Abstract
Lumpy skin disease (LSD), a current global concern, causes economic devastation in livestock industries, with cattle and water buffalo reported to have higher morbidity and lower mortality rates. LSD is caused by lumpy skin disease virus (LSDV), a member of the Poxviridae family. It is an enzootic, rapidly explorative and sometimes fatal infection, characterized by multiple raised nodules on the skin of infected animals. It was first reported in Zambia in 1929 and is considered endemic in Africa south of the Sahara desert. It has gradually spread beyond Africa into the Middle East, with periodic occurrences in Asian and East European countries. Recently, it has been spreading in most Asian countries including far East Asia and threatens incursion to LSD-free countries. Rapid and accurate diagnostic capabilities, virus identification, vaccine development, vector control, regional and international collaborations and effective biosecurity policies are important for the control, prevention, and eradication of LSD infections. This review critically evaluates the global burden of LSD, the chronological historical outbreaks of LSD, and future directions for collaborative global actions.
1. Background
The increasing emergence or recurrence of major transboundary and emerging animal diseases in recent decades has become a matter of great economic and public health concern worldwide. These diseases affect food security by reducing the availability and affordability of high-quality animal products [1]. In recent years, lumpy skin disease (LSD) has been identified as one of the most devastating and emerging threats to large domesticated ruminants such as cattle, water buffalo and wild bovine species [2,3]. The World Organization for Animal Health (WOAH) has listed LSD as one of the most economically important and notifiable trans-boundary viral animal diseases. The disease is also known as pseudo-urticaria, Neethling pox virus disease, Exanthema nodularis bovis and Knopvelsiekte [4]. LSD is caused by lumpy skin disease virus (LSDV), a member of the genus Capripoxvirus within the Family Poxviridae. LSDV is antigenically closely related to sheep and goat poxviruses but differs from them phylogenetically [5,6].
LSD was reported for the very first time in 1929 (in Zambia) and, since then, was considered to be confined to various areas of Africa, where periodic outbreaks would be recorded until 1986 [7]. The first occurrence of LSD outside of Africa was reported in Israel between 1986 and 1988 and has gradually spread to the Middle East, then Eastern Europe and Russia [8], with subsequent spread through the Balkans [9]. In 2019, new cases were reported in South and East Asia [2], presenting an ongoing threat to all the Indo-Asian countries including Afghanistan, Pakistan and India [10,11]. Current active case identification in Asia (China, Cambodia, Singapore and Indonesia) has raised concerns about the intrusion of this virus into LSD-free countries with large, naïve cattle populations, such as Australia [12].
This contagious disease is transmitted by a variety of vectors such as biting flies, lice, ticks, mosquitoes, and wasps, but also through close contact with infected animals or contaminated feed and water troughs [5,13]. Hot and humid weather is responsible for vector multiplication and an increase in activity, making the rainy summer and autumn season and low marshy land epidemiologically more suitable for the occurrence of this disease [9,14].
LSD is characterized by high morbidity and low mortality rates and, depending on the host immune response, affected animals can display acute or chronic clinical disease [13]. Fever, anorexia, lymphadenopathy, rhinorrhea and distinct skin lesions define the acute infection stage, whereas animals in the chronic infection stage display poor production and infertility. The economic impact of LSD is realized though a drop in milk production, poor-quality hides and meat, abortion and death [15,16,17].
Preventing the spread of LSD to a disease-free area or stamping out of the disease in an area would require several control measures including strict quarantine, restriction of animal movement, vaccination with live attenuated vaccines (either homologous or heterologous or both), isolation and slaughter of affected animals, proper disposal of carcasses, cleaning and disinfection of the premises and, very importantly, insect control [14,18,19]. Occasionally, whole herd depopulation has been recommended, but in endemic scenarios, the affected farms often isolate sick animals and provide supportive treatment that may include wound dressings to prevent fly infestations and secondary infections [18,19,20].
The emergence pattern, re-emergence record and risk of incursion of LSD to uninfected countries are gradually increasing, and therefore the importance of identification of new measures which could be beneficial for rapid tracing of the infection and formation of definite control strategies takes on greater urgency. Genomic information is a vital resource that has recently been used for quick tracing, typing and identification of the mutation point of infectious agents and to develop appropriate control and eradication strategies [21,22]. This review, therefore, also provides a perspective on the use of the genetic basis of LSDV to establish convenient diagnostic and control measures.
