Mpox Resurgence: A Multifaceted Analysis for Global Preparedness
Abstract
:1. Introduction
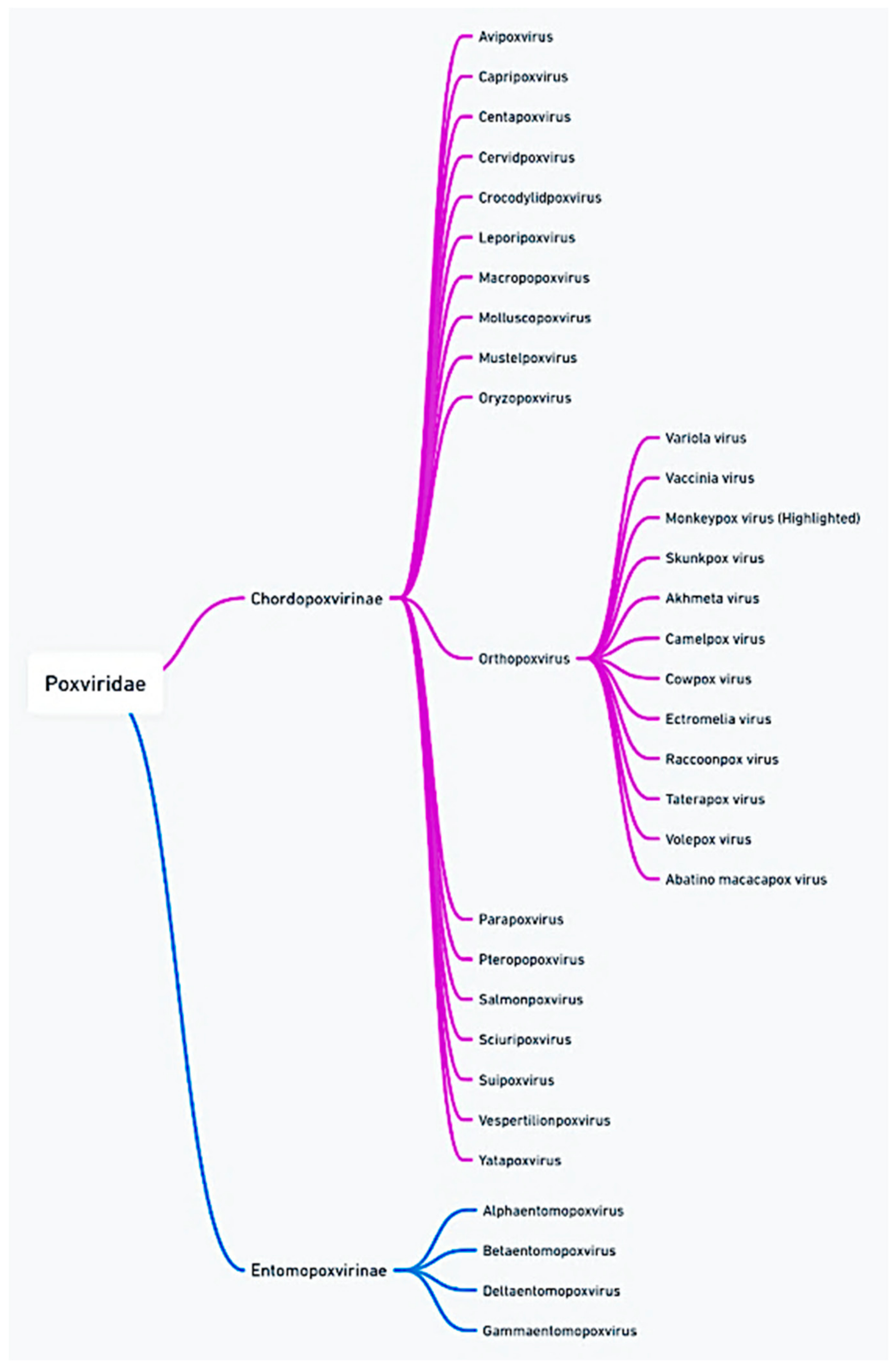
2. Molecular Characteristics of Mpox
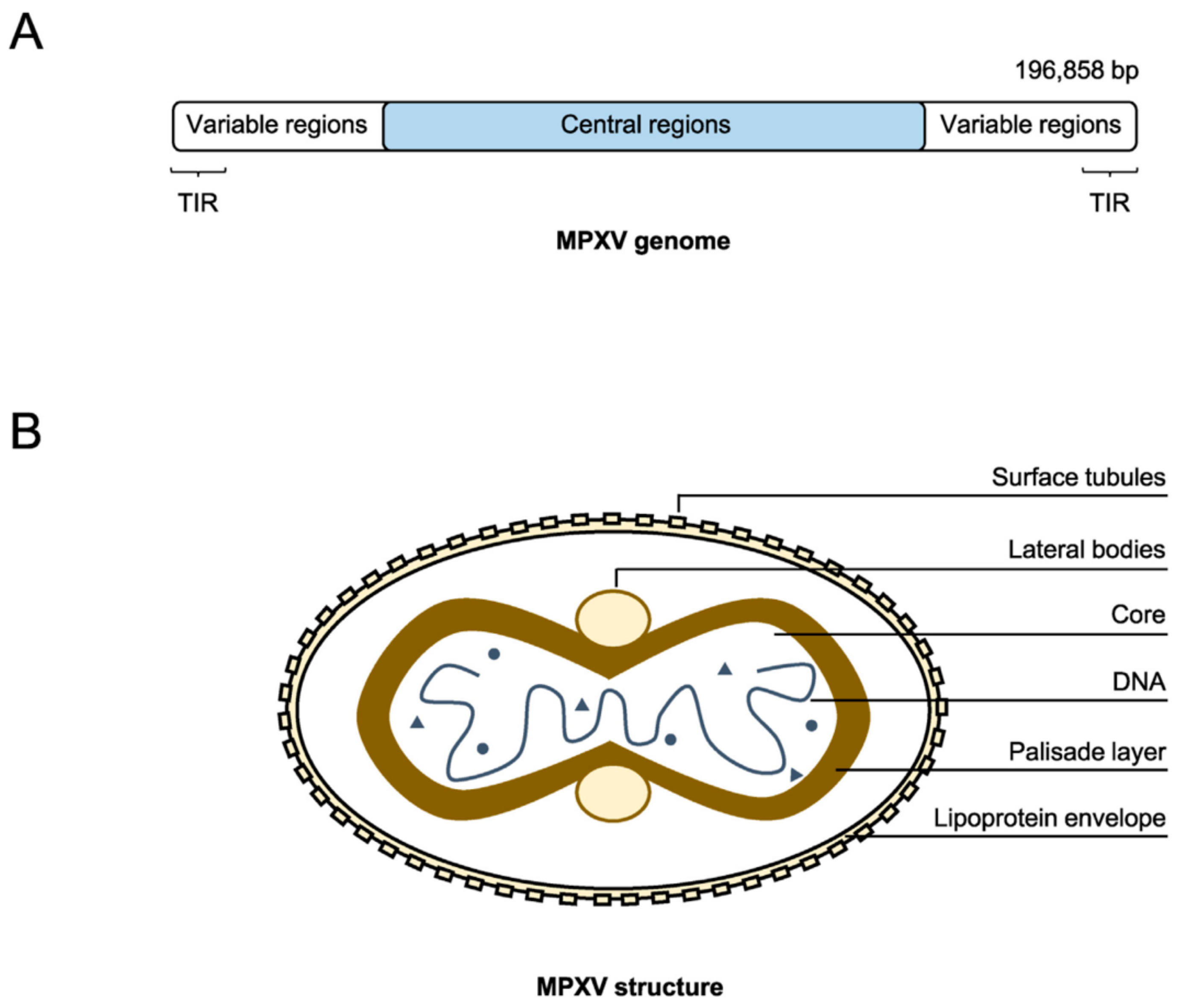
2.1. Genomic Structure
2.2. Evolutionary Clades and Mutations
2.3. Mechanisms of Viral Infection
2.4. Environmental Stability and Resistance
3. Epidemiology of Mpox
3.1. Historical Epidemiology (1970–2020)
3.2. Global Outbreaks and Trends (2022–2024)
3.3. Transmission Patterns
3.4. High-Risk Populations and Susceptibility
4. Clinical Features and Disease Progression
4.1. Incubation Period and Early Symptoms
4.2. Clinical Manifestations and Complications
- Neurological complications, although rare, can include encephalitis, seizures, and focal neurological deficits, which may result from the virus’s ability to invade the central nervous system. These complications often require intensive supportive care and can lead to long-term neurological sequelae.
