Comparison of Gross Pathology between Classical and Recombinant Lumpy Skin Disease Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Experimental Design
3. Results
3.1. Clinical Disease in Cattle
3.1.1. Dagestan/2015 [4]
3.1.2. Saratov/2017 [13]
3.1.3. Udmurtiya 2019 [10]
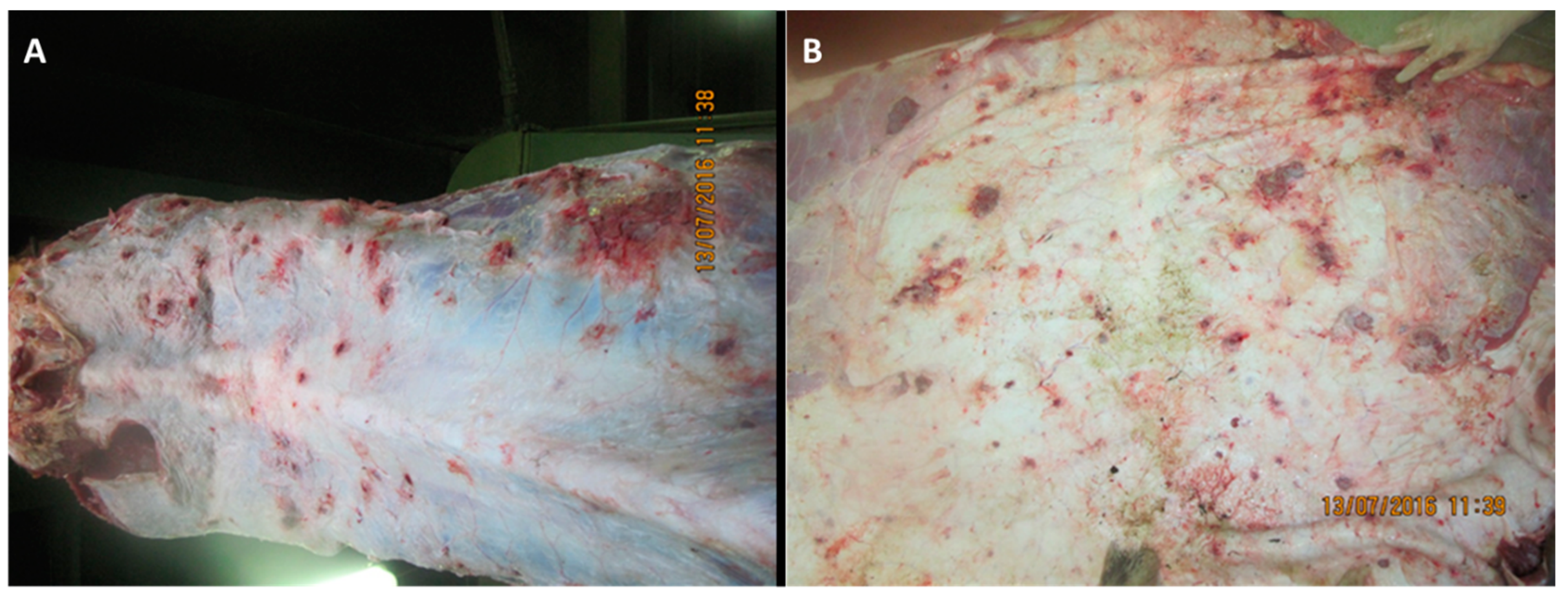
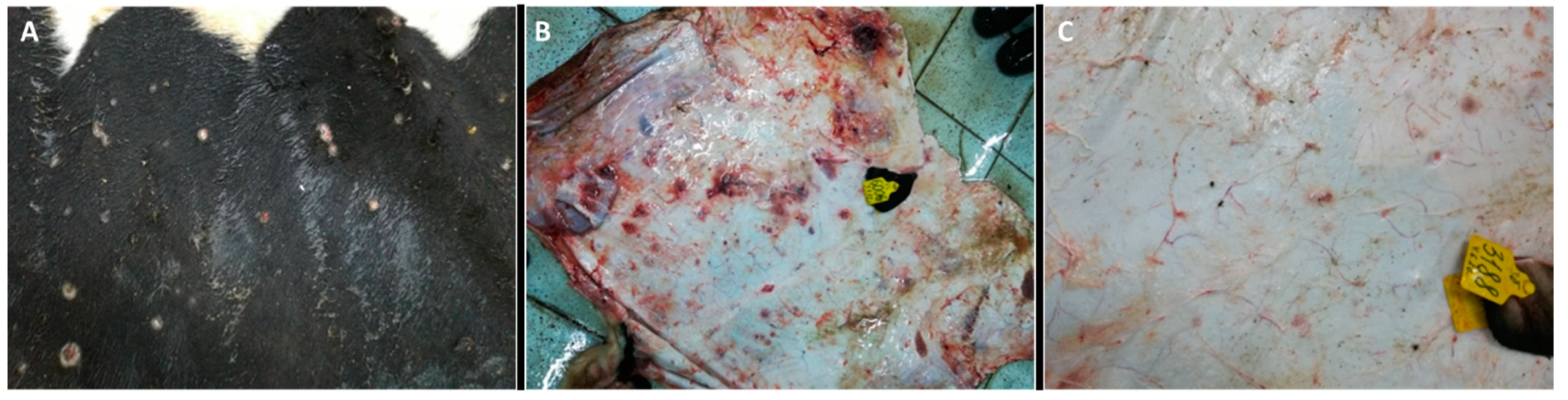
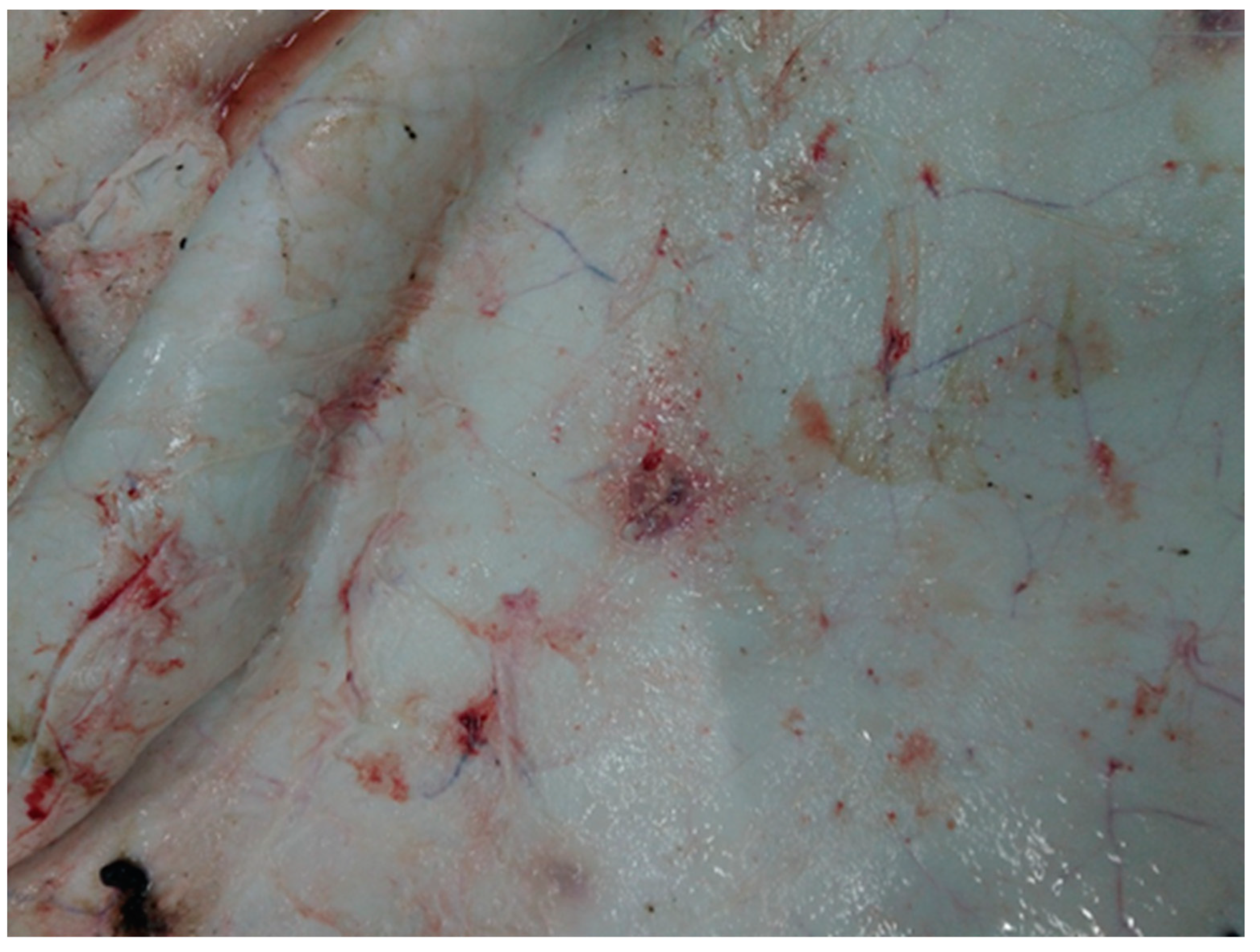
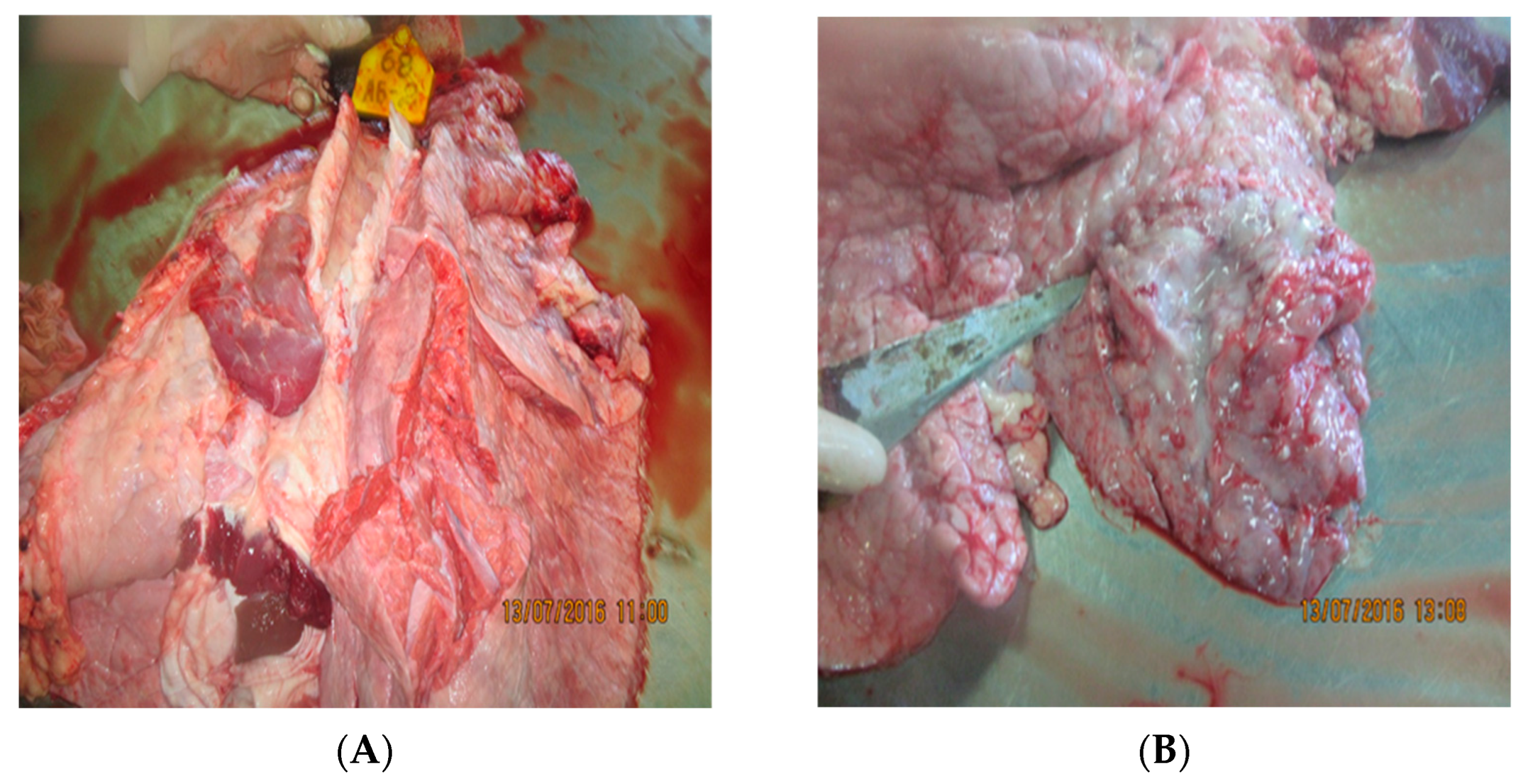
3.2. Post-Mortem Findings
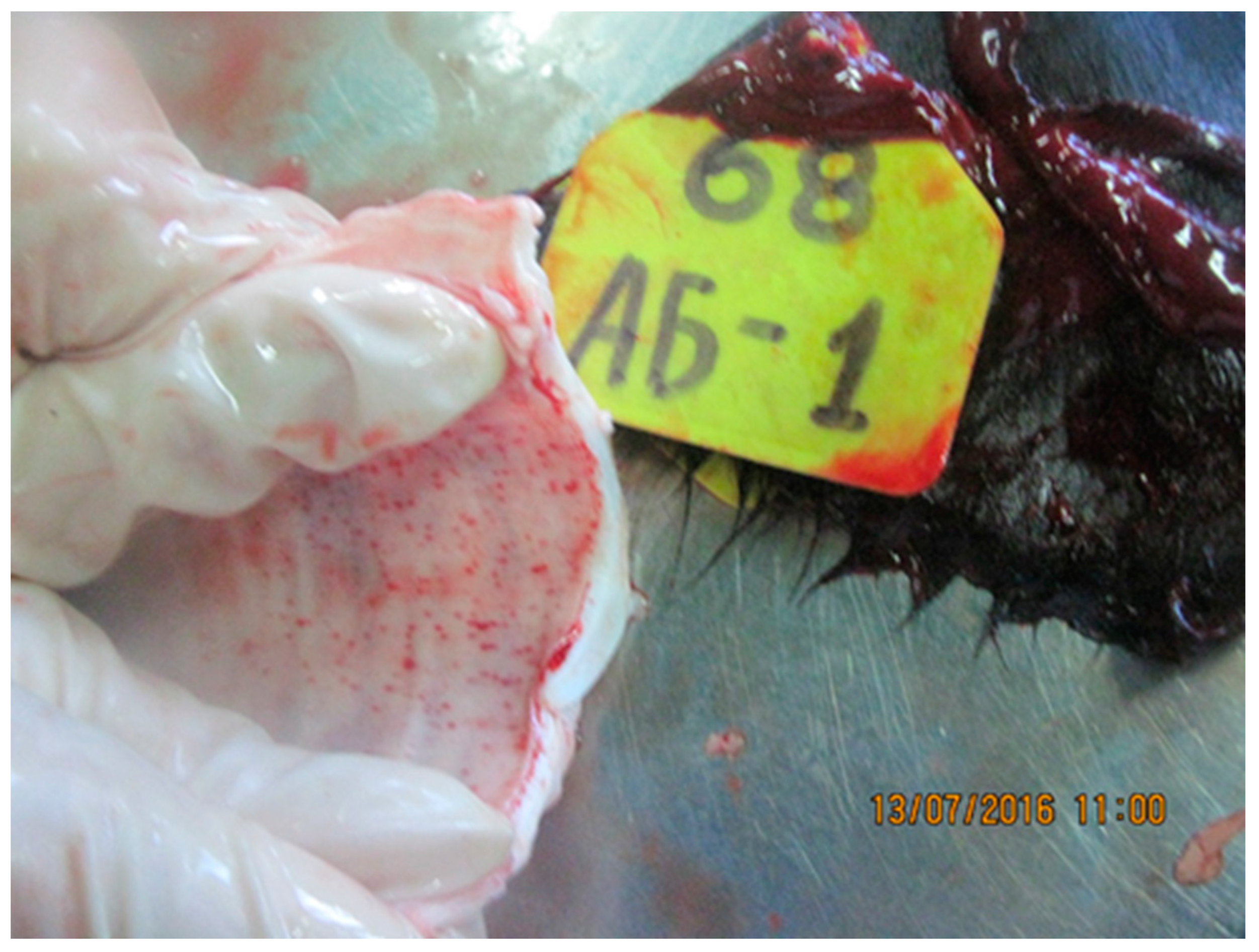
3.2.1. Skin and Muscle
3.2.2. Nasal Area
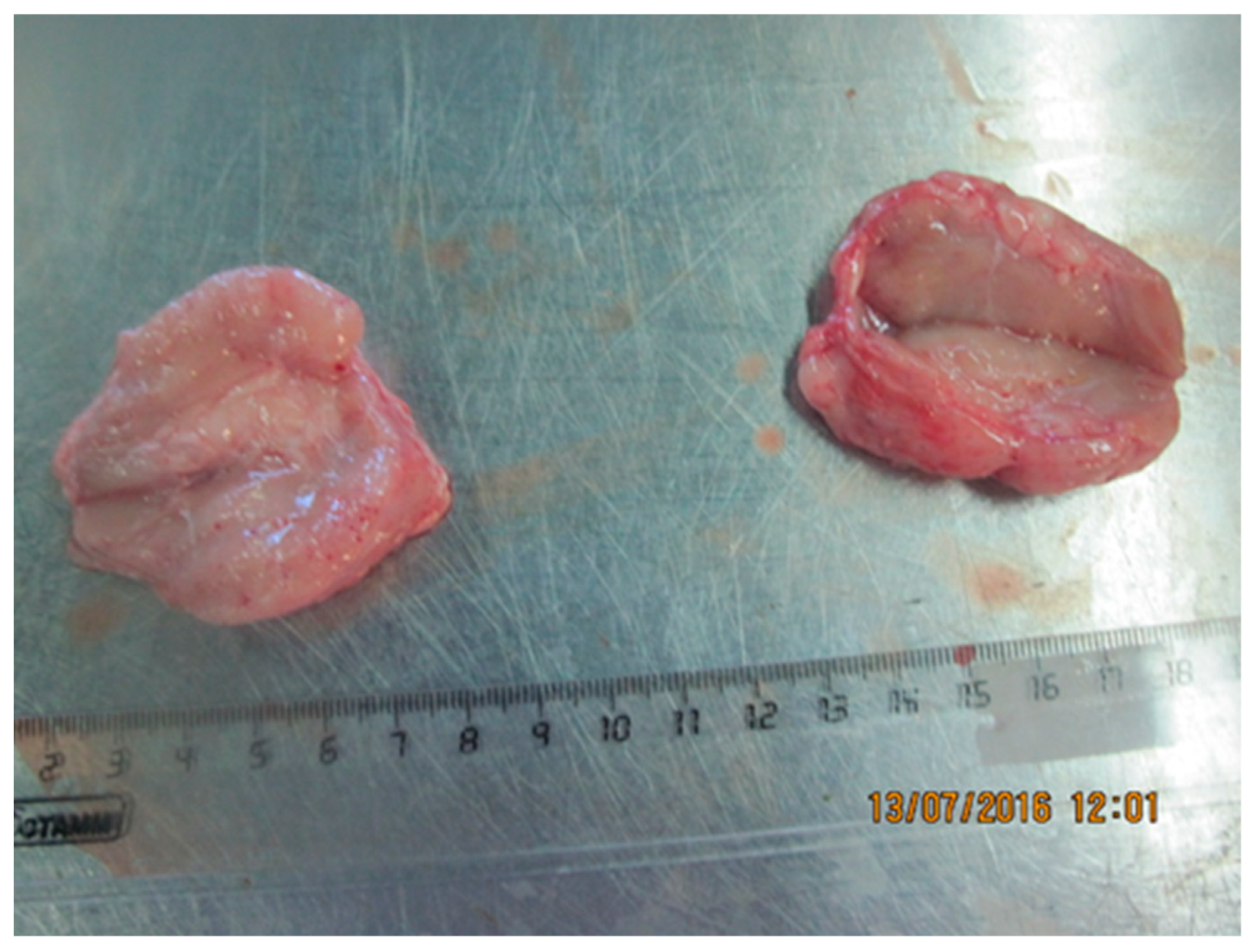
3.2.3. Tongue
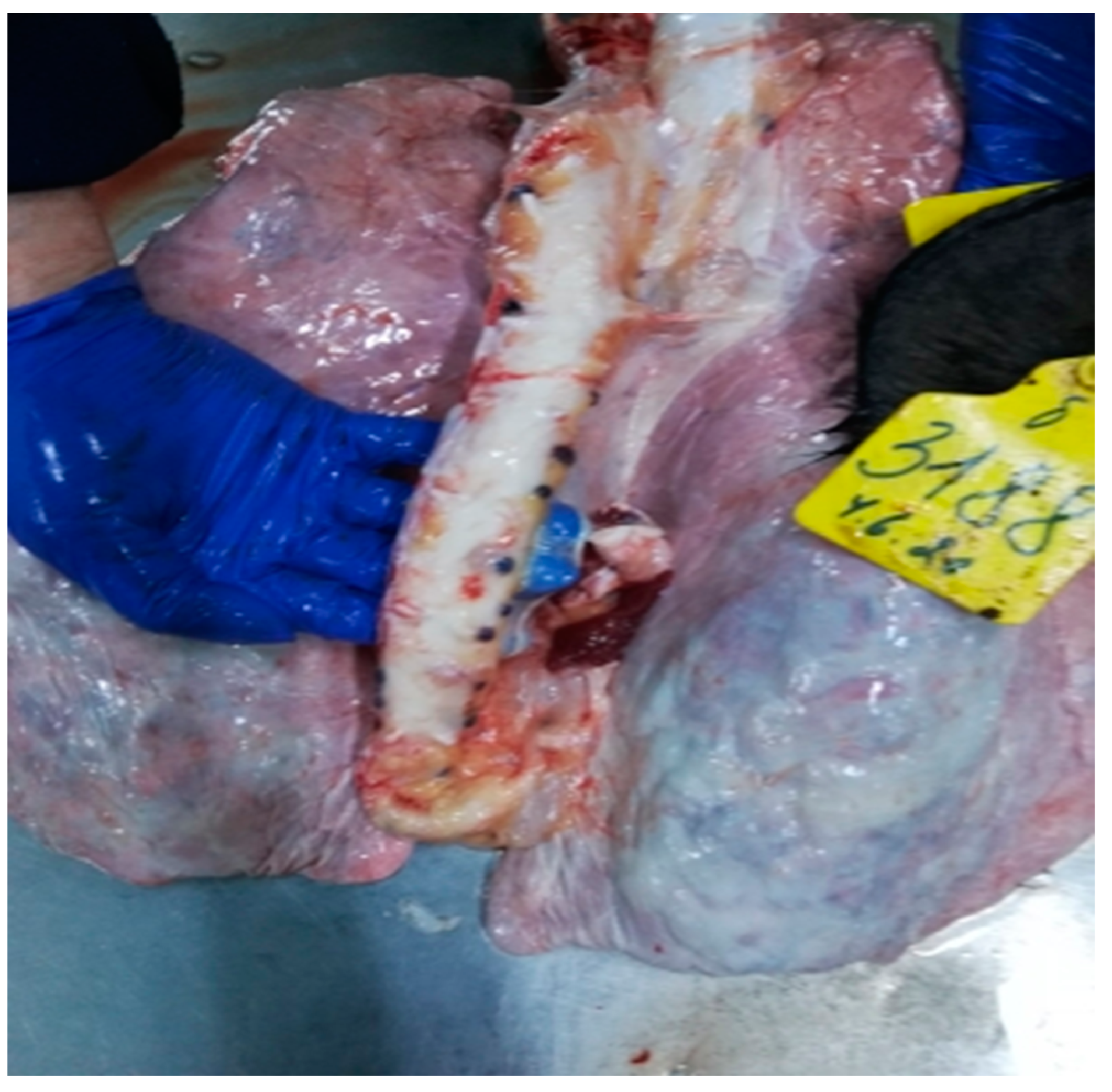
3.2.4. Lungs
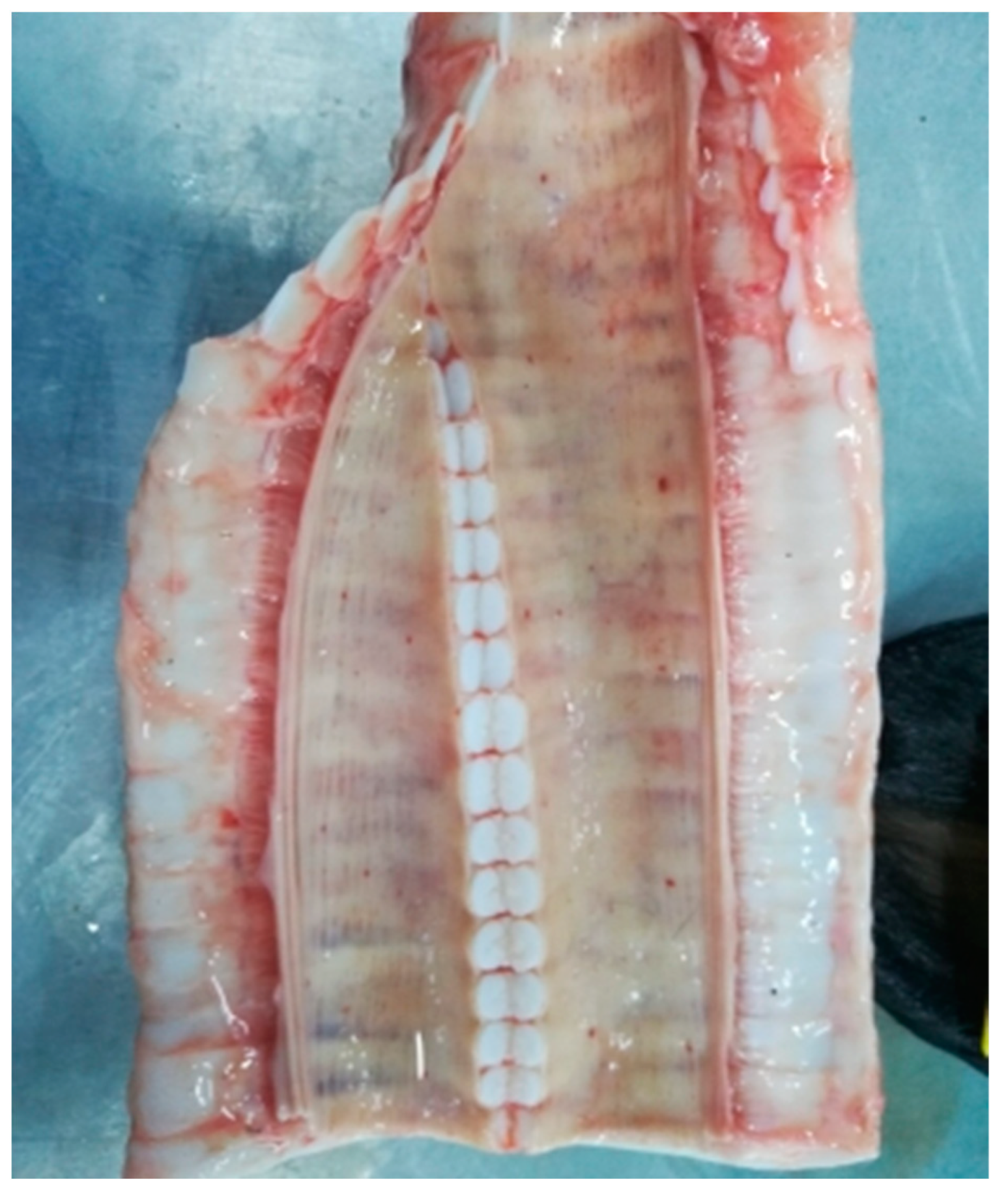
3.2.5. Trachea
3.2.6. Lymph Nodes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sprygin, A.; Sainnokhoi, T.; Gombo-Ochir, D.; Tserenchimed, T.; Tsolmon, A.; Byadovskaya, O.; Ankhanbaatar, U.; Mazloum, A.; Korennoy, F.; Chvala, I. Genetic characterization and epidemiological analysis of the first lumpy skin disease virus outbreak in Mongolia, 2021. Transbound. Emerg. Dis. 2022, 69, 3664–3672. [Google Scholar] [CrossRef]