2. Virus, Pathology and Transmission
2.1. Lumpy Skin Disease Virus
Lumpy skin disease is a WOAH-marked highly contagious vector-borne emerging transboundary pox-viral infection of bovine species [23,24]. The disease is caused by lumpy skin disease virus (LSDV), which belongs to the genus Capripoxvirus (CaPV) under the subfamily of Chordopoxvirinae within the family of Poxviridae [22]. The genus capripoxvirus is comprised of sheep pox virus (SPPV), goat pox virus (GTPV) and lumpy skin disease virus (LSDV) and they all bear approximately 96% vaccine cross-protection [22,25,26,27].
2.2. Viral Structure, Nature, and Genome Characteristics
LSDV is an oval- or brick-shaped virus with a length of 294 ± 20 nm and width of 262 ± 22 nm with bilateral body and covered by bilipid bilayer. The lineage of LSDV is reported as Viruses > Varidnaviria > Bamfordvirae > Nucleocytoviricota > Pokkesviricetes > Chitovirales > Poxviridae > Chordopoxvirinae > Capripoxvirus. This virus ports the double-stranded, linear DNA genome of 151 kbp in size. The central region codes for chordopoxvirinae conserve, open reading frames (ORFs) annotated as putative gene coding for polypeptides of 53 to 2025 amino acids. The LSDV genome consists of a central coding region bounded by identical 2.4 kbp-inverted terminal repeats and contains 156 putative genes. Among them are 146 conserved genes which encode proteins involved in transcription and mRNA biogenesis, nucleotide metabolism, DNA replication, protein processing, virion structure and assembly, and viral virulence and host range [22,27]. LSDV has an additional nine genes specifically adapted for cattle infection and which are noted to be inactive in SPPV and GTPV [28]. Although most of the genes are identical between members of CaPV, the variable genes named G-protein-coupled chemokine receptor (GPCR) gene is used for genetic differentiation among them [29]. These genes are noted as stable in the past, but gradually a high frequency of synonymous mutations by natural drift and non-synonymous mutations with highly cell passaged viruses become a subject of concern. The alterations are recorded and submitted by different researchers and stored in Genbank, National Center for Biotechnology Information (NCBI). Hence, genomic study using high-throughput sequencing is important to understand the host–pathogen interactions. Sequencing technology has become an important tool for accurate detection and characterization of specific mutations or genes responsible for pathogenicity, immune evasion, vaccine escape, recombination or reassortment, virulence, transmissibility, tissue trophism and replication factors of LSDV [21,30,31]. The genome sequence of LSDV can produce information about its geographical origins, spatio-temporal spreading and disease pattern, re-emergence and the nature of infection. The identification of specific markers may be used for contact tracing, to identify vaccine candidates, and virus control and prevention policies. The identification of alteration or deletion of a specific marker responsible for viral replication and pathogenicity could be used for vaccine production [31,32,33]. In addition, the mutation pattern of genomes can be used to uncover potential outbreaks and interlink the existing unrelated outbreaks [30]. Hence, there are possibilities of using high-throughput sequencing (HTS) for outbreak prognosis and defending biosecurity threats.
2.3. Viral Replication and Resistance
Similar to other members of the poxviridae family, LSDV exhibits a cytoplasmic replication cycle, where the double-stranded DNA (dsDNA) is largely enzymatically mediated for both messenger RNA (mRNA) production and genome copying for progeny virions [9,34]. Actual viral DNA synthesis begins 1.5 to 6 h after infection and two types of infectious virions (single membranous and double membranous) are released from the infected cells [35]. Recent studies on the susceptibility and resistance of LSDV describe the virus as greatly susceptible to a wide range of temperature (55–65 °C) and pH (6.6–8.6) variation and resistant to a wide range of physical and chemical components. It is resistant to inactivation and can remain viable for up to 35 days in desiccated skin crusts, for >33 days in skin necrotic nodules and for at least 18 days in air-dried hides [13,36]. It may persist in the environment for longer periods of time, especially in dark conditions in contaminated animal sheds, where it can persists for several months [37]. This persistent nature makes LSDV an important biosecurity threat for global livestock industries.
2.4. Host–Pathogen Interaction
Lumpy skin disease virus is highly host-specific and causes disease only in bovine species such as cattle and water buffalo [38,39], whereas the other domesticated species such as sheep, goats, pigs and horses are not affected due to host specificity [40,41]. Cattle are the definitive hosts but specific antibodies for LSDV have been found in various wild ruminants such as blue wildebeest, eland, giraffe, impala and greater kudu, whenever experimental inoculation was carried out [42,43,44]. However, it is necessary to observe and monitor disease outbreaks and virus mutations regularly to anticipate potential host jump.
