Immune Priming of Pacific Oysters (Crassostrea gigas) to Induce Resistance to Ostreid herpesvirus 1: Comparison of Infectious and Inactivated OsHV-1 with Poly I:C
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Experimental Design
2.2. Oysters
2.3. Aquarium Management
2.4. Immune Priming
2.5. OsHV-1 Challenge
2.6. Quantification of OsHV-1 DNA
2.7. Statistical Analysis
3. Results
3.1. Immune Priming
3.2. OsHV-1 Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA. Scientific opinion on the increased mortality events in Pacific oyster Crassotrea gigas. Eur. Food Saf. Auth. J. 2010, 8, 1894. [Google Scholar]
- Jenkins, C.; Hick, P.; Gabor, M.; Spiers, Z.; Fell, S.A.; Gu, X.; Read, A.; Go, J.; Dove, M.; O’Connor, W.; et al. Identification and characterisation of an Ostreid herpesvirus-1 microvariant (OsHV-1 micro-var) in Crassostrea gigas (Pacific oysters) in Australia. Dis. Aquat. Org. 2013, 105, 109–126. [Google Scholar] [CrossRef]
- Keeling, S.; Brosnahan, C.; Williams, R.; Gias, E.; Hannah, M.; Bueno, R.; McDonald, W.; Johnston, C. New Zealand juvenile oyster mortality associated with Ostreid herpesvirus 1—An opportunistic longitudinal study. Dis. Aquat. Org. 2014, 109, 231–239. [Google Scholar] [CrossRef]
- Trancart, S.; Tweedie, A.; Liu, O.; Paul-Pont, I.; Hick, P.; Houssin, M.; Whittington, R.J. Diversity and molecular epidemiology of Ostreid herpesvirus 1 in farmed Crassostrea gigas in Australia: Geographic clusters and implications for “microvariants” in global mortality events. Virus Res. 2022, 323, 198994. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Castinel, A.; Cheslett, D.; Nozal, D.F.; Whittington, R.J. The impacts of Ostreid herpesvirus 1 microvariants on Pacific oyster aquaculture in the Northern and Southern Hemispheres since 2008. Rev. Sci. Et. Tech. Off. Int. Des Epizoot. 2019, 38, 491–509. [Google Scholar] [CrossRef]
- Pernet, F.; Lupo, C.; Bacher, C.; Whittington, R.J. Infectious diseases in oyster aquaculture require a new integrated approach. Phil. Trans. Royal Soc. B 2016, 371, 20150213. [Google Scholar] [CrossRef]
- Degremont, L.; Nourry, M.; Maurouard, E. Mass selection for survival and resistance to OsHV-1 infection in Crassostrea gigas spat in field conditions: Response to selection after four generations. Aquaculture 2015, 446, 111–121. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Dhand, N.K.; Whittington, R.J. Influence of husbandry practices on OsHV-1 associated mortality of Pacific oysters Crassostrea gigas. Aquaculture 2013, 412, 202–214. [Google Scholar] [CrossRef]
- Pernet, F.; Barret, J.; Le Gall, P.; Corporeau, C.; Dégremont, L.; Lagarde, F.; Pépin, J.-F.; Keck, N. Mass mortalities of Pacific oysters Crassostrea gigas reflect infectious diseases and vary with farming practices in the Mediterranean Thau lagoon, France. Aquac. Env. Interact 2012, 2, 215–237. [Google Scholar] [CrossRef]
