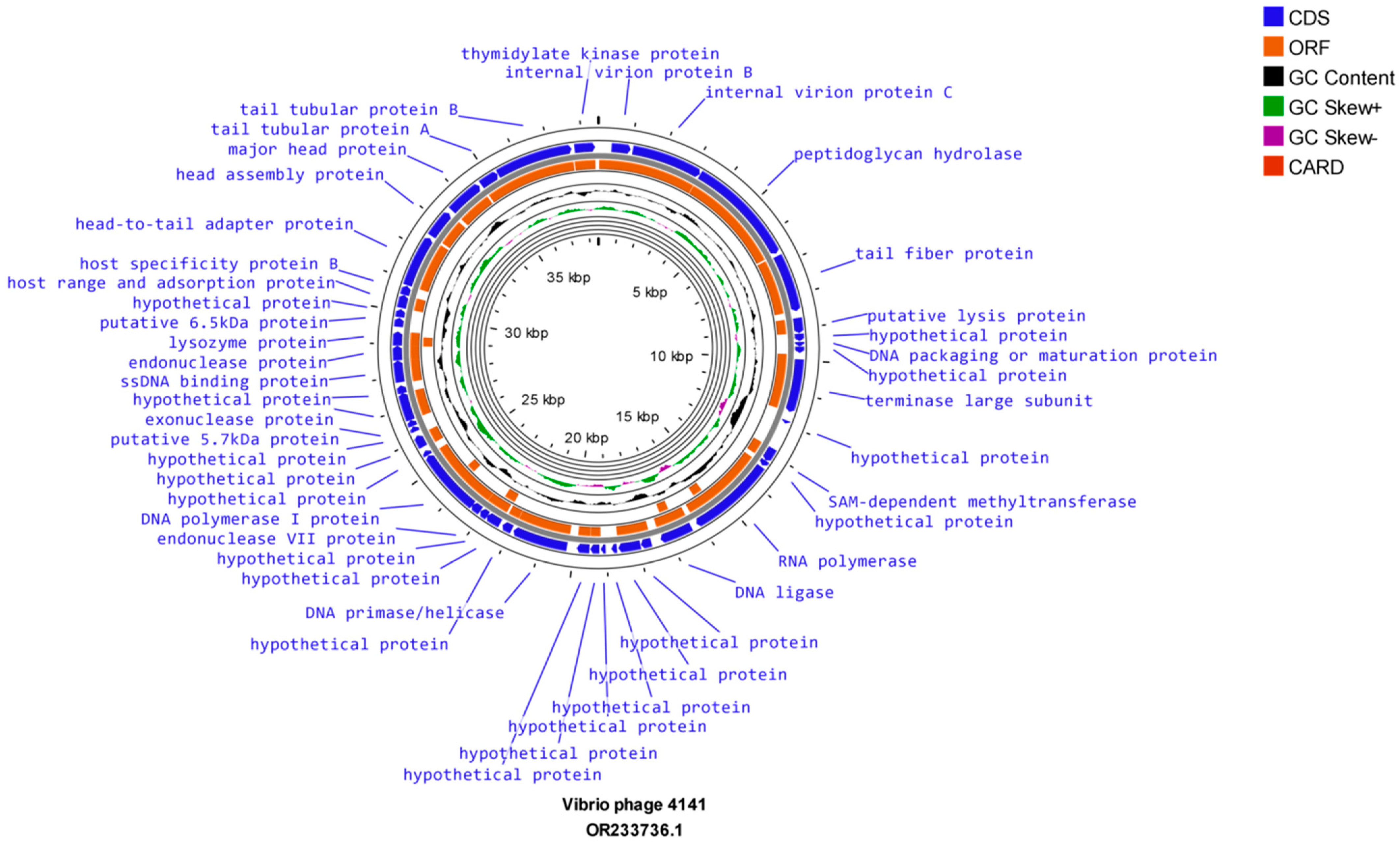
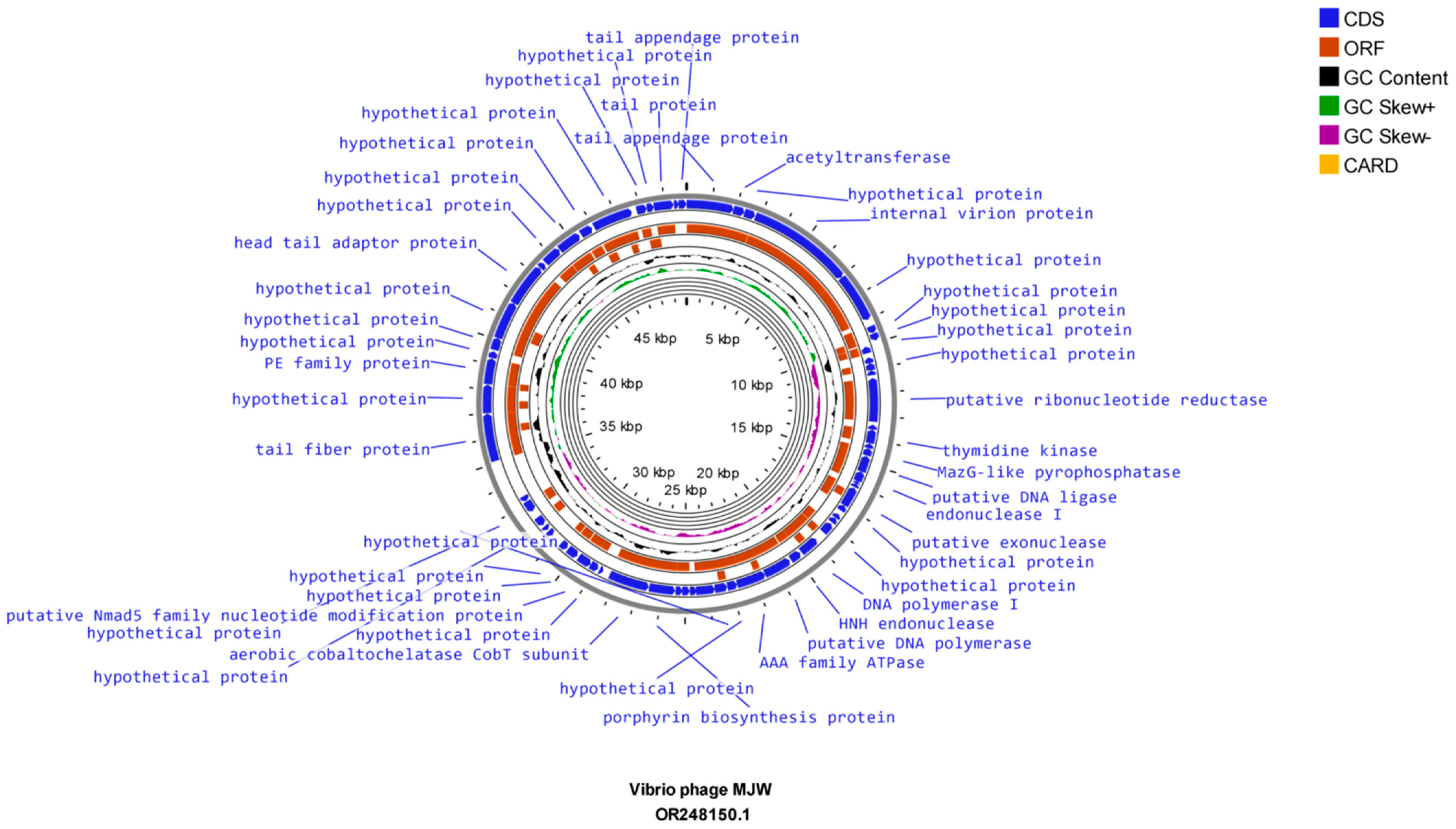
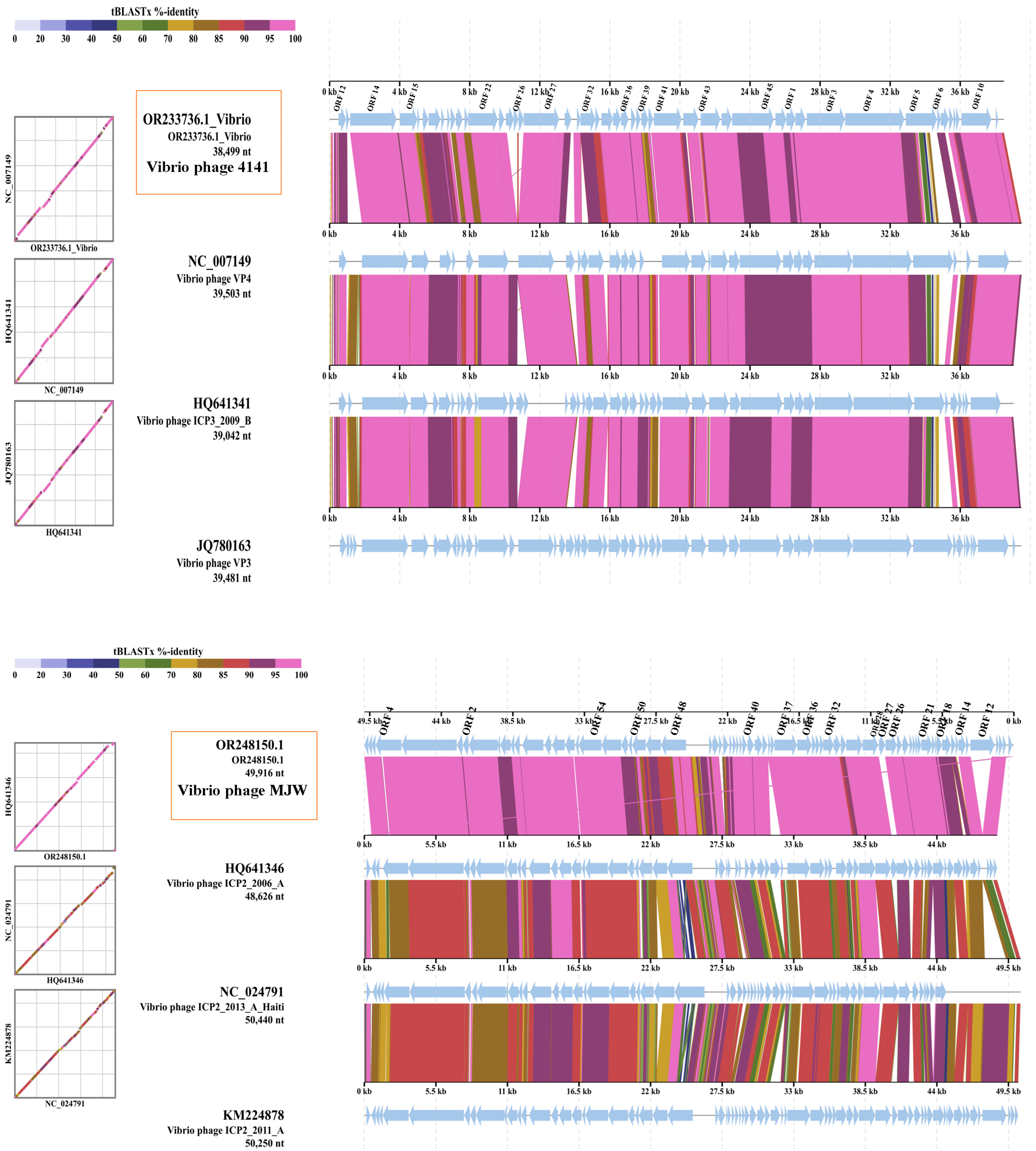
3.8. Genome Sequencing and Bioinformatics Analysis
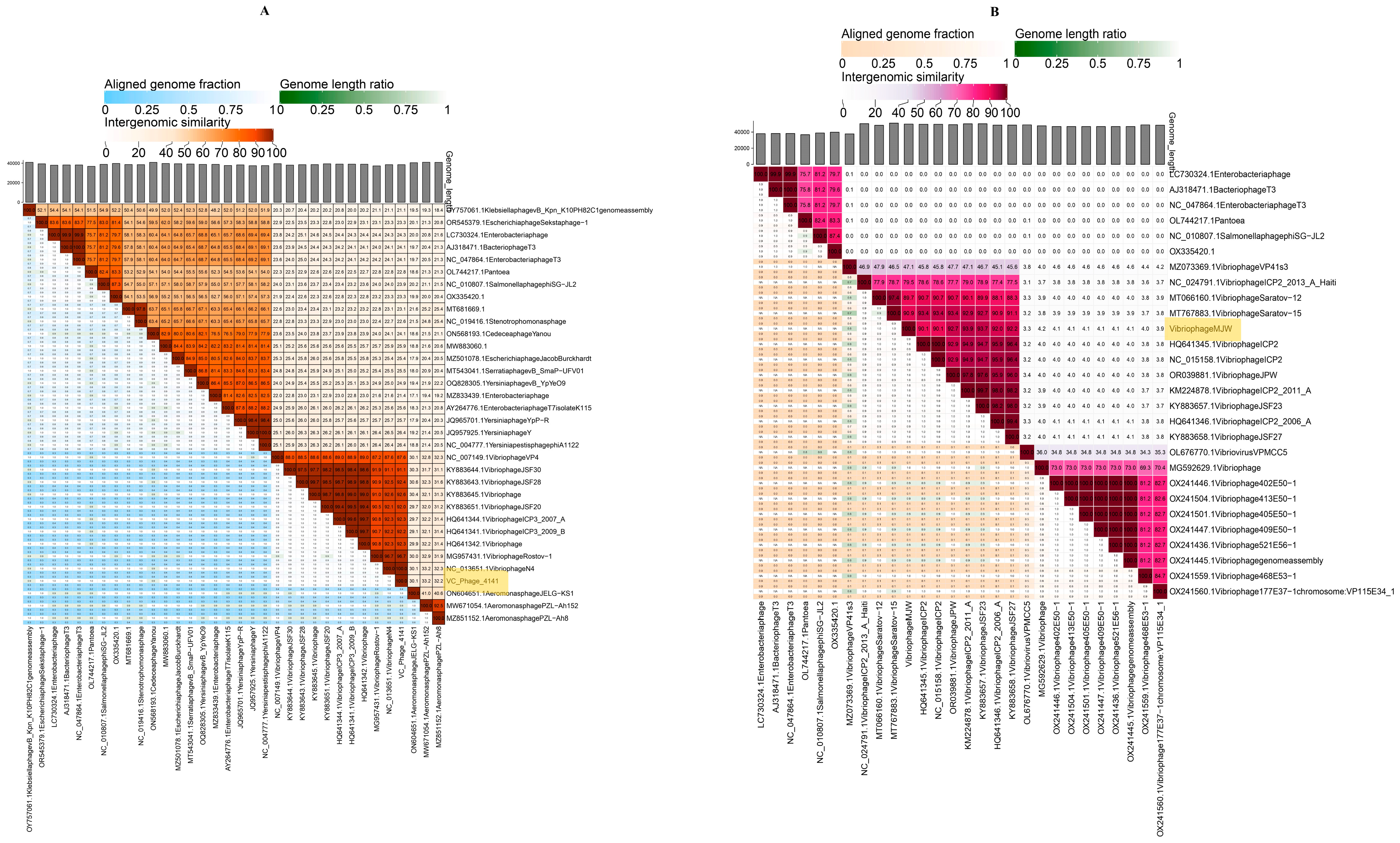
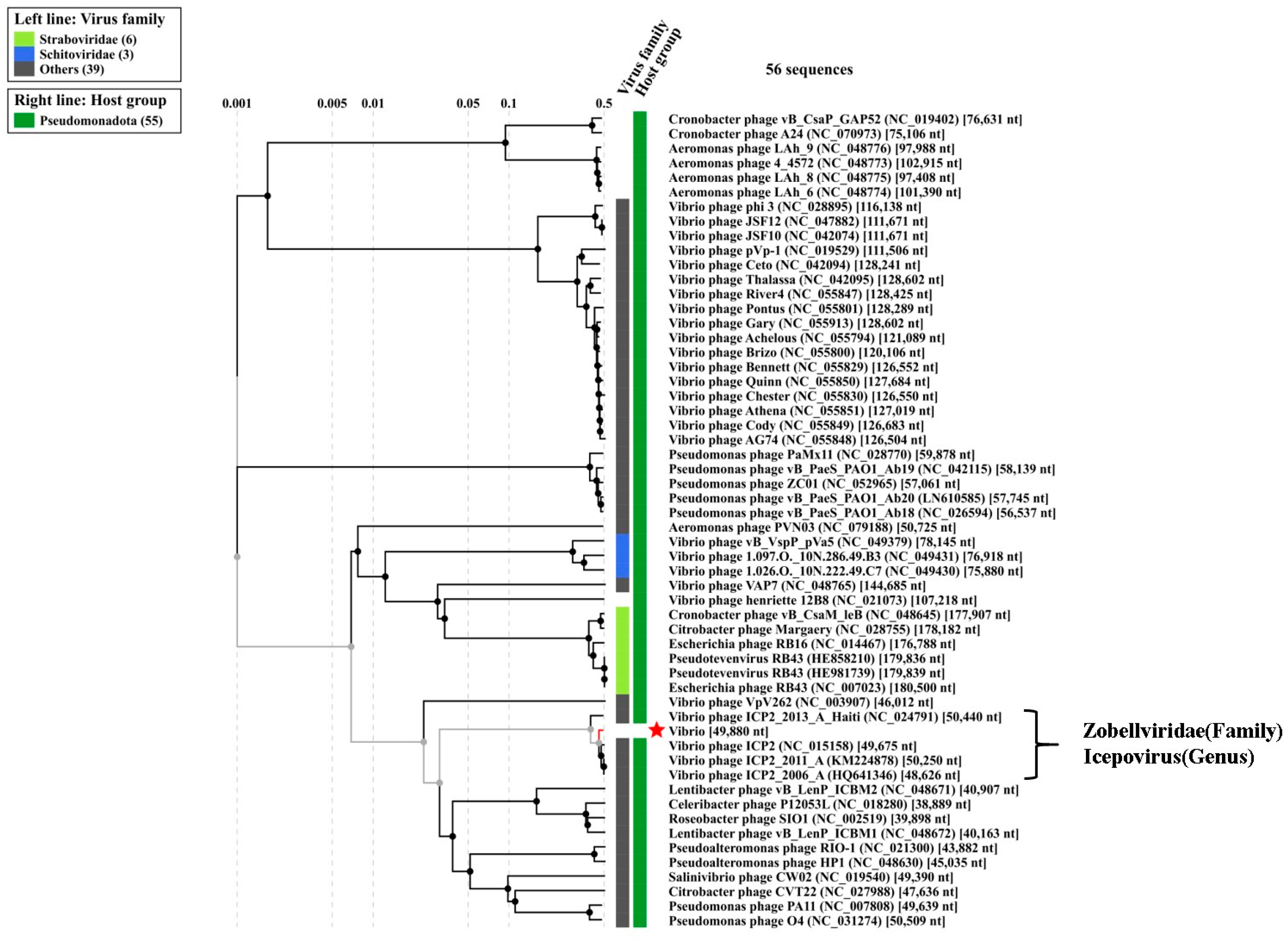
3.9. Phylogenetic Tree Analysis
4. Discussions
5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TEM | Transmission Electron Microscopy |
| WGS | Whole-Genome Sequencing |
| LA | Luria Agar |
| LB | Luria Broth |
| ORF | Open Reading Frame |
| MOI | Multiplicity of Infection |
| PFU | Plaque Forming Unit |
| MJW | Vibrio phage MJW |
| 4141 | Vibrio phage 4141 |
References
- Hraib, M.; Alaidi, S.; Jouni, S.; Saad, S.; Muna, M.; Alaidi, N.; Alshehabi, Z. Cholera: An Overview with Reference to the Syrian Outbreak. Avicenna J. Med. 2023, 13, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.V.; Matte, M.H.; Matte, G.R.; Jiang, S.; Sabeena, F.; Shukla, B.N.; Sanyal, S.C.; Huq, A.; Colwell, R.R. Molecular analysis of Vibrio cholerae O1, O139, non-O1, and non-O139 strains: Clonal relationships between clinical and environmental isolates. Appl. Environ. Microbiol. 2001, 67, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Nelson, A.R.; Lopez, A.L.; Sack, D.A. Updated global burden of cholera in endemic countries. PLoS Neglected Trop. Dis. 2015, 9, e0003832. [Google Scholar] [CrossRef] [PubMed]
- Muzembo, B.A.; Kitahara, K.; Debnath, A.; Ohno, A.; Okamoto, K.; Miyoshi, S.I. Cholera Outbreaks in India, 2011–2020: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5738. [Google Scholar] [CrossRef]
- Miller, J.; Birnbaum, M.L. Characterization of Interventional Studies of the Cholera Epidemic in Haiti. Prehospital Disaster Med. 2018, 33, 176–181. [Google Scholar] [CrossRef]
- Leibovici-Weissman, Y.; Neuberger, A.; Bitterman, R.; Sinclair, D.; Salam, M.A.; Paul, M. Antimicrobial drugs for treating cholera. Cochrane Database Syst. Rev. 2014, 2014, CD008625. [Google Scholar] [CrossRef]
- Das, B.; Verma, J.; Kumar, P.; Ghosh, A.; Ramamurthy, T. Antibiotic resistance in Vibrio cholerae: Understanding the ecology of resistance genes and mechanisms. Vaccine 2020, 38, A83–A92. [Google Scholar] [CrossRef]