The degree of severity of LSD in hosts depends upon several host and environmental factors [45]. Although breed, age and sex variation do not seem to play a role in determining the severity of disease [13,39,46], some studies found Bos taurus more susceptible to severe disease than indigenous (Bos indicus) and Zebu cattle [47] due to their thin-skin characteristics and also high-producing milk production [48]. Male zebu cattle often become susceptible due to working as draught animals, resulting in skin scratches which become the site of vector attraction [6]. Calves tend to display more severe clinical signs than adults [6,49].
Under ideal environmental conditions, vector populations may proliferate and come into more regular contact with susceptible hosts. Environmental and land use policies therefore play an important role in management of vector-borne diseases such as LSD. Farming in low land with continuous watercourses provides a humid condition relatively suitable for the higher proliferation of these vectors [2,6,14]. The farming system designed for high yielding cows combined with favorable environmental condition could create the stressful condition, which may provoke host immunosuppression and production of disease [50]. Comparatively larger farms with intensive housing, shared feeding and watering facilities may also help in spread of LSDV [51].
Due to the remarkable stability of the virus in different infective sites and environmental conditions, with better resistance against most of the physical and chemical inactivators, LSDV can persist in skin lesions for up to 35 days at ambient temperature [52]. The virus can be shed through the nasal and lacrimal secretions, milk and semen, and are noted to be viable for up to 11 days in milk and 22 days in semen [53,54]. There is no evidence of virus presence in the meat from infected animals but the virus can be isolated from infected fomites including animal rearing and transportation equipment [55]. There is a lack of evidence of viral presence in the vector body after 4 days of inoculation [49].
The host immune response is not clearly understood but, as with most of the capripox viruses, a lifelong immunity against reinfection with LSDV has been demonstrated [56]. After infection, the growth and replication of LSDV occurs intracellularly [57,58], so the humoral immunity cannot resist the viral proliferation but innate immunity has the capacity to mount an immune response by stimulating the adaptive immune system and mediating several immune cells such as lymphocytes, macrophages and neutrophils, responsible for inactivating the causal agent and controlling the disease [28,56,59]. Animals that have recovered from natural infection produce specific antibodies, capable of neutralizing up to 3 logs of the virus and are also resistant to re-infection [52]. The maternal immunity may support calves to resist the clinical infection of LSD for up to 6 months [60]. Vaccination can stimulate the humoral immunity of the animal and give protection for more than 7 months [59], so an annual vaccination schedule with a booster dose is recommended due to the unknown duration of both cellular and humoral immunity [61]. However, LSDV–host interaction remains unclear at this time.
2.5. Viral Transmission
In general, LSDV is thought to be transmitted by the indirect route, with a blood-sucking arthropod as the vector [62]. The vector associated with transmission transmits the viral particle through its mouth parts without any viral replication in the host itself, hence this transmission is considered mechanical, instead of biological [6]. Some biting flies such as Stable flies (Stomoxy calictrans) and biting files (Biomyia fasciata), mosquitoes such as Culex mirificens and female Aedes egypti and the biting midge, Culicoides nubeculosus, are commonly involved in the transmission of LSDV [43,63]. Additionally, house flies (Musca domestica) and tsetse flies (Glossina Sp.) may also play a role in LSDV transmission [64]. Several hard ticks (Amblyomma hebraeum, Rhipicephalus appendiculatus and Rhipicephalus decoloratus) may also serve as reservoirs as well as mechanical vectors for LSDV [64]. Recent studies have shown that Boophilus decoloratus is one of the tick species which can transmit LSDV by both transstadial and transovarian means [63,65]. However, the vector availability and distribution vary in different geographical and climatic regions, so further detailed studies in other geographical areas are warranted.
Direct contact with infected animals has been shown to be an effective method of LSDV transmission [66,67] since they shed the virus through skin lesions, nasal, oral and ocular secretions, and from lesions containing mucosa [68]. Direct transmission of this disease then occurs through the introduction of shared feeding and watering pots contaminated with these body secretions. It can also be transmitted iatrogenically during mass treatment using common treatment tools such as needles [6,55,69]. Lactating cows can also transmit LSDV to suckling calves by milk contaminated with udder skin lesions, while vertical transmission has been demonstrated experimentally through the transmission of infected semen during natural mating or artificial insemination; the virus may remain persistent for up to 42 days post-infection in semen [70]. An intrauterine route of infection was recorded as vertical transmission from dam to calves [68,71] and it is assumed that this virus can be shed through vaginal secretion [48,72]. Direct transmission accounts for short route infection, whereas indirect or vector borne transmission can be applicable for both short and long-distance infection followed by uncontrolled transboundary animal movement [45,73,74]. A representation of the possible mode of transmission of LSDV is shown in Figure 1.