- Fagbo, S.; Coetzer, J.A.W.; Venter, E.H. Seroprevalence of Rift Valley fever and lumpy skin disease in African buffalo (Syncerus caffer) in the Kruger National Park and Hluhluwe-iMfolozi Park, South Africa. J. S. Afr. Vet. Assoc. 2014, 85, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, C.; Lamien, C.E.; Fakhfakh, E.; Chadeyras, A.; Aba-Adulugba, E.; Libeau, G.; Tuppurainen, E.; Wallace, D.B.; Adam, T.; Silber, R.; et al. Capripoxvirus G-protein-coupled chemokine receptor: A host-range gene suitable for virus animal origin discrimination. J. Gen. Virol. 2009, 90, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Kononov, A.; Prutnikov, P.; Shumilova, I.; Kononova, S.; Nesterov, A.; Byadovskaya, O.; Pestova, Y.; Diev, V.; Sprygin, A. Determination of lumpy skin disease virus in bovine meat and offal products following experimental infection. Transbound. Emerg. Dis. 2019, 66, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Sprygin, A.; Pestova, Y.; Wallace, D.B.; Tuppurainen, E.; Kononov, A.V. Transmission of lumpy skin disease virus: A short review. Virus Res. 2019, 269, 197637. [Google Scholar] [CrossRef]
- Aleksandr, K.; Pavel, P.; Olga, B.; Svetlana, K.; Vladimir, R.; Yana, P.; Alexander, S. Emergence of a new lumpy skin disease virus variant in Kurgan Oblast, Russia, in 2018. Arch. Virol. 2020, 165, 1343–1356. [Google Scholar] [CrossRef]