- Whittington, R.; Hick, P.; Evans, O.; Rubio, A.; Alford, B.; Dhand, N.; Paul-Pont, I. Protection of Pacific oyster (Crassostrea gigas) spat from mortality due to Ostreid herpesvirus-1 (OsHV-1 µVar) using simple treatments of incoming seawater in land-based upwellers. Aquaculture 2015, 437, 10–20. [Google Scholar] [CrossRef]
- Whittington, R.; Dhand, N.; Evans, O.; Paul-Pont, I. Further observations on the influence of husbandry practices on OsHV-1 μVar mortality in Pacific oysters Crassostrea gigas: Age, cultivation structures and growing height. Aquaculture 2015, 438, 82–97. [Google Scholar] [CrossRef]
- Whittington, R.J.; Hick, P.; Fuhrmann, M.; Liu, O.; Paul-Pont, I. Removal of oyster pathogens from seawater. Environ. Int. 2021, 150, 106258. [Google Scholar] [CrossRef]
- Green, T.J.; Helbig, K.; Speck, P.; Raftos, D.A. Primed for success: Oyster parents treated with poly(I:C) produce offspring with enhanced protection against Ostreid herpesvirus type I infection. Mol. Immunol. 2016, 78, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Montagnani, C. Poly I:C induces a protective antiviral immune response in the Pacific oyster (Crassostrea gigas) against subsequent challenge with Ostreid herpesvirus (OsHV-1 mu var). Fish Shellfish Immunol. 2013, 35, 382–388. [Google Scholar] [CrossRef]
- Lafont, M.; Petton, B.; deLorgeril, J.; Vergnes, A.; Vidal-Dupiol, J.; Gueguen, Y.; Haffner, P.; Mitta, G.; Gourbal, B.; Montagnani, C. Efficient and long-lasting protection against the pacific oyster mortality syndrome through antiviral immune priming. Fish Shellfish Immunol. 2019, 91, 461. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Song, L. The oyster immunity. Dev. Comp. Immunol. 2018, 80, 99–118. [Google Scholar] [CrossRef]
- Namikoshi, A.; Wu, J.L.; Yamashita, T.; Nishizawa, T.; Nishioka, T.; Arimoto, M.; Muroga, K. Vaccination trials with Penaeus japonicus to induce resistance to white spot syndrome virus. Aquaculture 2004, 229, 25–35. [Google Scholar] [CrossRef]
- Rowley, A.F.; Pope, E.C. Vaccines and crustacean aquaculture—A mechanistic exploration. Aquaculture 2012, 334–337, 1–11. [Google Scholar] [CrossRef]
- Kurtz, J.; Franz, K. Evidence for memory in invertebrate immunity. Nature 2003, 425, 37–38. [Google Scholar] [CrossRef]
- Milutinović, B.; Kurtz, J. Immune memory in invertebrates. Semin. Immunol. 2016, 28, 328–342. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, L.; Sun, Z.; Wang, L.; Zhou, Z.; Liu, R.; Yue, F.; Sun, R.; Song, L. The specifically enhanced cellular immune responses in Pacific oyster (Crassostrea gigas) against secondary challenge with Vibrio splendidus. Dev. Comp. Immunol. 2014, 45, 141–150. [Google Scholar] [CrossRef]