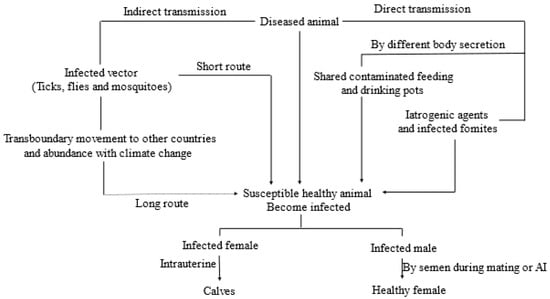
Figure 1.
Schematic representation of the possible mode of transmission of LSDV.
2.6. Pathogenesis and Effects on Host Body
There is limited knowledge on the pathogenesis of LSD [71,75] compared to other viral diseases. LSD shows a progressive pattern of pathogenesis from viral inoculation followed by implantation and multiplication locally, then development of viremia resulting in viral transportation to the specific tissues and organs [57,76]. The incubation period of LSDV is approximately 5 weeks in the case of natural infections, while it ranges from 4 to 7 days experimentally [13]. Similar to the other members of Capripoxvirus, LSDV has a tissue trophism for keratinocytes [77]. Just after inoculation into the susceptible animals, either naturally or experimentally, the virus starts to replicate in most abundant cells such as keratinocytes, hair follicle epithelium, fibroblasts, interstitial macrophages and pericytes of the predilection site such as the skin of the head, neck, genitalia, limb and udder [76]. In keratinocytes, initially hyperplasia and ballooning degeneration occur, which may extend to the epidermis and several micro-vesicles form, then, larger vesicles form by the coalescence of micro-vesicles and attract the inflammatory cells to accumulate in the epidermis, dermis and subcutaneous layer [78]. Finally, there is ulceration with exudation resulting in scab and crust formation with different degrees of hemorrhage, congestion and edema formation in the surroundings [9,49]. The viral particles may be transported to secondary sites of infection including lungs, liver, kidney and other lymph-nodes [75,79] through systemic circulation; especially monocytes are considered as the carrier of LSDV and intermittent fever is the indication of extreme viremia. During virama there may be the possibility of the development of vasculitis and lymphangitis resulting from endothelial injury with viral replication [80].
2.7. Clinical Manifestation
Depending on the lump count, vector load, host susceptibility and immunity, managerial and environmental factors, clinical manifestations of LSD can be divided into mild and acute forms and starts with biphasic fever, rise to a peak of 40–40.5 °C, which can persist for 3 days [9,81]. In the mild form, very few number of nodular lesions of 1–5 cm in diameter are found within 2–3 days after the onset of fever along with anorexia, depression, hypersalivation, nasal and ocular secretion, decreased production and emaciation. The nodules are usually round, raised, hard, painful and hyperemic and usually observed in the skin of the muzzle, neck, back, legs, scrotum, perineum, eyelids, lower ear, nasolacrimal mucosa, and tail [72]. The acute form is more severe, with continuous high pyrexia, severe anorexia and depression, often many uniform nodules may be observed over the animal body within 2–3 weeks of onset of the disease. The raised, nodular lesions are usually approximately 1–7 cm in size and most commonly found in the head, neck, udder, genitalia, perineum and legs and can easily be separated from the surrounding healthy skin by a hemorrhagic rim [81]. The smaller nodules often coalesce to form larger lesions and slough off creating a condition named as ‘sit-fasts’, which serve as the nidus for further vector attraction and secondary bacterial infection [78,82]. Due to involvement of the whole skin and muscles, severe pain results in the animal becoming reluctant to move; occasionally swelling of the face, brisket and limb are also observed [54,83]. Affected animals also exhibit some typical LSD lesions in the oral cavity, conjunctiva and nasal cavity resulting in profuse mucosal secretion [39,50]. In affected cows, severe loss of milk production, abortion and persistent anestrous may develop, whereas, in male animals, due to testicular tissue involvement, clinical swelling of the scrotum is observed, which may lead to temporary or permanent infertility [39,72].