- Verma, J.; Bag, S.; Saha, B.; Kumar, P.; Ghosh, T.S.; Dayal, M.; Senapati, T.; Mehra, S.; Dey, P.; Desigamani, A.; et al. Genomic plasticity associated with antimicrobial resistance in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2019, 116, 6226–6231. [Google Scholar] [CrossRef]
- Wozniak, R.A.; Waldor, M.K. Integrative and conjugative elements: Mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat. Rev. Microbiol. 2010, 8, 552–563. [Google Scholar] [CrossRef]
- Siddique, A.; Akram, K.; Zaman, K.; Laston, S.; Salam, A.; Majumdar, R.N.; Islam, M.; Fronczak, N. Why treatment centres failed to prevent cholera deaths among Rwandan refugees in Goma, Zaire. Lancet 1995, 345, 359–361. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-Sensing Regulation of Antimicrobial Resistance in Bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Pauzé-Foixet, J.; Mathieu-Denoncourt, A.; Duperthuy, M. Elevated concentrations of polymyxin B elicit a biofilm-specific resistance mechanism in Vibrio cholerae. Res. Microbiol. 2024, 175, 104179. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Buliva, E.; Elnossery, S.; Okwarah, P.; Tayyab, M.; Brennan, R.; Abubakar, A. Cholera prevention, control strategies, challenges and World Health Organization initiatives in the Eastern Mediterranean Region: A narrative review. Heliyon 2023, 9, e15598. [Google Scholar] [CrossRef] [PubMed]
- Górski, A.; Jończyk-Matysiak, E.; Miedzybrodzki, R.; Weber-Dabrowska, B.; Łusiak-Szelachowska, M.; Bagińska, N.; Borysowski, J.; Łobocka, M.B.; Węgrzyn, A.; Węgrzyn, G. Phage therapy: Beyond antibacterial action. Front. Med. 2018, 5, 146. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, Y.; Wang, R.; Lou, J.; Zhang, L.; Li, J.; Bi, Z.; Kan, B. Multiple antibiotic resistance of Vibrio cholerae serogroup O139 in China from 1993 to 2009. PLoS ONE 2012, 7, e38633. [Google Scholar] [CrossRef][Green Version]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Wittebole, X.; De Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2014, 5, 226–235. [Google Scholar] [CrossRef]