- Ocular complications are another serious concern, with patients potentially developing conjunctivitis, keratitis, and, in severe cases, corneal scarring, which can lead to permanent vision loss if not promptly treated.
- Respiratory complications, such as bronchopneumonia, can occur when the virus spreads to the respiratory tract, either through direct viral invasion or secondary bacterial infection, and are a leading cause of mortality in severe cases of mpox.
- The management of skin lesions to prevent bacterial superinfection;
- Monitoring for signs of neurological or respiratory involvement;
- Providing appropriate ophthalmologic care when ocular manifestations are present (Supplementary Figures S7 and S8; Supplementary Table S5).
4.3. Outcomes and Mortality Rates
5. Diagnosis of Mpox
5.1. Specimen Collection and Laboratory Techniques
5.2. Molecular Methods
6. Treatment and Management Strategies
6.1. Supportive Care
6.2. Antiviral Therapies
6.3. Immunotherapy and Vaccination
7. Prevention and Public Health Implications
7.1. Vaccination Strategies and Efficacy
7.2. Public Health Policies and Recommendations
7.3. Future Directions in Prevention and Control
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Meyer, H.; Perrichot, M.; Stemmler, M.; Emmerich, P.; Schmitz, H.; Varaine, F.; Shungu, R.; Tshioko, F.; Formenty, P. Outbreaks of disease suspected of being due to human Monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002, 40, 2919–2921. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wagner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Faitley, J.A.; et al. The detection of Monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox virus in Nigeria Infection biology, epidemiology, and evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- Shchelkunov, S.N. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog. 2013, 9, e1003756. [Google Scholar] [CrossRef]
- Faye, O.; Pratt, C.B.; Faye, M.; Fall, G.; Chitty, J.; Diagne, M.; Wiley, M.R.; Yinka-Ogunleye, A.F.; Aruna, S.; Etebu, E.N.; et al. Genomic characterisation of human Monkeypox virus in Nigeria. Lancet Infect. Dis. 2018, 18, 246. [Google Scholar] [CrossRef]
- Yu, Z.; Zou, X.; Deng, Z.; Zhao, M.; Gu, C.; Fu, L.; Xiao, W.; He, W.; He, M.; Yang, Q.; et al. Genome analysis of the mpox virus and characterization of core/variable regions. Genomics 2024, 116, 110763. [Google Scholar] [CrossRef]
- Shchelkunov, S.N.; Totmenin, A.V.; Babkin, I.V. Human Monkeypox and smallpox viruses Genomic comparison. FEBS Lett. 2001, 509, 66–70. [Google Scholar] [CrossRef]
- Shchelkunov, S.N.; Totmenin, A.V.; Babkin, I.V.; Mikheev, M.V.; Gutorov, V.V.; Ryazankina, O.I.; Moss, B. Comparative analysis of orthopoxvirus genomes and implications for poxvirus evolution. J. Virol. 2002, 76, 3920–3932. [Google Scholar]
- Yi, X.M.; Lei, Y.L.; Li, M.; Li, Z.; Li, S. Les interactions entre le virus de la variole du singe et l’hôte. Cell Insight 2024, 3, 100185. [Google Scholar] [CrossRef]
- Essbauer, S.; Meyer, H.; Pfeffer, M. Zoonotic poxviruses. Vet. Microbiol. 2010, 140, 229–236. [Google Scholar] [CrossRef]
