Accuracy of Human Papillomavirus (HPV) Testing on Urine and Vaginal Self-Samples Compared to Clinician-Collected Cervical Sample in Women Referred to Colposcopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design and Sample Collection
2.3. Pre-Analytical Sample Processing
2.4. Nucleic Acid Extraction
2.5. HPV DNA Detection
2.6. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Comparison of the Two Different Nucleic Acid Extraction Methods
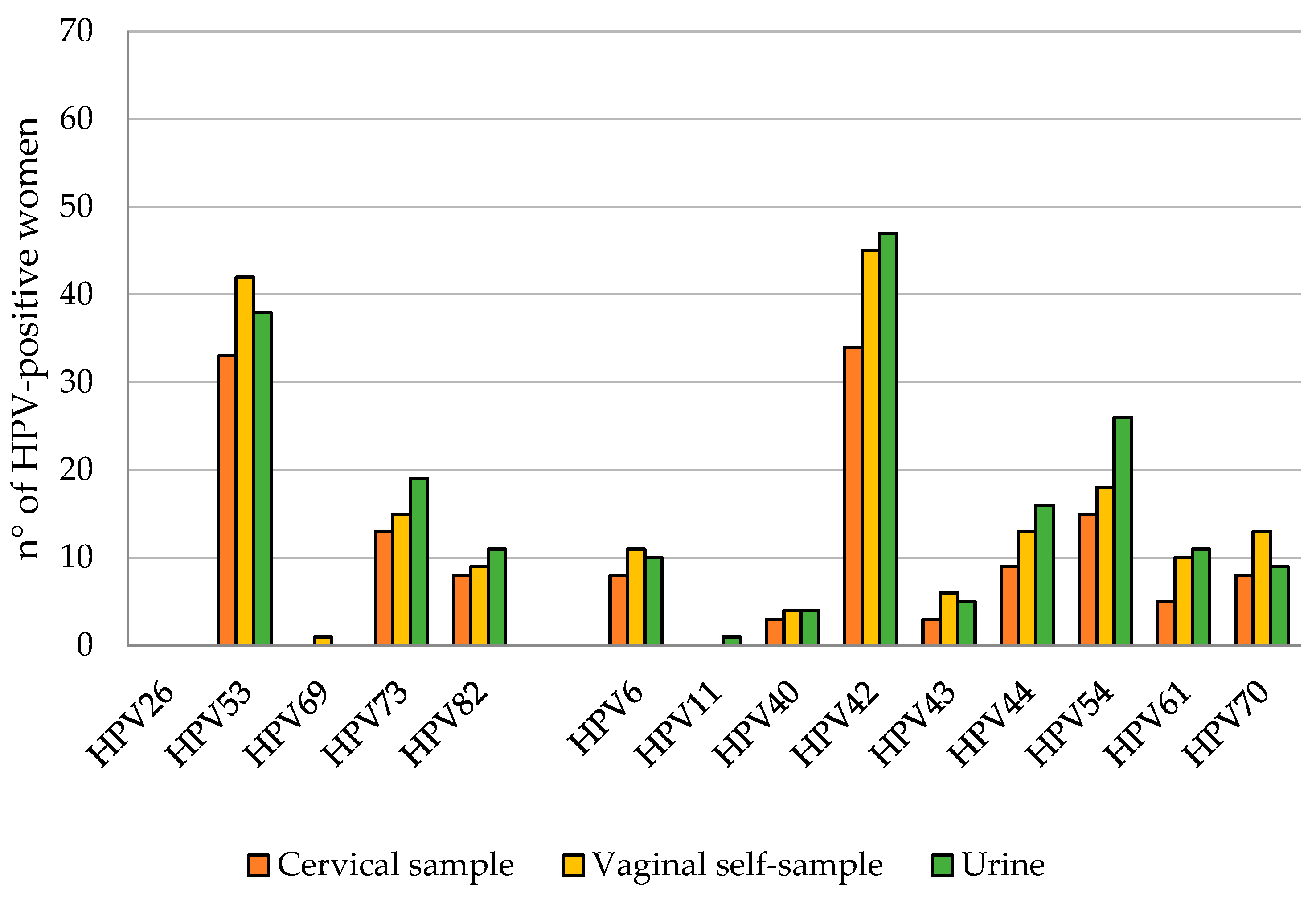
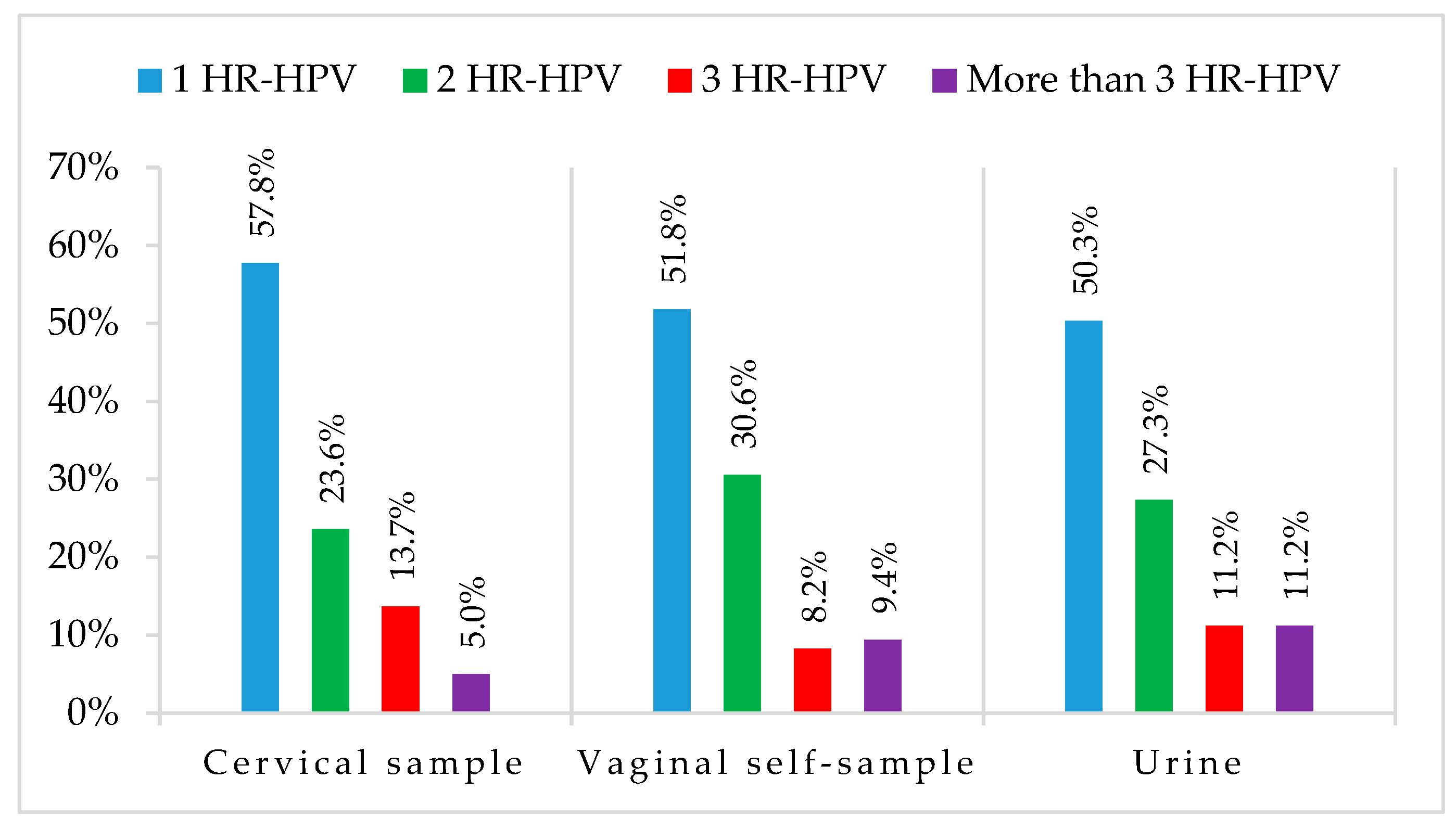
3.3. HPV DNA Detection
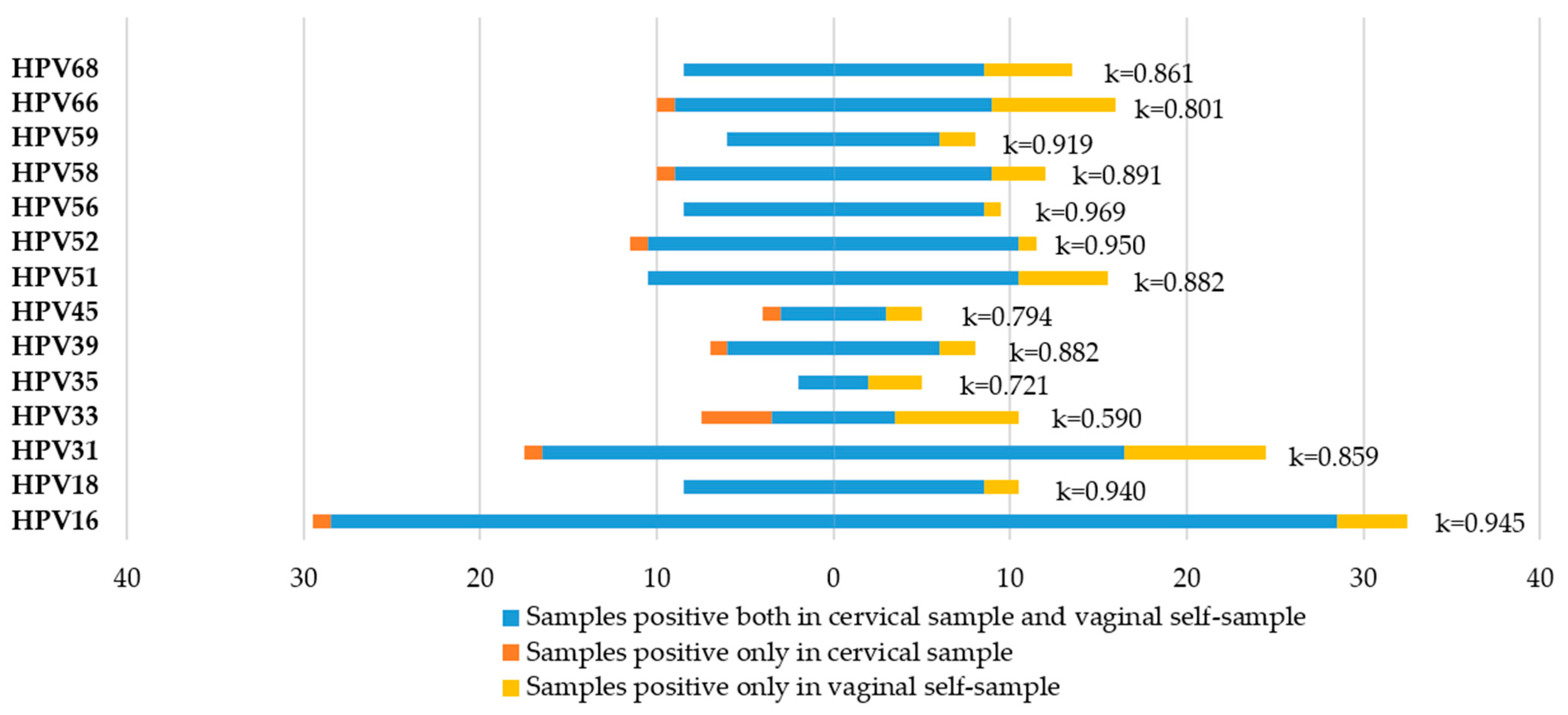
3.4. Agreement between Cervical Samples and Vaginal Self-Samples
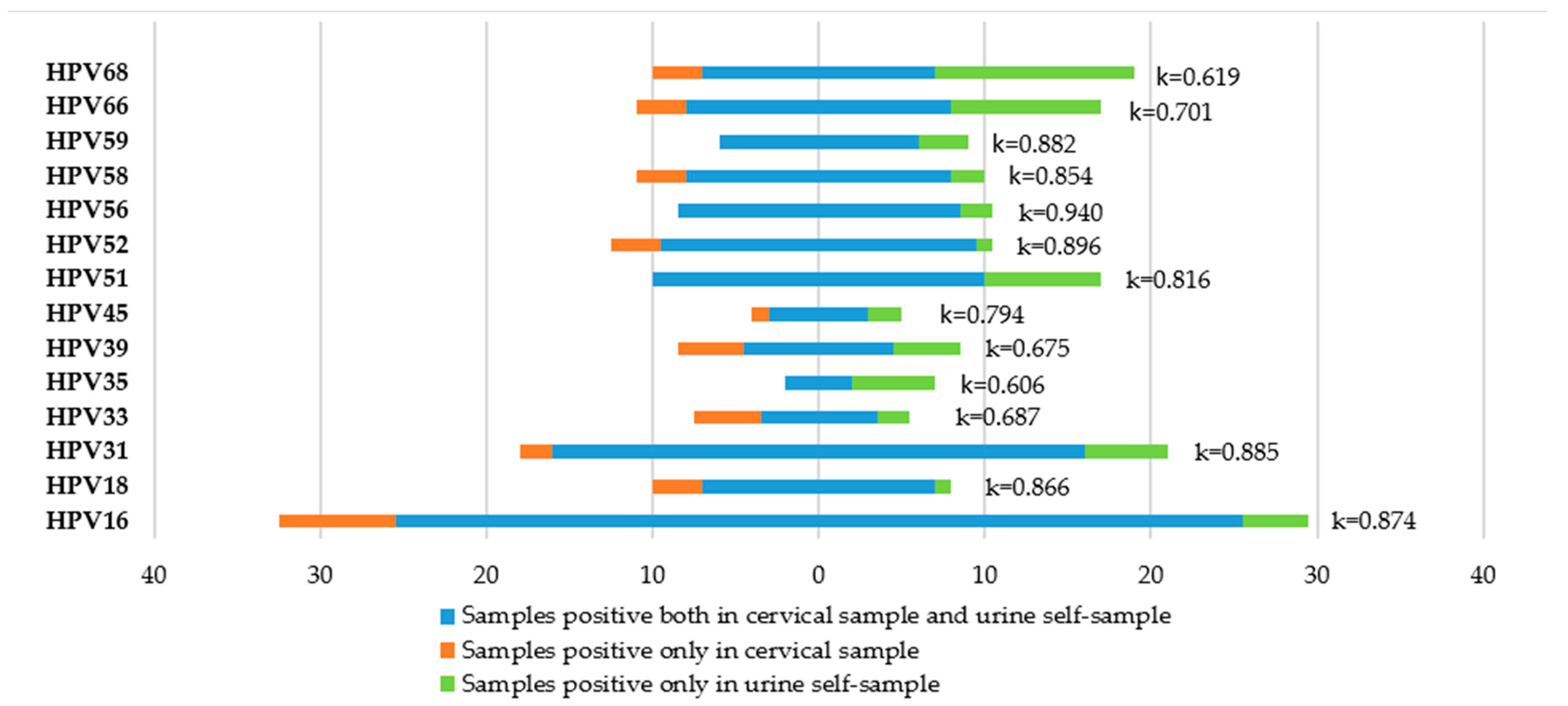
3.5. Agreement between Cervical Samples and Urine Samples