- de Kantzow, M.C.; Whittington, R.J.; Hick, P. Prior exposure to Ostreid herpesvirus 1 (OsHV-1) at 18 degrees C is associated with improved survival of juvenile Pacific oysters (Crassostrea gigas) following challenge at 22 degrees C. Aquaculture 2019, 507, 443–450. [Google Scholar] [CrossRef]
- Kurtz, J. Memory in the innate and adaptive immune systems. Microbes Infect. 2004, 6, 1410–1417. [Google Scholar] [CrossRef]
- Schulenburg, H.; Boehnisch, C.; Michiels, N.K. How do invertebrates generate a highly specific innate immune response? Mol. Immunol. 2007, 44, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, S.H.; La Rocca, G.; Gruber, J.J.; Thompson, C.B. Long-lived microRNA–Argonaute complexes in quiescent cells can be activated to regulate mitogenic responses. Proc. Natl. Acad. Sci. 2013, 110, 157–162. [Google Scholar] [CrossRef]
- Escobedo-Bonilla, C.M.; Vega-Peña, S.; Mejía-Ruiz, C.H. Efficacy of double-stranded RNA against white spot syndrome virus (WSSV) non-structural (orf89, wsv191) and structural (vp28, vp26) genes in the Pacific white shrimp Litopenaeus vannamei. J. King Saud Univ. Sci. 2015, 27, 182–188. [Google Scholar] [CrossRef]
- Lafont, M.; Goncalves, P.; Guo, X.M.; Montagnani, C.; Raftos, D.; Green, T. Transgenerational plasticity and antiviral immunity in the Pacific oyster (Crassostrea gigas) against Ostreid herpesvirus 1 (OsHV-1). Dev. Comp. Immunol. 2019, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Pauletto, M.; Segarra, A.; Montagnani, C.; Quillien, V.; Faury, N.; Le Grand, J.; Miner, P.; Petton, B.; Labreuche, Y.; Fleury, E.; et al. Long dsRNAs promote an anti-viral response in Pacific oyster hampering ostreid herpesvirus 1 replication. J. Exp. Biol. 2017, 220, 3671–3685. [Google Scholar] [CrossRef]
- Lafont, M.; Petton, B.; Vergnes, A.; Pauletto, M.; Segarra, A.; Gourbal, B.; Montagnani, C. Long-lasting antiviral innate immune priming in the Lophotrochozoan Pacific oyster, Crassostrea gigas. Sci. Rep. 2017, 7, 13143. [Google Scholar] [CrossRef]
- Green, T.J.; Speck, P. Antiviral Defense and Innate Immune Memory in the Oyster. Viruses 2018, 10, 133. [Google Scholar] [CrossRef]
- Green, T.J.; Benkendorff, K.; Robinson, N.; Raftos, D.; Speck, P. Anti-viral gene induction is absent upon secondary challenge with double-stranded RNA in the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2014, 39, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Evans, O.; Hick, P.; Whittington, R.J. Detection of Ostreid herpesvirus-1 microvariants in healthy Crassostrea gigas following disease events and their possible role as reservoirs of infection. J. Invert. Pathol. 2017, 148, 20–33. [Google Scholar] [CrossRef]
- Liu, O.; Hick, P.; Whittington, R. The resistance to lethal challenge with Ostreid herpesvirus-1 of Pacific oysters (Crassostrea gigas) previously exposed to this virus. Viruses 2023, 15, 1706. [Google Scholar] [CrossRef] [PubMed]