- Kugelman, J.R.; Johnston, S.C.; Mulembakani, P.M.; Kisalu, N.; Lee, M.S.; Koroleva, G.; Mccarthy, S.E.; Grxtole, M.C.; Wolfe, N.D.; Fair, J.N.; et al. Genomic variability of Monkeypox virus among humans in the Democratic Republic of the Congo. Emerg. Infect. Dis. 2014, 20, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Mauldin, M.R.; Antwerpen, M.; Emerson, G.L.; Li, Y.; Zoeller, G.; Carroll, D.S.; Meyer, H. Cowpox Virus: What’s in a Name? Viruses 2017, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Symons, J.A.; Khanna, A.; Vanderplasschen, A.; Alcamí, A. Vaccinia virus immune evasion. Immunol. Rev. 1997, 159, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Venturi, C.; Guadagno, A.; Varesano, S.; Ricucci, V.; Nigro, N.; Di Biagio, A.; Ciccarese, G. Histopathologic and Transmission Electron Microscopic Findings in Monkeypox Cutaneous Lesions. Le Infezioni in Medicina 2024, 32, 76. [Google Scholar]
- Schwartz, D.A.; Mbala-Kingebeni, P.; Patterson, K.; Huggins, J.W.; Pittman, P.R. Congenital Mpox Syndrome (Clade I) in stillborn fetus after placental infection and intrauterine transmission, Democratic Republic of the Congo, 2008. Emerg. Infect. Dis. 2023, 29, 2198. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Carroll, D.S.; Karem, K.L. Factors affecting the likelihood of Monkeypox’s emergence and spread in the post-smallpox era. Curr. Opin. Virol. 2010, 1, 334–343. [Google Scholar] [CrossRef]
- Kannan, S.R.; Sachdev, S.; Reddy, A.S.; Kandasamy, S.L.; Byrareddy, S.N.; Lorson, C.L.; Singh, K. Mutations in the monkeypox virus replication complex: Potential contributing factors to the 2022 outbreak. J. Autoimmun. 2022, 133, 102928. [Google Scholar] [CrossRef]
- Alakunle, E.; Kolawole, D.; Diaz-Canova, D.; Alele, F.; Adegboye, O.; Moens, U.; Okeke, M.I. A comprehensive review of monkeypox virus and mpox characteristics. Front. Cell. Infect. Microbiol. 2024, 14, 1360586. [Google Scholar] [CrossRef]
- Moss, B. Understanding the Biology of Monkeypox Virus to Prevent Future Outbreaks. Nat. Microbiol. 2024, 9, 1408–1416. [Google Scholar] [CrossRef]
- Tajudeen, Y.A.; Oladipo, H.J.; Muili, A.O.; Ikebuaso, J.G. Monkeypox: A review of a zoonotic disease of global public health concern. Health Promot. Perspect. 2023, 13, 1. [Google Scholar] [CrossRef]
- Moss, B. Poxvirus cell entry How many proteins does it take? Viruses 2013, 4, 688–707. [Google Scholar] [CrossRef] [PubMed]
- Lucena-Neto, F.D.; Falcão, L.F.; Vieira-Junior, A.S.; Moraes, E.C.; David, J.P.; Silva, C.C.; Sousa, J.R.; Duarte, M.I.S.; Vasconcelos, P.F.C.; Quaresma, J.A.S. Monkeypox virus immune evasion and eye manifestation: Beyond eyelid implications. Viruses 2023, 15, 2301. [Google Scholar] [CrossRef] [PubMed]
- Seet, B.T.; Johnston, J.B.; Brunetti, C.R.; McFadden, G. Poxviruses and immune evasion. Annu. Rev. Immunol. 2003, 21, 377–423. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.B.; McFadden, G. Poxvirus immunomodulatory strategies Current perspectives. J. Virol. 2004, 78, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Carroll, D.S.; Gallardo-Romero, N.; Weiss, S.; Clemmons, C.; Hughes, C.M.; Salzer, J.; Olson, V.A.; Abel, J.; Karem, K.; et al. Monkeypox disease transmission and the importance of residential proximity to a wildlife reservoir. J. Infect. Dis. 2013, 207, 1186–1195. [Google Scholar]
- Sagripanti, J.L.; Lytle, C.D. Sensitivity of Monkeypox virus to chemical disinfectants. J. Virol. Methods 2007, 141, 32–37. [Google Scholar]