3.6. Accuracy of CIN2+ Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ervik, M.; Lam, F.; Ferlay, J.; Mery, L.; Soerjomataram, I.; Bray, F. Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Munoz, J.; Bosch, F.X.; de Sanjosé, S. Human Papillomavirus and Related Diseases in the World; Summary Report 10 March 2023; ICO/IARC Information Centre on HPV: Lyon, France, 2023. [Google Scholar]
- Aiom-Airtum, I. Numeri del Cancro in Italia; Edizione: Treviso, Italy, 2020. [Google Scholar]
- Arbyn, M.; Ronco, G.; Anttila, A.; Meijer, C.; Poljak, M.; Ogilvie, G.; Koliopoulos, G.; Naucler, P.; Sankaranarayanan, R.; Peto, J. Evidence Regarding Human Papillomavirus Testing in Secondary Prevention of Cervical Cancer. Vaccine 2012, 30, F88–F99. [Google Scholar] [CrossRef]
- Ronco, G.; Dillner, J.; Elfstrom, K.M.; Tunesi, S.; Snijders, P.J.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Arbyn, M.; Simon, M.; Peeters, E.; Xu, L.; Meijer, C.J.L.M.; Berkhof, J.; Cuschieri, K.; Bonde, J.; Ostrbenk Vanlencak, A.; Zhao, F.H.; et al. 2020 list of human papillomavirus assays suitable for primary cervical cancer screening. Clin. Microbiol. Infect. 2021, 27, 1083–1095. [Google Scholar] [CrossRef]
- Arbyn, M.; Depuydt, C.; Benoy, I.; Bogers, J.; Cuschieri, K.; Schmitt, M.; Pawlita, M.; Geraets, D.; Heard, I.; Gheit, T.; et al. VALGENT: A protocol for clinical validation of human papillomavirus assays. J. Clin. Virol. 2016, 76 (Suppl. 1), S14–S21. [Google Scholar] [CrossRef]
- Meijer, C.J.; Berkhof, J.; Castle, P.E.; Hesselink, A.T.; Franco, E.L.; Ronco, G.; Arbyn, M.; Bosch, F.X.; Cuzick, J.; Dillner, J.; et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int. J. Cancer 2009, 124, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Adcock, R.; Cuzick, J.; Hunt, W.C.; McDonald, R.M.; Wheeler, C.M.; Joste, N.E.; Kinney, W.; Wheeler, C.M.; Hunt, W.C.; McDonald, R.M.; et al. Role of HPV Genotype, Multiple Infections, and Viral Load on the Risk of High-Grade Cervical Neoplasia. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Zappacosta, R.; Sablone, F.; Pansa, L.; Buca, D.; Rosini, S. Analytic and Diagnostic Performances of Human Papillomavirus E6/E7 mRNA Test on up-to 11-Year-Old Liquid-Based Cervical Samples. A Biobank-Based Longitudinal Study. Int. J. Mol. Sci. 2017, 18, 1480. [Google Scholar] [CrossRef]
- Epicentro Il portale dell’epidemiologia per la Sanità Pubblica. La sorveglianza Passi. Available online: https://www.epicentro.iss.it/passi/dati/ScreeningCervicale#dati (accessed on 15 July 2023).