- Hick, P.M.; Evans, O.; Rubio, A.; Dhand, N.K.; Whittington, R.J. Both age and size influence susceptibility of Pacific oysters (Crassostrea gigas) to disease caused by Ostreid herpesvirus-1 (OsHV-1) in replicated field and laboratory experiments. Aquaculture 2018, 489, 110–120. [Google Scholar] [CrossRef]
- Petton, B.; Pernet, F.; Robert, R.; Boudry, P. Temperature influence on pathogen transmission and subsequent mortalities in juvenile Pacific oysters Crassostrea gigas. Aquac. Env. Interact 2013, 3, 257–273. [Google Scholar] [CrossRef]
- Clegg, T.A.; Morrissey, T.; Geoghegan, F.; Martin, S.W.; Lyons, K.; Ashe, S.; More, S.J. Risk factors associated with increased mortality of farmed Pacific oysters in Ireland during 2011. Prev. Vet. Med. 2014, 113, 257–267. [Google Scholar] [CrossRef]
- Hick, P.; Evans, O.; Looi, R.; English, C.; Whittington, R.J. Stability of Ostreid herpesvirus-1 (OsHV-1) and assessment of disinfection of seawater and oyster tissues using a bioassay. Aquaculture 2016, 450, 412–421. [Google Scholar] [CrossRef]
- Martenot, C.; Oden, E.; Travaillé, E.; Malas, J.P.; Houssin, M. Comparison of two real-time PCR methods for detection of ostreid herpesvirus 1 in the Pacific oyster Crassostrea gigas. J. Virol. Methods 2010, 170, 86–89. [Google Scholar] [CrossRef]
- Evans, O.; Paul-Pont, I.; Hick, P.; Whittington, R.J. A simple centrifugation method for improving the detection of Ostreid herpesvirus-1 (OsHV-1) in natural seawater samples with an assessment of the potential for particulate attachment. J. Virol. Methods 2014, 210, 59–66. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Jarp, J.; Tverdal, A. Statistical aspects of fish vaccination trials. Dev. Biol. Stand. 1997, 90, 311–320. [Google Scholar]
- Delisle, L.; Rolton, A.; Vignier, J. Inactivated ostreid herpesvirus-1 induces an innate immune response in the Pacific oyster, Crassostrea gigas, hemocytes. Front. Immunol. 2023, 14, 1161145. [Google Scholar] [CrossRef] [PubMed]
- de Kantzow, M.; Hick, P.; Becker, J.; Whittington, R. Effect of water temperature on mortality of Pacific oysters Crassostrea gigas associated with microvariant Ostreid herpesvirus 1 (OsHV-1 μVar). Aquac. Env. Interact 2016, 8, 419–428. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Evans, O.; Dhand, N.K.; Whittington, R.J. Experimental infections of Pacific oyster Crassostrea gigas using the Australian Ostreid herpesvirus-1 (OsHV-1) uVar strain. Dis. Aquat. Org. 2015, 113, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Evans, O.; Hick, P.; Dhand, N.; Whittington, R.J. Transmission of Ostreid herpesvirus-1 in Crassostrea gigas by cohabitation: Effects of food and number of infected donor oysters. Aquac. Env. Interact 2015, 7, 281–295. [Google Scholar] [CrossRef]