- Sanz-Bernardo, B.; Haga, I.R.; Wijesiriwardana, N.; Hawes, P.C.; Simpson, J.; Morrison, L.R.; MacIntyre, N.; Brocchi, E.; Atkinson, J.; Haegeman, A.; et al. Lumpy Skin Disease Is Characterized by Severe Multifocal Dermatitis With Necrotizing Fibrinoid Vasculitis Following Experimental Infection. Vet. Pathol. 2020, 57, 388–396. [Google Scholar] [CrossRef]
- Calistri, P.; de Clercq, K.; Gubbins, S.; Klement, E.; Stegeman, A.; Abrahantes, J.C.; Marojevic, D.; Antoiniou, S.-E.; Broglia, A. Lumpy skin disease epidemiological report IV: Data collection and analysis. Eur. Food Saf. Auth. 2020, 18, e06010. [Google Scholar] [CrossRef]
- Krotova, A.; Byadovskaya, O.; Shumilova, I.; van Schalkwyk, A.; Sprygin, A. An in-depth bioinformatic analysis of the novel recombinant lumpy skin disease virus strains: From unique patterns to established lineage. BMC Genom. 2022, 23, 396. [Google Scholar] [CrossRef]
- Nesterov, A.; Mazloum, A.; Byadovskaya, O.; Shumilova, I.; Van Schalkwyk, A.; Krotova, A.; Kirpichenko, V.; Donnik, I.; Chvala, I.; Sprygin, A. Experimentally controlled study indicates that the naturally occurring recombinant vaccine-like lumpy skin disease strain Udmurtiya/2019, detected during freezing winter in northern latitudes, is transmitted via indirect contact. Front. Vet. Sci. 2022, 9, 1001426. [Google Scholar] [CrossRef]