- Liu, C.; Hong, Q.; Chang, R.Y.K.; Kwok, P.C.L.; Chan, H.K. Phage-Antibiotic Therapy as a Promising Strategy to Combat Multidrug-Resistant Infections and to Enhance Antimicrobial Efficiency. Antibiotics 2022, 11, 570. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Petrovic Fabijan, A.; Iredell, J.; Danis-Wlodarczyk, K.; Kebriaei, R.; Abedon, S.T. Translating phage therapy into the clinic: Recent accomplishments but continuing challenges. PLoS Biol. 2023, 21, e3002119. [Google Scholar] [CrossRef] [PubMed]
- Gorodnichev, R.; Kornienko, M.; Kuptsov, N.; Malakhova, M.; Bespiatykh, D.; Veselovsky, V.; Shitikov, E.; Ilina, E. Molecular genetic characterization of three new Klebsiella pneumoniae bacteriophages suitable for phage therapy. Extrem. Med. 2021, 23, 84–90. [Google Scholar] [CrossRef]
- Wintachai, P.; Surachat, K.; Singkhamanan, K. Isolation and Characterization of a Novel Autographiviridae Phage and Its Combined Effect with Tigecycline in Controlling Multidrug-Resistant Acinetobacter baumannii-Associated Skin and Soft Tissue Infections. Viruses 2022, 14, 194. [Google Scholar] [CrossRef]
- Kar, P.; Das, T.K.; Ghosh, S.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Characterization of a Vibrio-infecting bacteriophage, VPMCC5, and proposal of its incorporation as a new genus in the Zobellviridae family. Virus Res. 2022, 321, 198904. [Google Scholar] [CrossRef]
- Gorodnichev, R.B.; Kornienko, M.A.; Malakhova, M.V.; Bespiatykh, D.A.; Manuvera, V.A.; Selezneva, O.V.; Veselovsky, V.A.; Bagrov, D.V.; Zaychikova, M.V.; Osnach, V.A.; et al. Isolation and Characterization of the First Zobellviridae Family Bacteriophage Infecting Klebsiella pneumoniae. Int. J. Mol. Sci. 2023, 24, 4038. [Google Scholar] [CrossRef]
- Yen, M.; Cairns, L.S.; Camilli, A. A cocktail of three virulent bacteriophages prevents Vibrio cholerae infection in animal models. Nat. Commun. 2017, 8, 14187. [Google Scholar] [CrossRef]
- Tewari, D.N.; Biswas, S.; Chakrabarti, A.K.; Dutta, S. Viral Inactivation and Biocompatibility Study of Electrically Activated Water Mist. Microbiol. Insights 2022, 15, 11786361221096651. [Google Scholar] [CrossRef]