- Hahon, N.; Kozikowski, E. Inactivation of poxviruses by heat and ultraviolet light. J. Bacteriol. 1961, 82, 886–891. [Google Scholar]
- Deere, J.R.; Lonsdorf, E.V.; Clennon, J.A.; Gillespie, T.R. Bridging the Gap: Integrating Knowledge from the Study of Social Network Analysis and Infectious Disease Dynamics in Human and Nonhuman Primates. Annu. Rev. Anthropol. 2024, 53, 37–53. [Google Scholar] [CrossRef]
- Boulanger, L.L.; Liverman, C.T. Review of the Centers for Disease Control and Prevention’s Smallpox Vaccination Program; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Breman, J.G.; Ruti, K.; Steniowski, M.V. Human Monkeypox A clinical and epidemiological study of 51 cases in the African region. Bull. World Health Organ. 1980, 58, 273–282. [Google Scholar]
- Damon, I.K. Status of human Monkeypox: Clinical characterization and public health concerns. Annu. Rev. Virol. 2011, 5, 345–357. [Google Scholar]
- Vaughan, A.M.; Cenciarelli, O.; Colombe, S.; de Sousa, L.A.; Fischer, N.; Gossner, C.M.; Haussig, J.M. A Large Multi-Country Outbreak of Monkeypox Across 41 Countries in the WHO European Region, 7 March to 23 August 2022. Eurosurveillance 2022, 27, 2200620. [Google Scholar] [CrossRef] [PubMed]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of Monkeypox virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Al-Mandhari, A.; Kodama, C.; Abubakar, A.; Hajjeh, R.; Brennan, R. Monkeypox Outbreak and Response Efforts in the Eastern Mediterranean Region. East. Mediterr. Health J. 2022, 28, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Baskaran, P.; Raghav, P.; Naveen, K.H. Monkeypox: Situation actuelle en Inde. Indian J. Community Med. 2022, 47, 628–630. [Google Scholar] [CrossRef]
- Sharma, S.K.; Dash, P.K.; Yadav, R.G.; Shrivastava, A.; Menon, R.; Kumar, J.S.; Sharma, S.; Dhankher, S.; Dhiman, S.; Kumari, D.; et al. Isolement et caractérisation du virus mpox émergent en Inde. J. Med. Virol. 2023, 95, e28911. [Google Scholar] [CrossRef]
- World Health Organization. Monkeypox Global Surveillance, Case Tracking, and Vaccine Coverage; WHO Report: Geneva, Switzerland, 2024. [Google Scholar]
- Hutin, Y.J.F.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of human Monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434–438. [Google Scholar] [CrossRef]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.; Reynolds, M.G.; et al. A tale of two clades Monkeypox viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef]
- Minhaj, F.S.; McCollum, A.M. Outbreak of Monkeypox virus infections in the United States, June 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 713–719. [Google Scholar]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Human Monkeypox virus infection in humans Global trends and transmission dynamics during the 2022–2023 outbreaks. Clin. Infect. Dis. 2023, 77, 1722–1730. [Google Scholar]
- Berríos-Torres, S.I.; Mu, Y.; Edwards, J.R.; Horan, T.C.; Fridkin, S.K. Improved risk adjustment in public reporting: Coronary artery bypass graft surgical site infections. Infect. Control. Hosp. Epidemiol. 2012, 33, 463–469. [Google Scholar] [CrossRef]