- Sprygin, A.; Babin, Y.; Pestova, Y.; Kononova, S.; Byadovskaya, O.; Kononov, A. Complete genome sequence of the lumpy skin disease virus recovered from the first outbreak in the Northern Caucasus Region of Russia in 2015. Microbiol. Resour. Announc. 2019, 8, e01733-18. [Google Scholar] [CrossRef] [PubMed]
- Sprygin, A.; Van Schalkwyk, A.; Shumilova, I.; Nesterov, A.; Kononova, S.; Prutnikov, P.; Byadovskaya, O.; Kononov, A. Full-length genome characterization of a novel recombinant vaccine-like lumpy skin disease virus strain detected during the climatic winter in Russia, 2019. Arch. Virol. 2020, 165, 2675–2677. [Google Scholar] [CrossRef]
- Aleksandr, K.; Olga, B.; David, W.B.; Pavel, P.; Yana, P.; Svetlana, K.; Alexander, N.; Vladimir, R.; Dmitriy, L.; Alexander, S. Non-vector-borne transmission of lumpy skin disease virus. Sci. Rep. 2020, 10, 7436. [Google Scholar] [CrossRef]
- Kumar, N.; Chander, Y.; Kumar, R.; Khandelwal, N.; Riyesh, T.; Chaudhary, K.; Shanmugasundaram, K.; Kumar, S.; Kumar, A.; Gupta, M.K. Isolation and characterization of lumpy skin disease virus from cattle in India. PLoS ONE 2021, 16, e0241022. [Google Scholar] [CrossRef]