- Chakrabarti, A.K.; Ghosh, A.N.; Nair, G.B.; Niyogi, S.K.; Bhattacharya, S.K.; Sarkar, B.L. Development and evaluation of a phage typing scheme for Vibrio cholerae O139. J. Clin. Microbiol. 2000, 38, 44–49. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, M.; Bhowmick, T.S.; Koley, H.; Atterbury, R.; Chakrabarti, A.K.; Sarkar, B.L. Isolation and Characterization of Novel Broad Host Range Bacteriophages of Vibrio cholerae O1 from Bengal. J. Glob. Infect. Dis. 2018, 10, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.K.; Biswas, A.; Tewari, D.N.; Mondal, P.P.; Dutta, S. Phage Types of Vibrio cholerae 01 Biotype ElTor Strains Isolated from India during 2012-2017. J. Glob. Infect. Dis. 2020, 12, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Chhibber, S.; Kaur, P.; Gondil, V.S. Simple drop cast method for enumeration of bacteriophages. J. Virol. Methods 2018, 262, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Esmael, A.; Azab, E.; Gobouri, A.A.; Nasr-Eldin, M.A.; Moustafa, M.M.A.; Mohamed, S.A.; Badr, O.A.M.; Abdelatty, A.M. Isolation and Characterization of Two Lytic Bacteriophages Infecting a Multi-Drug Resistant Salmonella Typhimurium and Their Efficacy to Combat Salmonellosis in Ready-to-Use Foods. Microorganisms 2021, 9, 423. [Google Scholar] [CrossRef]
- Mirzaei, M.K.; Nilsson, A.S. Correction: Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE 2015, 10, e0127606. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Shi, K.; Fang, Z.; Yang, Y.; Zhang, R. Isolation and characterization of a novel phage belonging to a new genus against Vibrio parahaemolyticus. Virol. J. 2023, 20, 81. [Google Scholar] [CrossRef]
- Ranveer, S.A.; Dasriya, V.; Ahmad, F.; Dhillon, H.S.; Samtiya, M.; Shama, E.; Anand, T.; Dhewa, T.; Chaudhary, V.; Chaudhary, P.; et al. Positive and negative aspects of bacteriophages and their immense role in the food chain. NPJ Sci. Food 2024, 8, 1. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, P.; Kalia, N.; Singh, J.; Kaur, S. Anti-biofilm Properties of the Fecal Probiotic Lactobacilli Against Vibrio spp. Front. Cell. Infect. Microbiol. 2018, 8, 120. [Google Scholar] [CrossRef]