- Verdoodt, F.; Jentschke, M.; Hillemanns, P.; Racey, C.S.; Snijders, P.J.; Arbyn, M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: A systematic review and meta-analysis of randomised trials. Eur. J. Cancer 2015, 51, 2375–2385. [Google Scholar] [CrossRef]
- Racey, C.S.; Withrow, D.R.; Gesink, D. Self-collected HPV Testing Improves Participation in Cervical Cancer Screening: A Systematic Review and Meta-analysis. Can. J. Public Health-Rev. Can. Sante Publique 2013, 104, E159–E166. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Castle, P.E.; Schiffman, M.; Wentzensen, N.; Heckman-Stoddard, B.; Sahasrabuddhe, V.V. Meta-analysis of agreement/concordance statistics in studies comparing self- vs clinician-collected samples for HPV testing in cervical cancer screening. Int. J. Cancer 2022, 151, 308–312. [Google Scholar] [CrossRef]
- Costa, S.; Verberckmoes, B.; Castle, P.E.; Arbyn, M. Offering HPV self-sampling kits: An updated meta-analysis of the effectiveness of strategies to increase participation in cervical cancer screening. Br. J. Cancer 2023, 128, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Van Keer, S.; Pattyn, J.; Tjalma, W.A.A.; Van Ostade, X.; Ieven, M.; Van Damme, P.; Vorsters, A. First-void urine: A potential biomarker source for triage of high-risk human papillomavirus infected women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 216, 1–11. [Google Scholar] [CrossRef]
- Van Keer, S.; Tjalma, W.A.A.; Pattyn, J.; Biesmans, S.; Pieters, Z.; Van Ostade, X.; Ieven, M.; Van Damme, P.; Vorsters, A. Human papillomavirus genotype and viral load agreement between paired first-void urine and clinician-collected cervical samples. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Vorsters, A.; Van Damme, P.; Clifford, G. URINARY TESTING FOR HPV Urine testing for HPV: Rationale for using first void. BMJ-Br. Med. J. 2014, 349, g6252. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Lee, B.; Hwang, S.H.; Lee, D.O.; Sung, N.Y.; Park, J.Y.; Jun, J.K. Evaluation of satisfaction with three different cervical cancer screening modalities: Clinician-collected Pap test vs. HPV test by self-sampling vs. HPV test by urine sampling. J. Gynecol. Oncol. 2019, 30, e76. [Google Scholar] [CrossRef]
- Khoo, S.P.; Lim, W.T.; Rajasuriar, R.; Nasir, N.H.; Gravitt, P.; Woo, Y.L. The Acceptability and Preference of Vaginal Self-sampling for Human Papillomavirus (HPV) Testing among a Multi-ethnic Asian Female Population. Cancer Prev. Res. 2021, 14, 105–112. [Google Scholar] [CrossRef]
- Ørnskov, D.; Jochumsen, K.; Steiner, P.H.; Grunnet, I.M.; Lykkebo, A.W.; Waldstrøm, M. Clinical performance and acceptability of self-collected vaginal and urine samples compared with clinician-taken cervical samples for HPV testing among women referred for colposcopy. A cross-sectional study. BMJ Open 2021, 11, e041512. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. World Health Organization Classification of Tumours, Female Genital Tumours, 5th ed.; IARC: Lyon, France, 2020. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Koliopoulos, G.; Arbyn, M.; Martin-Hirsch, P.; Kyrgiou, M.; Prendiville, W.; Paraskevaidis, E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: A systematic review and meta-analysis of non-randomized studies. Gynecol. Oncol. 2007, 104, 232–246. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Arbyn, M.; Bergeron, C.; Bosch, F.X.; Dillner, J.; Jit, M.; Kim, J.; Poljak, M.; Nieminen, P.; Sasieni, P.; et al. Cervical screening: ESGO-EFC position paper of the European Society of Gynaecologic Oncology (ESGO) and the European Federation of Colposcopy (EFC). Br. J. Cancer 2020, 123, 510–517. [Google Scholar] [CrossRef]