- Green, T.J.; Rolland, J.-L.; Vergnes, A.; Raftos, D.; Montagnani, C. OsHV-1 countermeasures to the Pacific oyster’s anti-viral response. Fish Shellfish Immunol. 2015, 47, 435–443. [Google Scholar] [CrossRef]
- Strodthoff, D.; Ma, Z.; Wirström, T.; Strawbridge, R.J.; Ketelhuth, D.F.J.; Engel, D.; Clarke, R.; Falkmer, S.; Hamsten, A.; Hansson, G.K.; et al. Toll-Like Receptor 3 Influences Glucose Homeostasis and β-Cell Insulin Secretion. Diabetes 2015, 64, 3425–3438. [Google Scholar] [CrossRef] [PubMed]
- Lafont, M.; Vergnes, A.; Vidal-Dupiol, J.; de Lorgeril, J.; Gueguen, Y.; Haffner, P.; Petton, B.; Chaparro, C.; Barrachina, C.; Destoumieux-Garzon, D.; et al. A Sustained Immune Response Supports Long-Term Antiviral Immune Priming in the Pacific Oyster, Crassostrea gigas. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Langland, J.O. When Two Strands Are Better Than One: The Mediators and Modulators of the Cellular Responses to Double-Stranded RNA. Virology 1996, 219, 339–349. [Google Scholar] [CrossRef]
- Weber, F.; Wagner, V.; Rasmussen, S.; Hartmann, R.; Paludan, S. Double-Stranded RNA Is Produced by Positive-Strand RNA Viruses and DNA Viruses but Not in Detectable Amounts by Negative-Strand RNA Viruses. J. Virol. 2006, 80, 5059–5064. [Google Scholar] [CrossRef]
- Segarra, A.; Baillon, L.; Tourbiez, D.; Benabdelmouna, A.; Faury, N.; Bourgougnon, N.; Renault, T. Ostreid herpesvirus type 1 replication and host response in adult Pacific oysters, Crassostrea gigas. Vet. Res. 2014, 45, 103. [Google Scholar] [CrossRef]
- Segarra, A.; Mauduit, F.; Faury, N.; Trancart, S.; Degremont, L.; Tourbiez, D.; Haffner, P.; Barbosa-Solomieu, V.; Pepin, J.-F.; Travers, M.-A.; et al. Dual transcriptomics of virus-host interactions: Comparing two Pacific oyster families presenting contrasted susceptibility to ostreid herpesvirus 1. BMC Genom. 2014, 15, 580. [Google Scholar] [CrossRef]
- Grundhoff, A.; Sullivan, C.S. Virus-encoded microRNAs. Virology 2011, 411, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Lancz, G.J. Physical integrity of herpes simplex virus following thermal inactivation. Arch. Virol. 1980, 64, 375–381. [Google Scholar] [CrossRef] [PubMed]
- de Kantzow, M.C.; Whittington, R.J.; Hick, P.M. Different in vivo growth of ostreid herpesvirus 1 at 18 degrees C and 22 degrees C alters mortality of Pacific oysters (Crassostrea gigas). Arch. Virol. 2019, 164, 3035–3043. [Google Scholar] [CrossRef]
- Green, T.J.; Montagnani, C.; Benkendorff, K.; Robinson, N.; Speck, P. Ontogeny and water temperature influences the antiviral response of the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2014, 36, 151–157. [Google Scholar] [CrossRef]