- Parker, S.; Buller, R.M. A review of experimental and natural infections of animals with Monkeypox virus between 1958 and 2012. Future Virol. 2013, 8, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Lee, K.N.; Abel, J.; Carroll, D.S.; Montgomery, J.M.; Olson, V.A.; Li, Y.; Vidson, W.; Hughes, C.; Dillon, M.; et al. Monkeypox zoonotic associations insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am. J. Trop. Med. Hyg. 2007, 76, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Marennikova, S.S.; Seluhina, E.M. Susceptibility of some rodent species to Monkeypox virus, and course of the infection. Bull. World Health Organ. 1976, 53, 13–20. [Google Scholar]
- Arita, I.; Henderson, D.A. Smallpox and Monkeypox in non-human primates. Bull. World Health Organ. 1968, 39, 277–283. [Google Scholar]
- Shelukhina, E.M.; Shenkman, L.S.; Rozina, E.E.; Marennikova, S.S. Possible mechanism of orthopoxvirus preservation in nature. Vopr. Virusol. 1979, 24, 368–372. [Google Scholar]
- Earl, P.L.; Americo, J.L.; Cotter, C.A.; Moss, B. Comparative live bioluminescence imaging of Monkeypox virus dissemination in a wild-derived inbred mouse (Mus musculus castaneus) and outbred African dormouse (Graphiurus kelleni). Virology 2015, 475, 150–158. [Google Scholar] [CrossRef]
- Jezek, Z.; Fenner, F. Human Monkeypox. Monogr. Virol. 1988, 17, 1–140. [Google Scholar]
- Falendysz, E.A.; Lopera, J.G.; Lorenzsonn, F.; Salzer, J.S.; Hutson, C.L.; Doty, J.; Romeo, N.G.; Carroll, D.S.; Osorio, J.E.; Rocke, T.E. Further assessment of Monkeypox virus infection in Gambian pouched rats (Cricetomys gambianus) using in vivo bioluminescent imaging. PLoS Neglected Trop. Dis. 2015, 9, e0004130. [Google Scholar] [CrossRef]
- Lahariya, C.; Thakur, A.; Dudeja, N. Monkeypox disease outbreak (2022): Epidemiology, challenges, and the way forward. Indian Pediatr. 2022, 59, 636–642. [Google Scholar] [CrossRef]
- McCollum, A.M.; Damon, I.K.; Moses, C. The clinical and epidemiological features of a major human Monkeypox outbreak in the Democratic Republic of the Congo. J. Clin. Virol. 2012, 55, 334–340. [Google Scholar]
- Zlámal, M.; Bartovská, Z.; Burantová, A.; Zákoucká, H.; Jiřincová, H.; Chmel, M.; Holub, M. Monkeypox and Herpes Simplex Virus Type 2 Coinfection: Case Report of Perianal Lesions in HIV-Positive Patient. Sex. Transm. Dis. 2022, 49, 769–770. [Google Scholar] [CrossRef] [PubMed]
- Khallafallah, O.; Grose, C. Reassessment of evidence about coinfection of chickenpox and monkeypox (mpox) in African children. Viruses 2022, 14, 2800. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef]
- Sigha, O.B.; Medi, S.C.; Nkoro, G.A.; Ndom, M.S.; Mbenoun, M.-L.; Kamdem, J.; Essomba, N.E.; Kouotou, E.A.S. Challenges in the Management of Monkeypox in a Developing Country: Case of Cameroon. Our Dermatol. Online 2024, 15 (Supp. S1), 24–29. [Google Scholar] [CrossRef]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Kuehnert, M.J. Clinical characteristics of human Monkeypox, and risk factors for severe disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef]
- Mailhe, M.; Beaumont, A.L.; Thy, M.; Le Pluart, D.; Perrineau, S.; Houhou-Fidouh, N.; Peiffer-Smadja, N. Clinical Characteristics of Ambulatory and Hospitalized Patients with Monkeypox Virus Infection: An Observational Cohort Study. Clin. Microbiol. Infect. 2023, 29, 233–239. [Google Scholar] [CrossRef]