- Sias, C.; Garbuglia, A.R.; Piselli, P.; Cimaglia, C.; Lapa, D.; Del Nonno, F.; Baiocchini, A.; Capobianchi, M.R. Comparison of the Abbott RealTime High Risk HPV with Genomica HPV Clinical Array for the detection of human papillomavirus DNA. APMIS 2013, 121, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, M.; Alonso, I.; Rodriguez-Trujillo, A.; Bernal, S.; Geraets, D.; Guimerà, N.; Torne, A.; Ordi, J. Comparison of the analytical and clinical performance of five tests for the detection of human papillomavirus genital infection. J. Virol. Methods 2017, 248, 238–243. [Google Scholar] [CrossRef]
- Donà, M.G.; Benevolo, M.; Pimpinelli, F.; Battista, M.; Rollo, F.; Stivali, F.; Moscarelli, A.; Giuliani, M.; Di Carlo, A.; Vocaturo, A. Comparative evaluation of different DNA extraction methods for HPV genotyping by linear array and INNO-LiPA. J. Med. Virol. 2011, 83, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, M.; Musumeci, R.; Sechi, I.; Sotgiu, G.; Piana, A.; Perdoni, F.; Sina, F.; Fruscio, R.; Landoni, F.; Cocuzza, C.E. Prevalence of Human Papillomavirus (HPV) and Other Sexually Transmitted Infections (STIs) among Italian Women Referred for a Colposcopy. Int. J. Environ. Res. Public Health 2019, 16, 5000. [Google Scholar] [CrossRef]
- Martinelli, M.; Musumeci, R.; Rizzo, A.; Muresu, N.; Piana, A.; Sotgiu, G.; Landoni, F.; Cocuzza, C. Prevalence of Chlamydia trachomatis Infection, Serovar Distribution and Co-Infections with Seven High-Risk HPV Types among Italian Women with a Recent History of Abnormal Cervical Cytology. Int. J. Environ. Res. Public Health 2019, 16, 3354. [Google Scholar] [CrossRef]
- Baasland, I.; Romundstad, P.R.; Eide, M.L.; Jonassen, C.M. Clinical performance of Anyplex II HPV28 by human papillomavirus type and viral load in a referral population. PLoS ONE 2019, 14, e0210997. [Google Scholar] [CrossRef]
- Thomsen, L.T.; Frederiksen, K.; Munk, C.; Junge, J.; Castle, P.E.; Iftner, T.; Kjaer, S.K. High-risk and low-risk human papillomavirus and the absolute risk of cervical intraepithelial neoplasia or cancer. Obstet. Gynecol. 2014, 123, 57–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Latsuzbaia, A.; Vanden Broeck, D.; Van Keer, S.; Weyers, S.; Donders, G.; Doyen, J.; Tjalma, W.; De Sutter, P.; Peeters, E.; Vorsters, A.; et al. Validation of BD Onclarity HPV assay on vaginal self-samples versus cervical samples using the VALHUDES protocol. Cancer Epidemiol. Biomark. Prev. 2022, 31, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Van Keer, S.; Peeters, E.; Vanden Broeck, D.; De Sutter, P.; Donders, G.; Doyen, J.; Tjalma, W.A.A.; Weyers, S.; Vorsters, A.; Arbyn, M. Clinical and analytical evaluation of the RealTime High Risk HPV assay in Colli-Pee collected first-void urine using the VALHUDES protocol. Gynecol. Oncol. 2021, 162, 575–583. [Google Scholar] [CrossRef]