- Kloosterman, W.P.; Plasterk, R.H. The diverse functions of microRNAs in animal development and disease. Dev. Cell 2006, 11, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Pernet, F.; Lagarde, F.; Jeannee, N.; Daigle, G.; Barret, J.; Le Gall, P.; Quere, C.; D’orbcastel, E.R. Spatial and temporal dynamics of mass mortalities in oysters is influenced by energetic reserves and food quality. PLoS ONE 2014, 9, e88469. [Google Scholar] [CrossRef] [PubMed]
- Pernet, F.; Tamayo, D.; Fuhrmann, M.; Petton, B. Deciphering the effect of food availability, growth and host condition on disease susceptibility in a marine invertebrate. J. Exp. Biol. 2019, 222 Pt 17, jeb210534. [Google Scholar] [CrossRef]
- ABARES. Australian Fisheries and Aquaculture Statistics 2017. 2018, Volume 2018. Available online: https://www.agriculture.gov.au/sites/default/files/sitecollectiondocuments/abares/publications/AustFishAquacStats_2017_v1.2.0.pdf (accessed on 30 June 2023).
- Paul-Pont, I.; Dhand, N.K.; Whittington, R.J. Spatial distribution of mortality in Pacific oysters Crassostrea gigas: Reflection on mechanisms of OsHV-1 transmission. Dis. Aquat. Org. 2013, 105, 127–138. [Google Scholar] [CrossRef]
- Whittington, R.J.; Paul-Pont, I.; Evans, O.; Hick, P.; Dhand, N.K. Counting the dead to determine the source and transmission of the marine herpesvirus OsHV-1 in Crassostrea gigas. Vet. Res. 2018, 49, 34. [Google Scholar] [CrossRef]
- Hartmann, D.; Adams, J.S.; Meeker, A.K.; Schneider, M.A.; Lenz, B.F.; Talmadge, J.E. Dissociation of Therapeutic and Toxic Effects of Polyinosinic-Polycytidylic Acid Admixed with Poly-l-lysine and Solubilized with Carboxymethyl Cellulose in Tumor-bearing Mice1. Cancer Res. 1986, 46, 1331–1338. [Google Scholar] [PubMed]
- Hartmann, D.; Schneider, M.A.; Lenz, B.F.; Talmadge, J.E. Toxicity of Polyinosinic-Polycytidylic Acid Admixed with Poly-L-Lysine and Solubilized with Carboxymethylcellulose in Mice. Pathol. Immunopathol. Res. 2008, 6, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Homan, E.R.; Zendzian, R.P.; Schott, L.D.; Levy, H.B.; Adamson, R.H. Studies on Poly I:C toxicity in experimental animals. Toxicol. Appl. Pharmacol. 1972, 23, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Krown, S.E.; Kerr, D.; Stewart, W.E.I.; Field, K.A.; Oettgen, H.F. Phase I Trials of Poly(I,C) Complexes in Advanced Cancer. J. Immunother. 1985, 4, 640–649. [Google Scholar]

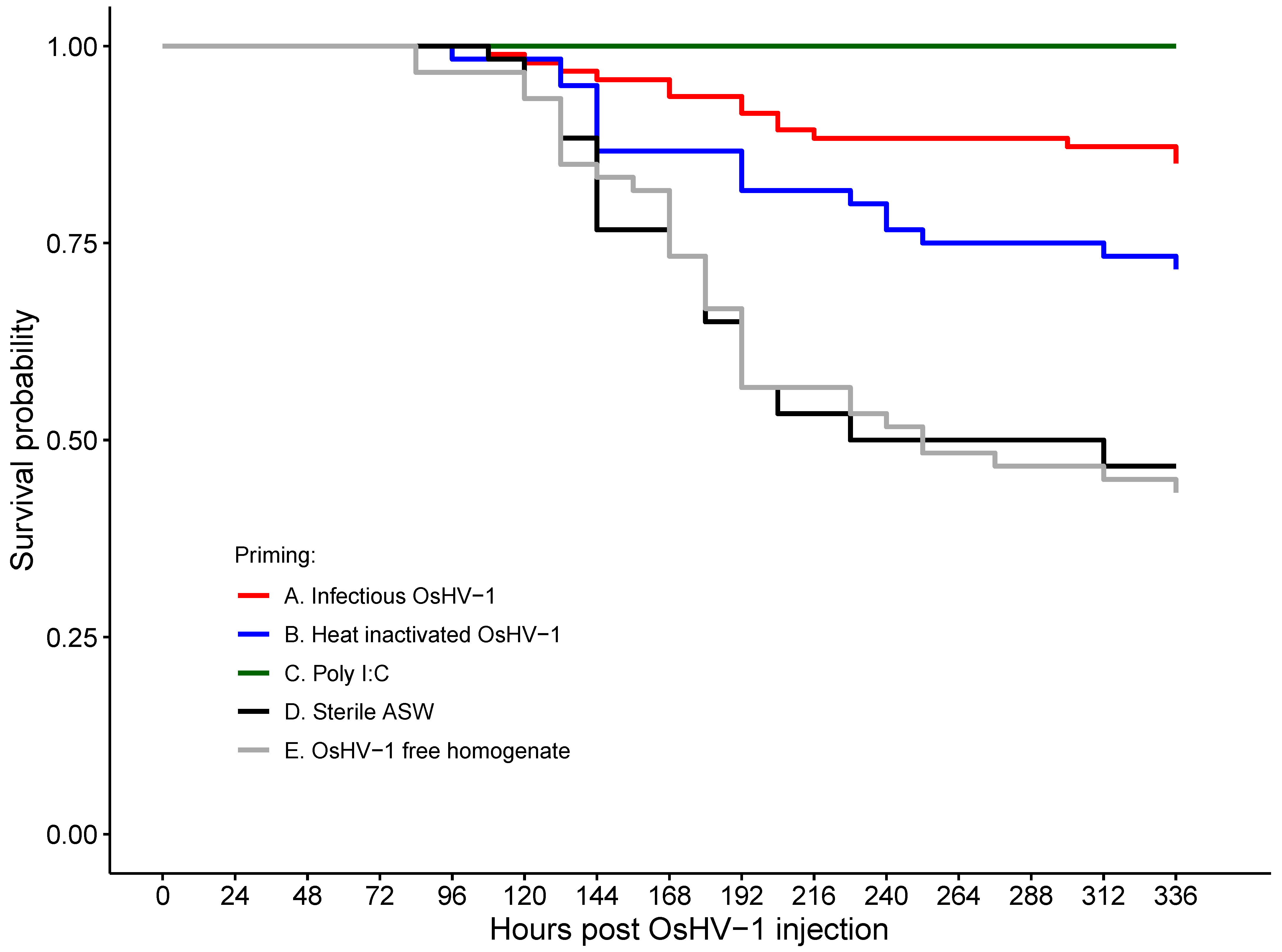

| Challenge | Treatment Group | N | Mortality % (95% CI) | Prevalence % (95% CI) |
|---|---|---|---|---|
| Negative control | Heat-inactivated OsHV-1 | 20 | 0 (0–16.8) | - |
| OsHV-1-free homogenate | 20 | 0 (0–16.8) | - | |
| Infectious OsHV-1 | 30 | 0 (0–11.6) | - | |
| Poly I:C | 20 | 0 (0–16.8) | - | |
| Sterile ASW | 20 | 0 (0–16.8) | - | |
| OsHV-1 | Heat-inactivated OsHV-1 | 60 | 26.7 (17.0–39.2) | 55.56 (33.0–76.0) |
| OsHV-1-free homogenate | 60 | 55.0 (42.4–67.0) | 55.00 (33.6–74.7) | |
| Infectious OsHV-1 | 94 | 12.77 (7.4–21.2) | 55.56 (33.0–76.0) | |
| Poly I:C | 60 | 0 (0–6.0) | 0.00 (0.0–16.8) * | |