- Ochwo, S.; VanderWaal, K.; Ndekezi, C.; Nkamwesiga, J.; Munsey, A.; Witto, S.G.; Nantima, N.; Mayanja, F.; Okurut, A.R.A.; Atuhaire, D.K. Molecular detection and phylogenetic analysis of lumpy skin disease virus from outbreaks in Uganda 2017–2018. BMC Vet. Res. 2020, 16, 66. [Google Scholar] [CrossRef]
- Arjkumpa, O.; Suwannaboon, M.; Boonrod, M.; Punyawan, I.; Liangchaisiri, S.; Laobannue, P.; Lapchareonwong, C.; Sansri, C.; Kuatako, N.; Panyasomboonying, P.; et al. The First Lumpy Skin Disease Outbreak in Thailand (2021): Epidemiological Features and Spatio-Temporal Analysis. Front Vet. Sci. 2021, 8, 799065. [Google Scholar] [CrossRef]
- Parvin, R.; Chowdhury, E.H.; Islam, M.T.; Begum, J.A.; Nooruzzaman, M.; Globig, A.; Dietze, K.; Hoffmann, B.; Tuppurainen, E. Clinical Epidemiology, Pathology, and Molecular Investigation of Lumpy Skin Disease Outbreaks in Bangladesh during 2020–2021 Indicate the Re-Emergence of an Old African Strain. Viruses 2022, 14, 2529. [Google Scholar] [CrossRef]
- Kononova, S.; Kononov, A.; Shumilova, I.; Byadovskaya, O.; Nesterov, A.; Prutnikov, P.; Babiuk, S.; Sprygin, A. A lumpy skin disease virus which underwent a recombination event demonstrates more aggressive growth in primary cells and cattle than the classical field isolate. Transbound. Emerg. Dis. 2021, 68, 1377–1383. [Google Scholar] [CrossRef]