- Van Keer, S.; Latsuzbaia, A.; Vanden Broeck, D.; De Sutter, P.; Donders, G.; Doyen, J.; Tjalma, W.A.A.; Weyers, S.; Arbyn, M.; Vorsters, A. Analytical and clinical performance of extended HPV genotyping with BD Onclarity HPV Assay in home-collected first-void urine: A diagnostic test accuracy study. J. Clin. Virol. 2022, 155, 105271. [Google Scholar] [CrossRef]
- Arbyn, M.; Peeters, E.; Benoy, I.; Broeck, D.V.; Bogers, J.; De Sutter, P.; Donders, G.; Tjalma, W.; Weyers, S.; Cuschieri, K.; et al. VALHUDES: A protocol for validation of human papillomavirus assays and collection devices for HPV testing on self-samples and urine samples. J. Clin. Virol. 2018, 107, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.K.; Ci, P.W.; Velicer, C.; Kang, L.N.; Liu, B.; Cui, J.F.; Chen, F.; Zhang, X.; Chang, I.J.; Roberts, C.C.; et al. Comparison of HPV genotypes and viral load between different sites of genital tract: The significance for cervical cancer screening. Cancer Epidemiol. 2014, 38, 168–173. [Google Scholar] [CrossRef]
- Sundström, K.; Dillner, J. How Many Human Papillomavirus Types Do We Need to Screen For? J. Infect. Dis. 2021, 223, 1510–1511. [Google Scholar] [CrossRef] [PubMed]
- Nygård, M.; Hansen, B.T.; Kjaer, S.K.; Hortlund, M.; Tryggvadóttir, L.; Munk, C.; Lagheden, C.; Sigurdardottir, L.G.; Campbell, S.; Liaw, K.L.; et al. Human papillomavirus genotype-specific risks for cervical intraepithelial lesions. Hum. Vaccin. Immunother. 2021, 17, 972–981. [Google Scholar] [CrossRef]
- Xu, H.; Yu, Y.; George, W.; Smith, J.S.; Hu, S.; Dang, L.; Zhang, X.; Pan, Q.; Qiao, Y.; Zhao, F. Comparison of the performance of paired urine and cervical samples for cervical cancer screening in screening population. J. Med. Virol. 2020, 92, 234–240. [Google Scholar] [CrossRef]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P.; Collaboration Self-Sampling, H.P.V.T. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ-Br. Med. J. 2018, 363, k4823. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.; Park, N.J.; Park, J.Y. Comparison of Seegene Anyplex II HPV28 assay with BD Onclarity HPV assay for human papillomavirus genotyping. PLoS ONE 2022, 17, e0267836. [Google Scholar] [CrossRef] [PubMed]


| Cytology | n (%) Total: 245 |
|---|---|
| HSIL | 33 (13.5%) |
| ASC-H | 19 (7.7%) |
| LSIL | 107 (43.7%) |
| ASC-US | 57 (23.3%) |
| AGC | 11 (4.5%) |
| NILM | 18 (7.3%) |
| Biopsy/Conization | n (%) Total: 60 |
| Adenocarcinoma | 1 (1.7%) |
| Adenosquamous carcinoma | 1 (1.7%) |
| CIN3 | 33 (55.0%) |
| CIN2 | 9 (15.0%) |
| CIN1 | 7 (11.6%) |
| Negative | 9 (15%) |
| Cytology Results | n° of Samples | Cervical Samples | Vaginal Self-Samples | Urine Self-Samples |
|---|---|---|---|---|
| Hr-HPV-Positive | Hr-HPV-Positive | Hr-HPV-Positive | ||
| NILM | 18 | 5 (27.8%) | 5 (27.8%) | 4 (22.2%) |
| ASC-US/LSIL | 164 | 107 (65.2%) | 112 (68.3%) | 108 (65.9%) |
| AGC/ASC-H/HSIL | 63 | 49 (77.8%) | 51 (81.5%) | 49 (77.8%) |
| Histology Result | n° of Samples | Cervical Samples | Vaginal Self-Samples | Urine Self-Samples |
|---|---|---|---|---|
| Hr-HPV-Positive | Hr-HPV-Positive | Hr-HPV-Positive | ||
| <CIN2 | 16 | 11 (68.8%) | 11 (68.8%) | 11 (68.8%) |
| CIN2+ | 44 | 42 (95.5%) | 42 (95.5%) | 40 (90.9%) |
| HPV Genotype | TP | TN | FP | FN | PPA % (n) | NPA % (n) | OPA % (n) | k Value (95% CI) |
|---|---|---|---|---|---|---|---|---|
| hr-HPV | 160 | 74 | 10 | 1 | 99.4% (160) | 88.1% (74) | 95.5% (234) | 0.898 (0.839–0.957) |
| HPV16 | 57 | 183 | 4 | 1 | 98.3% (57) | 97.96% (183) | 98.0% (240) | 0.945 (0.896–0.993) |