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef]
- Mbala, P.K.; Huggins, J.W.; Riu-Rovira, T.; Ahuka-Mundeke, S.; Muyembe-Tamfum, J.J.; Wemakoy, O. Maternal and fetal outcomes among pregnant women with Monkeypox in the Democratic Republic of Congo. Clin. Infect. Dis. 2017, 64, 748–752. [Google Scholar] [CrossRef]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Hughes, C.M.; Kabamba, J.; Malekani, J.; et al. Extended human-to-human transmission during a Monkeypox outbreak in the Democratic Republic of the Congo. Emerg. Infect. Dis. 2016, 22, 1014–1021. [Google Scholar] [CrossRef]
- Brown, K.; Leggat, P.A. Human Monkeypox: Current state of knowledge and implications for the future. Trop. Med. Infect. Dis. 2016, 1, 8. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Wilkins, K.; Hughes, C.; Damon, I.K. Real-time PCR assays for the specific detection of Monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods 2006, 136, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.; Nitsche, A. Multicolour, multiplex real-time PCR assay for the detection of human-pathogenic poxviruses. Mol. Cell. Probes 2010, 24, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, I.; Saijo, M.; Shiota, T.; Ami, Y.; Suzaki, Y.; Nagata, N.; Hasegawa, H.; Sakai, K.; Fukushi, S.; Mizutani, T.; et al. Loop-mediated isothermal amplification-based diagnostic assay for Monkeypox virus infections. J. Med. Virol. 2009, 81, 1102–1108. [Google Scholar] [CrossRef]
- Euler, M.; Wang, Y.; Otto, P.; Tomaso, H.; Escudero, R.; Anda, P.; Hufert, F.T.; Weidmann, M. Recombinase polymerase amplification assay for rapid detection of Yersinia pestis. J. Clin. Microbiol. 2012, 50, 2234–2238. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.G.; Yorita, K.L.; Kuehnert, M.J.; Davidson, W.L.; Huhn, G.D.; Holman, R.C.; Damon, I.K. Clinical management of human Monkeypox Lessons from recent cases in the United States. J. Infect. Dis. 2006, 194, 773–780. [Google Scholar] [CrossRef] [PubMed]
- McCollum, A.M.; Damon, I.K.; Moses, C. The role of nutritional support in the management of Monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar]
- Jezek, Z.; Szczeniowski, M.; Paluku, K.M. Human Monkeypox Clinical features of 282 patients. J. Infect. Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Dunning, J. Psychological impact and supportive care in Monkeypox A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1022–1030. [Google Scholar] [CrossRef]
- Huggins, J.; Goff, A.; Hensley, L.; Mucker, E.; Shamblin, J.; Wlazlowski, C.; Johnson, W.; Chapman, J.; Larsen, T.; Twenhafel, N.; et al. Nonhuman primates are protected from smallpox virus or Monkeypox virus challenges by the antiviral drug ST-246. J. Virol. 2009, 83, 7253–7264. [Google Scholar] [CrossRef]
- Chittick, G.; Morrison, M.; Brundage, T.; Nichols, W.G.; Short, W.R. Brincidofovir A review of its use in preventing and treating viral infections. Drugs 2017, 77, 1531–1540. [Google Scholar]
- De Clercq, E. Cidofovir in the treatment of poxvirus infections. Antivir. Res. 2002, 55, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.W.; Damon, I.K.; Pertowski, C.A. Clinical use of Vaccinia immune globulin intravenous (VIGIV) in treating adverse events following smallpox vaccination and other orthopoxvirus-related infections. Vaccine 2015, 33, 4963–4972. [Google Scholar]