- Ivanova, L.A.; Egorov, V.V.; Zabrodskaya, Y.A.; Shaldzhyan, A.A.; Baranchikov, A.Y.; Tsvigun, N.V.; Lykholay, A.N.; Yapryntsev, A.D.; Lebedev, D.V.; Kulminskaya, A.A. Matrix is everywhere: Extracellular DNA is a link between biofilm and mineralization in Bacillus cereus planktonic lifestyle. NPJ Biofilms Microbiomes 2023, 9, 9. [Google Scholar] [CrossRef]
- Monninger, M.K.; Nguessan, C.A.; Blancett, C.D.; Kuehl, K.A.; Rossi, C.A.; Olschner, S.P.; Williams, P.L.; Goodman, S.L.; Sun, M.G. Preparation of viral samples within biocontainment for ultrastructural analysis: Utilization of an innovative processing capsule for negative staining. J. Virol. Methods 2016, 238, 70–76. [Google Scholar] [CrossRef]
- Chevallet, M.; Luche, S.; Rabilloud, T. Silver staining of proteins in polyacrylamide gels. Nat. Protoc. 2006, 1, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. ViPTree: The viral proteomic tree server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef] [PubMed]
- Lomsadze, A.; Gemayel, K.; Tang, S.; Borodovsky, M. Modeling leaderless transcription and atypical genes results in more accurate gene prediction in prokaryotes. Genome Res. 2018, 28, 1079–1089. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Mihara, T.; Nishimura, Y.; Shimizu, Y.; Nishiyama, H.; Yoshikawa, G.; Uehara, H.; Hingamp, P.; Goto, S.; Ogata, H. Linking Virus Genomes with Host Taxonomy. Viruses 2016, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence Alignment High Accuracy High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Moraru, C.; Varsani, A.; Kropinski, A.M. VIRIDIC-A Novel Tool to Calculate the Intergenomic Similarities of Prokaryote-Infecting Viruses. Viruses 2020, 12, 1268. [Google Scholar] [CrossRef]
- Steven, A.; Trus, B.; Maizel, J.; Unser, M.; Parry, D.; Wall, J.; Hainfeld, J.; Studier, F. Molecular substructure of a viral receptor-recognition protein. The gp17 tail-fiber of bacteriophage T7. J. Mol. Biol. 1988, 200, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.; Chen, Y.; Biswas, S.; Canfield, E.; Abdolvahabi, A.; Feldman, D.E. Bacteriophage antidefense genes that neutralize TIR and STING immune responses. Cell Rep. 2023, 42, 112305. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.; Prazak, J.; Que, Y.A.; Cameron, D.R. Progress and Pitfalls of Bacteriophage Therapy in Critical Care: A Concise Definitive Review. Crit. Care Explor. 2021, 3, e0351. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef]