- Babiuk, S.; Bowden, T.R.; Parkyn, G.; Dalman, B.; Manning, L.; Neufeld, J.; Embury-Hyatt, C.; Copps, J.; Boyle, D.B. Quantification of lumpy skin disease virus following experimental infection in cattle. Transbound. Emerg. Dis. 2008, 55, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Abutarbush, S.M.; Ababneh, M.M.; Al Zoubi, I.G.; Al Sheyab, O.M.; Al Zoubi, M.G.; Alekish, M.O.; AlGharabat, R.J. Lumpy Skin Disease in J ordan: Disease Emergence, Clinical Signs, Complications andPreliminary-associated Economic Losses. Transbound. Emerg. Dis. 2015, 62, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Annandale, C.H.; Holm, D.E.; Ebersohn, K.; Venter, E.H. Seminal transmission of lumpy skindisease virus in heifers. Transbound. Emerg. Dis. 2014, 61, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Shumilova, I.; Krotova, A.; Nesterov, A.; Byadovskaya, O.; van Schalkwyk, A.; Sprygin, A. Overwintering of recombinant lumpy skin disease virus in northern latitudes, Russia. Transbound. Emerg. Dis. 2022, 69, e3239–e3243. [Google Scholar] [CrossRef]
- Shumilova, I.; Nesterov, A.; Byadovskaya, O.; Prutnikov, P.; Wallace, D.B.; Mokeeva, M.; Pronin, V.; Kononov, A.; Chvala, I.; Sprygin, A. A Recombinant Vaccine-like Strain of Lumpy Skin Disease Virus Causes Low-Level Infection of Cattle through Virus-Inoculated Feed. Pathogens 2022, 11, 920. [Google Scholar] [CrossRef] [PubMed]
- Möller, J.; Moritz, T.; Schlottau, K.; Krstevski, K.; Hoffmann, D.; Beer, M.; Hoffmann, B. Experimental lumpy skin disease virus infection of cattle: Comparison of a field strain and a vaccine strain. Arch. Virol. 2019, 164, 2931–2941. [Google Scholar] [CrossRef] [PubMed]
- Katsoulos, P.D.; Chaintoutis, S.C.; Dovas, C.I.; Polizopoulou, Z.S.; Brellou, G.D.; Agianniotaki, E.I.; Tasioudi, K.E.; Chondrokouki, E.; Papadopoulos, O.; Karatzias, H.; et al. Investigation on the incidence of adverse reactions, viraemia and haematological changes following field immunization of cattle using a live attenuated vaccine against lumpy skin disease. Transbound. Emerg. Dis. 2018, 65, 174–185. [Google Scholar] [CrossRef]
- Hansen, S.; Pessôa, R.; Nascimento, A.; El-Tholoth, M.; Abd El Wahed, A.; Sanabani, S.S. Dataset of the microbiome composition in skin lesions caused by lumpy skin disease virus via 16s rRNA massive parallel sequencing. Data Brief 2019, 27, 104764. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, E.; Alexandrov, T.; Beltrán-Alcrudo, D.J.F.A.P. Lumpy skin disease-a manual for veterinarians. In FAO Animal Production and Health Manual; FAO: Rome, Italy, 2017; Volume 20. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumilova, I.; Sprygin, A.; Mazloum, A.; Pronin, V.; Byadovskaya, O.; Babiuk, S.; Donnik, I.; Chvala, I. Comparison of Gross Pathology between Classical and Recombinant Lumpy Skin Disease Viruses. Viruses 2023, 15, 1883. https://doi.org/10.3390/v15091883
Shumilova I, Sprygin A, Mazloum A, Pronin V, Byadovskaya O, Babiuk S, Donnik I, Chvala I. Comparison of Gross Pathology between Classical and Recombinant Lumpy Skin Disease Viruses. Viruses. 2023; 15(9):1883. https://doi.org/10.3390/v15091883
Chicago/Turabian StyleShumilova, Irina, Alexander Sprygin, Ali Mazloum, Valeriy Pronin, Olga Byadovskaya, Shawn Babiuk, Irina Donnik, and Ilya Chvala. 2023. "Comparison of Gross Pathology between Classical and Recombinant Lumpy Skin Disease Viruses" Viruses 15, no. 9: 1883. https://doi.org/10.3390/v15091883
APA StyleShumilova, I., Sprygin, A., Mazloum, A., Pronin, V., Byadovskaya, O., Babiuk, S., Donnik, I., & Chvala, I. (2023). Comparison of Gross Pathology between Classical and Recombinant Lumpy Skin Disease Viruses. Viruses, 15(9), 1883. https://doi.org/10.3390/v15091883