- Frey, S.E.; Newman, F.K.; Kennedy, J.S.; Sobek, V.; Ennis, F.; Hill, H.; Yan, L.; Chaplin, P.; Belshe, R.B. Clinical and immunologic responses to multiple doses of MVA-BN, an attenuated smallpox vaccine in vaccinia-naive individuals. J. Infect. Dis. 2007, 196, 493–500. [Google Scholar]
- Belongia, E.A.; Naleway, A.L.; Baxter, R. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. J. Am. Med. Assoc. 2008, 300, 642–650. [Google Scholar]
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Herry, C.R.; Meyer, T.P.H.; et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef]
- Nalca, A.; Zumbrun, E.E.; Hatkin, J.M. ACAM2000 The new smallpox vaccine for United States Strategic National Stockpile. Drug Des. Dev. Ther. 2010, 4, 71–79. [Google Scholar] [CrossRef]
- Cono, J.; Casey, C.G.; Bell, D.M. Smallpox vaccination and adverse reactions: Guidance for clinicians. Morb. Mortal. Wkly. Rep. 2003, 52, 1–28. [Google Scholar]
- Bolken, T.C.; Hruby, D.E.; Jordan, R. The role of vaccination in controlling Monkeypox outbreaks. Expert Rev. Vaccines 2009, 8, 1069–1075. [Google Scholar]
- World Health Organization. Monkeypox Surveillance, case investigation and contact tracing—Interim guidance. In WHO Guidelines; WHO: Lyon, France, 2022. [Google Scholar]
- Mitjà, O.; Alemany, A.; Marks, M.; Mora, J.I.L.; Rodríguez-Aldama, J.C.; Silva, M.S.T.; Herrear, E.A.C.; Crabtree-Ramirez, B.; Blanco, J.L.; Girometti, N.; et al. Mpox in people with advanced HIV infection: A global case series. Lancet 2023, 401, 939–949. [Google Scholar] [CrossRef]


| Feature | MPXV Clade I | MPXV Clade IIa | MPXV Clade IIb | VARV e |
|---|---|---|---|---|
| Endemic Region | Central Africa a | West Africa b | West Africa c | Eradicated |
| Global Outbreak | No | 2003 | 2018–2023 | Eradicated |
| Animal Reservoir | Multiple species | Multiple species | Multiple species | None |
| Vesicular Lesions (Human) | Yes | Yes | Yes | Yes |
| Lethality (Human) | 10.6% | Low | 3.6% d | ~35% |
| Select Agent | Yes | No | No | Yes |
| Vaccine Available (Human) f | Yes | Yes | Yes | Yes |
| Therapeutics Available (Human) g | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Abdoul-Latif, F.; Ainane, A.; Mohamed, H.; Merito Ali, A.; Houmed Aboubaker, I.; Jutur, P.P.; Ainane, T. Mpox Resurgence: A Multifaceted Analysis for Global Preparedness. Viruses 2024, 16, 1737. https://doi.org/10.3390/v16111737
Mohamed Abdoul-Latif F, Ainane A, Mohamed H, Merito Ali A, Houmed Aboubaker I, Jutur PP, Ainane T. Mpox Resurgence: A Multifaceted Analysis for Global Preparedness. Viruses. 2024; 16(11):1737. https://doi.org/10.3390/v16111737
Chicago/Turabian StyleMohamed Abdoul-Latif, Fatouma, Ayoub Ainane, Houda Mohamed, Ali Merito Ali, Ibrahim Houmed Aboubaker, Pannaga Pavan Jutur, and Tarik Ainane. 2024. "Mpox Resurgence: A Multifaceted Analysis for Global Preparedness" Viruses 16, no. 11: 1737. https://doi.org/10.3390/v16111737
APA StyleMohamed Abdoul-Latif, F., Ainane, A., Mohamed, H., Merito Ali, A., Houmed Aboubaker, I., Jutur, P. P., & Ainane, T. (2024). Mpox Resurgence: A Multifaceted Analysis for Global Preparedness. Viruses, 16(11), 1737. https://doi.org/10.3390/v16111737