- Fu, J.; Li, Y.; Zhao, L.; Wu, C.; He, Z. Characterization and Genomic Analysis of a Bacteriophage with Potential in Lysing Vibrio alginolyticus. Viruses 2023, 15, 135. [Google Scholar] [CrossRef]
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current State of Compassionate Phage Therapy. Viruses 2019, 11, 343. [Google Scholar] [CrossRef]
- Turner, D.; Kropinski, A.M.; Adriaenssens, E.M. A Roadmap for Genome-Based Phage Taxonomy. Viruses 2021, 13, 506. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Zhang, C.; Zhang, C.; Xu, X.; Zhou, X.; Zhao, Z. Autographiviridae phage HH109 uses capsular polysaccharide for infection of Vibrio alginolyticus. iScience 2024, 27, 110695. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Gordon, M.; Ramirez, P. Efficacy and Experience of Bacteriophages in Biofilm-Related Infections. Antibiotics 2024, 13, 125. [Google Scholar] [CrossRef]
- Liu, Y.; Demina, T.A.; Roux, S.; Aiewsakun, P.; Kazlauskas, D.; Simmonds, P.; Prangishvili, D.; Oksanen, H.M.; Krupovic, M. Diversity, taxonomy, and evolution of archaeal viruses of the class Caudoviricetes. PLoS Biol. 2021, 19, e3001442. [Google Scholar] [CrossRef] [PubMed]
- Lupo, D.; Leptihn, S.; Nagler, G.; Haase, M.; Molineux, I.J.; Kuhn, A. The T7 ejection nanomachine components gp15-gp16 form a spiral ring complex that binds DNA and a lipid membrane. Virology 2015, 486, 263–271. [Google Scholar] [CrossRef] [PubMed]
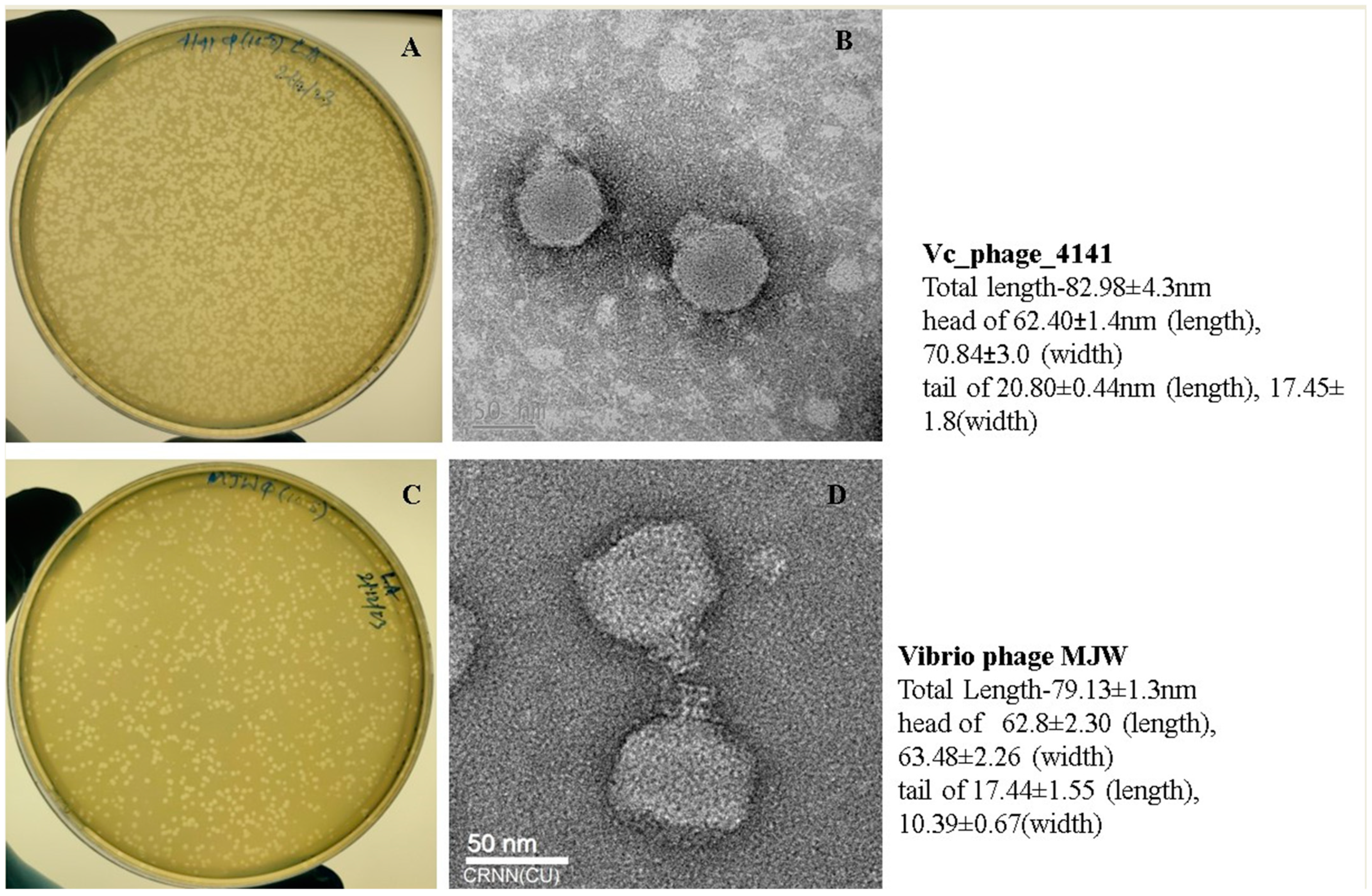


| Serial Number | Sample Number | State | Year | 4141 Phage | MJW Phage | EOP of 4141 Phage | EOP of MJW Phage |
|---|---|---|---|---|---|---|---|
| 1 | VC-314 | Narendra modi hospital, gujrat | 2023 | ++ | ++ | 1.0 | 0.98 |
| 2 | VC-317 | Narendra modi hospital, gujrat | 2023 | ++ | ++ | 1.0 | 0.81 |
| 3 | VC-272 | Narendra modi hospital, gujrat | 2023 | ++ | ++ | 1.0 | 0.68 |
| 4 | VC-288 | Narendra modi hospital, gujrat | 2023 | + | + | 0.87 | 0.89 |
| 5 | VC-321 | Narendra modi hospital, gujrat | 2023 | - | - | 0.08 | 0.04 |