| Sterile ASW | 60 | 53.33 (40.8–65.5) | 70.0 (47.3–85.9) |
| Treatment Group | Coefficient | Hazard Ratio (95% CI) |
|---|---|---|
| Sterile ASW | - | 1 |
| Heat-inactivated OsHV-1 | −0.895 | 0.41 (0.25–0.67) |
| OsHV-1-free homogenate | 0.025 | 1.03 (0.66–1.59) |
| Poly I:C | −3.802 | 0.02 (0.00–0.11) |
| Infectious OsHV-1 | −1.699 | 0.18 (0.11–0.32) |
| OsHV-1 Challenge Outcome | Treatment Group (Parameter) | OsHV-1 Genomes.mg−1 | Estimate (95% CI) | Std. Error | p-Value |
|---|---|---|---|---|---|
| Survivor | Sterile ASW (Intercept) | 17.91 | 1.253 (0.76–1.75) | 0.253 | <0.001 |
| Heat-inactivated OsHV-1 | 24.32 | 0.133 (−0.61–0.88) | 0.379 | 0.726 | |
| OsHV-1-free homogenate | 16.03 | −0.048 (−0.75–0.65) | 0.357 | 0.892 | |
| Infectious OsHV-1 | 13.8 | −0.113 (−0.83–0.61) | 0.367 | 0.759 | |
| Poly I:C | 1 | −1.253 (−1.95–−0.55) | 0.357 | <0.001 | |
| Mortality | Sterile ASW (Intercept) | 4.48 × 104 | 4.651 (4.33–4.97) | 0.162 | <0.001 |
| Heat-inactivated OsHV-1 | 2.89 × 104 | −0.19 (−0.73–0.35) | 0.277 | 0.495 | |
| OsHV-1-free homogenate | 3.54 × 104 | −0.102 (−0.54–0.33) | 0.221 | 0.645 | |
| Infectious OsHV-1 | 1.30 × 104 | −0.536 (−1.12–0.05) | 0.301 | 0.079 |
| Treatment Group (Parameter) | Estimate | Std. Error | p-Value | Odds Ratio (95% CI) |
|---|---|---|---|---|
| Sterile ASW (Reference) | 0.8473 | 0.488 | 0.082 | 1 |
| Heat-inactivated OsHV-1 | −0.6242 | 0.6805 | 0.359 | 0.54 (0.14–2.02) |
| OsHV-1-free homogenate | −0.6466 | 0.6634 | 0.33 | 0.52 (0.14–1.9) |
| Infectious OsHV-1 | −0.6242 | 0.6805 | 0.359 | 0.54 (0.14–2.02) |
| Poly I:C | −3.7917 | 1.1361 | <0.001 | 0.02 (0–0.15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Kantzow, M.; Hick, P.M.; Whittington, R.J. Immune Priming of Pacific Oysters (Crassostrea gigas) to Induce Resistance to Ostreid herpesvirus 1: Comparison of Infectious and Inactivated OsHV-1 with Poly I:C. Viruses 2023, 15, 1943. https://doi.org/10.3390/v15091943
de Kantzow M, Hick PM, Whittington RJ. Immune Priming of Pacific Oysters (Crassostrea gigas) to Induce Resistance to Ostreid herpesvirus 1: Comparison of Infectious and Inactivated OsHV-1 with Poly I:C. Viruses. 2023; 15(9):1943. https://doi.org/10.3390/v15091943
Chicago/Turabian Stylede Kantzow, Maximilian, Paul M. Hick, and Richard J. Whittington. 2023. "Immune Priming of Pacific Oysters (Crassostrea gigas) to Induce Resistance to Ostreid herpesvirus 1: Comparison of Infectious and Inactivated OsHV-1 with Poly I:C" Viruses 15, no. 9: 1943. https://doi.org/10.3390/v15091943
APA Stylede Kantzow, M., Hick, P. M., & Whittington, R. J. (2023). Immune Priming of Pacific Oysters (Crassostrea gigas) to Induce Resistance to Ostreid herpesvirus 1: Comparison of Infectious and Inactivated OsHV-1 with Poly I:C. Viruses, 15(9), 1943. https://doi.org/10.3390/v15091943