| HPV18 | 17 | 226 | 2 | 0 | 100.0% (17) | 99.1% (226) | 99.2% (243) | 0.940 (0.857–1.000) |
| Other hrHPV | 122 | 108 | 13 | 2 | 98.4% (122) | 89.3% (122) | 93.9% (230) | 0.877 (0.818–0.937) |
| HPV Genotype | TP | TN | FP | FN | PPA % (n) | NPA % (n) | OPA % (n) | k Value (95% CI) |
|---|---|---|---|---|---|---|---|---|
| hr-HPV | 149 | 73 | 12 | 11 | 93.1% (149) | 85.9% (73) | 90.6% (222) | 0.792 (0.712–0.873) |
| HPV16 | 51 | 183 | 4 | 7 | 87.9% (51) | 97.9% (183) | 95.5% (234) | 0.874 (0.801–0.946) |
| HPV18 | 14 | 227 | 1 | 3 | 82.4% (14) | 99.6% (227) | 98.4% (241) | 0.866 (0.737–0.995) |
| Other hrHPV | 117 | 104 | 17 | 7 | 94.4% (117) | 86.0% (104) | 90.2% (221) | 0.804 (0.730–0.878) |
| CIN2+ Detection | ||||
|---|---|---|---|---|
| Sensitivity % for CIN2+ Detection (95% CI) | Specificity % for <CIN2 Detection (95% CI) | Relative Sensitivity to Cervical Samples for CIN2+ Detection (95% CI) | Relative Specificity to Cervical Samples for <CIN2 Detection (95% CI) | |
| Cervical Sample | 95.5% (89.3–100.0%) | 40.8% (34.0–47.6%) | - | - |
| Vaginal Self-Sample | 95.5% (89.3–100.0%) | 36.3% (29.7–43.0%) | 1.00 (1.00–1.00) | 0.89 (0.82–0.97) |
| Urine Sample | 90.9% (82.4–99.4%) | 39.8% (3.0–46.6%) | 0.95 (0.89–1.02) | 0.98 (0.87–1.09) |
| CIN2+ Detection | ||||
|---|---|---|---|---|
| Sensitivity % for CIN2+ Detection (95% CI) | Specificity % for <CIN2 Detection (95% CI) | Relative Sensitivity to Cervical Samples for CIN2+ Detection (95% CI) | Relative Specificity to Cervical Samples for <CIN2 Detection (95% CI) | |
| Cervical Sample | 95.5% (89.3–100.0%) | 31.3% (8.5–584.0%) | - | - |
| Vaginal Self-Sample | 95.5% (89.3–100.0%) | 31.3% (8.5–55.0%) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Urine Sample | 90.9% (82.4–99.4%) | 31.3% (8.5–54.0%) | 0.95 (0.89–1.02) | 1.00 (1.00–1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, M.; Giubbi, C.; Di Meo, M.L.; Perdoni, F.; Musumeci, R.; Leone, B.E.; Fruscio, R.; Landoni, F.; Cocuzza, C.E. Accuracy of Human Papillomavirus (HPV) Testing on Urine and Vaginal Self-Samples Compared to Clinician-Collected Cervical Sample in Women Referred to Colposcopy. Viruses 2023, 15, 1889. https://doi.org/10.3390/v15091889
Martinelli M, Giubbi C, Di Meo ML, Perdoni F, Musumeci R, Leone BE, Fruscio R, Landoni F, Cocuzza CE. Accuracy of Human Papillomavirus (HPV) Testing on Urine and Vaginal Self-Samples Compared to Clinician-Collected Cervical Sample in Women Referred to Colposcopy. Viruses. 2023; 15(9):1889. https://doi.org/10.3390/v15091889
Chicago/Turabian StyleMartinelli, Marianna, Chiara Giubbi, Maria Letizia Di Meo, Federica Perdoni, Rosario Musumeci, Biagio Eugenio Leone, Robert Fruscio, Fabio Landoni, and Clementina Elvezia Cocuzza. 2023. "Accuracy of Human Papillomavirus (HPV) Testing on Urine and Vaginal Self-Samples Compared to Clinician-Collected Cervical Sample in Women Referred to Colposcopy" Viruses 15, no. 9: 1889. https://doi.org/10.3390/v15091889
APA StyleMartinelli, M., Giubbi, C., Di Meo, M. L., Perdoni, F., Musumeci, R., Leone, B. E., Fruscio, R., Landoni, F., & Cocuzza, C. E. (2023). Accuracy of Human Papillomavirus (HPV) Testing on Urine and Vaginal Self-Samples Compared to Clinician-Collected Cervical Sample in Women Referred to Colposcopy. Viruses, 15(9), 1889. https://doi.org/10.3390/v15091889