| 6 | VC-274 | Narendra modi hospital, gujrat | 2023 | ++ | ++ | 1.01 | 0.88 |
| 7 | VC-344 | Narendra modi hospital, gujrat | 2023 | ++ | ++ | 1.05 | 0.91 |
| 8 | VC-268 | Narendra modi hospital, gujrat | 2023 | ++ | ++ | 1.0 | 1.0 |
| 9 | VC-319 | Narendra modi hospital, gujrat | 2023 | ++ | ++ | 1.1 | 0.77 |
| 10 | VC-301 | Narendra modi hospital, gujrat | 2023 | ++ | ++ | 1.0 | 1.01 |
| 11 | SPHL-14 | Narendra modi hospital, gujrat | 2023 | ++ | ++ | 1.02 | 0.99 |
| 12 | SPHL-13 | Narendra modi hospital, gujrat | 2023 | ++ | ++ | 1.08 | 0.92 |
| 13 | VC-01KK23 | Kolar, karnataka | 2023 | ++ | ++ | 1.03 | 1.0 |
| 14 | IS-07 | Mallapuram hospital | 2023 | + | - | 0.51 | 0.44 |
| 15 | IS-04 | Mallapuram hospital | 2023 | ++ | ++ | 1.01 | 1.02 |
| 16 | IS-05 | Mallapuram hospital | 2023 | ++ | ++ | 1.04 | 0.92 |
| 17 | IS-06 | Mallapuram hospital | 2023 | ++ | ++ | 1.0 | 0.81 |
| 18 | IS-12 | Mallapuram hospital | 2023 | ++ | ++ | 1.0 | 0.78 |
| 19 | IS-13 | Mallapuram hospital | 2023 | ++ | ++ | 0.98 | 0.93 |
| 20 | IS-11 | Mallapuram hospital | 2023 | ++ | + | 0.94 | 1.0 |
| 21 | IS-10 | Mallapuram hospital | 2023 | ++ | ++ | 0.91 | 0.69 |
| 22 | IS-09 | Mallapuram hospital | 2023 | ++ | ++ | 0.89 | 0.83 |
| 23 | IS-17 | Mallapuram hospital | 2023 | ++ | ++ | 0.79 | 0.97 |
| 24 | VC-65/D-37 | Delhi aiims | 2022 | ++ | ++ | 1.0 | 1.0 |
| 25 | VC-116/D-37 | Delhi aiims | 2022 | ++ | ++ | 1.02 | 1.06 |
| 26 | VC-26/D-37 | Delhi aiims | 2022 | ++ | ++ | 1.01 | 0.82 |
| 27 | VC-48/D-37 | Delhi aiims | 2022 | ++ | ++ | 1.0 | 0.87 |
| 28 | VC-149/D-37 | Delhi aiims | 2022 | ++ | ++ | 0.99 | 1.0 |
| 29 | VC-281/D-37 | Delhi aiims | 2022 | ++ | ++ | 0.91 | 1.0 |
| 30 | VC-242/D-37 | Delhi aiims | 2022 | + | - | 0.39 | 0 |
| 31 | VC-0095/D-37 | Delhi aiims | 2020 | + | + | 0.44 | 0.21 |
| 32 | VC-8939 | Rajkot/asalim | 2021 | ++ | ++ | 1.0 | 1.0 |
| 33 | VC-240 | Amehdabad | 2021 | ++ | ++ | 0.71 | 0.63 |
| 34 | VC-26/AMH | Amehdabad | 2021 | ++ | ++ | 0.89 | 0.75 |
| 35 | VC-274/AMH | Amehdabad | 2021 | + | + | 0.94 | 1.01 |
| 36 | VC-284/AMH | Amehdabad | 2021 | - | ++ | 1.04 | 1.0 |
| 37 | VC-15/AMH | Amehdabad | 2021 | + | + | 0.48 | 0.33 |
| 38 | Vc-455/AMH | Amehdabad | 2021 | ++ | ++ | 1.02 | 1.0 |
| 39 | PL-207/GTPR | Kolkata | 2019 | ++ | ++ | 1.0 | 0.88 |
| 40 | PL-453/GTPR | Kolkata | 2019 | ++ | ++ | 1.0 | 1.0 |
| 41 | VC-0094/D-37 | Delhi aiims | 2020 | ++ | ++ | 1.1 | 1.02 |
| 42 | VC-0084/D-37 | Delhi aiims | 2020 | ++ | ++ | 1.0 | 1.01 |
| 43 | VC-0079/D-37 | Delhi aiims | 2020 | ++ | ++ | 1.02 | 1.0 |
| 44 | VC-0093/D-37 | Delhi aiims | 2020 | ++ | ++ | 1.0 | 0.95 |
| 45 | PL-161/GTPR | Kolkata | 2019 | - | - | 0.07 | 0 |
| 46 | PL-159/GTPR | Kolkata | 2019 | - | - | 0 | 0 |
| 47 | PL-211 | Kolkata | 2019 | + | + | 0.69 | 0.55 |
| 48 | G-54/GTPR | Kolkata | 2019 | ++ | ++ | 1.0 | 0.69 |
| 49 | PL-471/GTPR | Kolkata | 2019 | ++ | ++ | 1.0 | 0.76 |
| 50 | VC-0087/D-37 | Delhi aiims | 2020 | ++ | ++ | 1.1 | 0.83 |
| 51 | MAK757-ELTOR OGAWA | Vibrio phage laboratory | ++ | ++ | 1.0 | 1.0 | |
| 52 | N16961-ELTOR INAWA | Vibrio phage laboratory | ++ | ++ | 1.0 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, S.; Tewari, D.N.; Chakrabarti, A.K.; Dutta, S. Biological Characterization and Evaluation of the Therapeutic Value of Vibrio Phages 4141 and MJW Isolated from Clinical and Sewage Water Samples of Kolkata. Viruses 2024, 16, 1741. https://doi.org/10.3390/v16111741
Biswas S, Tewari DN, Chakrabarti AK, Dutta S. Biological Characterization and Evaluation of the Therapeutic Value of Vibrio Phages 4141 and MJW Isolated from Clinical and Sewage Water Samples of Kolkata. Viruses. 2024; 16(11):1741. https://doi.org/10.3390/v16111741
Chicago/Turabian StyleBiswas, Sanjoy, Devendra Nath Tewari, Alok Kumar Chakrabarti, and Shanta Dutta. 2024. "Biological Characterization and Evaluation of the Therapeutic Value of Vibrio Phages 4141 and MJW Isolated from Clinical and Sewage Water Samples of Kolkata" Viruses 16, no. 11: 1741. https://doi.org/10.3390/v16111741
APA StyleBiswas, S., Tewari, D. N., Chakrabarti, A. K., & Dutta, S. (2024). Biological Characterization and Evaluation of the Therapeutic Value of Vibrio Phages 4141 and MJW Isolated from Clinical and Sewage Water Samples of Kolkata. Viruses, 16(11), 1741. https://doi.org/10.3390/v16111741