2.8. Hematological Assessment
The hemogram analysis shows no significant alteration in the blood profile in the early (within 1–2 days) stage of infection but as the disease progresses, several alterations are noted in the erythrogram, leucogram and others [84]. In prolonged cases, a marked decrease in total erythrocyte count (TEC), hematocrit value (HCT), hemoglobin value (Hb) and mean corpuscular hemoglobin concentration (MCHC) with an increased mean corpuscular volume (MCV) [85] may indicate the development of hemorrhagic or hemolytic anemia. A macrocytic hypochromic type [86] of anemia may develop due to several inflammatory cytokines (TNF, IL-1α, IL-1β, and IF-γ), initiating reduced erythropoiesis by the bone marrow. This type of anemia develops slowly and is usually mild in nature [84] and associated with anorexia, dietary iron deficiency or disturbed iron metabolism [87]. In acute infections, a large amount of endogenous corticosteroid is produced which leads to lymphopenia because of elevated viral load and leukopenia due to increased tissue demand and neutrophilic margination [88,89]. After 10–14 days of infection, leukocytosis (granulocytic) may occur, associated with the marked production and accumulation of neutrophils due to secondary pyogenic bacterial infection [88,89,90]. The platelet count may decrease leading to a marked thrombocytopenia, with a resultant short life span and extreme consumption by vasculitis and vascular thrombus formation [91].
2.9. Biochemical Assessment
The serum biochemical analysis is associated with alterations in the protein, creatinine, minerals and enzymatic level. Total serum protein and albumin are decreased during early LSDV infection, due to decreased dietary protein intake and reduced protein synthesis as a result of hepatocellular disturbances with higher protein catabolism [92]. Elevated globulin levels and blood urea nitrogen (BUN) are detected during the later stage of infection, which may be due to dehydration and the host immune response [91,93,94]. Elevated serum alanine amino transferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) in early infection originate primarily from hepatocytic damage by the virus directly [92]. Raised AST activity with cardiac troponin activity confirms the cardiac tissue effect of LSDV [95,96]; however, skeletal muscle and myocardial damage are also indicated by increased serum lactate dehydrogenase (LDH) and creatine phosphokinase (CPK) activity [28,97,98]. Total blood glucose level and serum creatinine levels are also increased in infected cattle due to higher glucose catabolism in anorexia and kidney damage, respectively [89].
2.10. Pathological Assessment
In sum, there are skin nodules of uniform size, small, raised with erected hair, often merging to larger, irregular ones, spread all over the body surface [9]. These nodules are commonly present in the epidermal and dermal layer but subcutis and often deep musculature are also involved [7,99]. The appearance of the nodular lesions is associated with the progression of disease with definite “sit-fast” lesion characterized by severe ulcerative and suppurative necrosis with external scab or crust formation in acute cases. The necrotic lesion can eventually be found in almost all the parts of alimentary (lips, gum, palate, fore stomach), respiratory (nasal mucosa, pharynx, larynx, trachea and bronchiolar structures) and urogenital tract of both male (testes) and female (uterus, vagina, teats and udder) animals. In severe infection, the deeper tissues such as the tendon sheaths, synovial joints and even muzzle bone are also be affected [13]. Both local and mediastinal lymph nodes enlarge severely due to edema [100]. Pneumonic lung, tracheal dysfunction, mastitis, metritis, pyometra, and orchitis are common complications found, whenever there is secondary bacterial or fungal involvement [69,101].
Histopathological observation of LSD displays the pathognomonic microscopic lesion of eosinophilic intracytoplasmic inclusion bodies found in keratinocytes, macrophages, endothelial cells and pericytes from skin nodules [78,102]. There is marked ballooning degeneration of the cellular layer of both the epidermis and dermis which extend to macrovesicle formation with accumulation of inflammatory exudates [103,104]. The presence of marked inflammatory cells in the infection sites including the lymphocytes (for viral infection), macrophages (for phagocytosis), eosinophils (for inflammation), and neutrophil in the case of secondary infection are reported [68]. Similar lesions extend towards the subcutis and Zenker’s necrosis is noted when there is involvement of subcutaneous muscular structure [52]. There is marked congested blood vessels and edema, accompanied by marked accumulation of epithelioid cells in the dermal stoma [14]. There is severe vasculitis characterized by thickened vascular walls with inflammatory cell accumulation which finally leads to thrombus formation and concurrent necrosis [105,106]. The microscopic lesions are remarkably similar throughout the body [107].
3. Historical Outbreaks and Re-Emergence
Historically, LSD was first reported in Northern Rhodesia (now Zambia) in 1929 (MacDonald, 1931) as a condition of either poisoning or insect bite hypersensitivity, known as ‘pseudo-urticaria’ [108]. The infective nature of this condition was demonstrated between 1943 and 1945, when Zimbabwe (Southern Rhodesia), Botswana, and the Republic of South Africa reported cases [100,109]. By 1946, it had extended eastwards to Mozambique [46], then to central African countries such as Angola and Zaire in 1950, Madagascar in 1954 and finally Namibia, Tanzania and Uganda in 1956 [69]. Over the next two decades research of this infection was undertaken in different African countries. LSD first emerged in Kenya in 1957 and another re-emergence was recorded in 1977 [108,110]. After a gap of 14 years, another African country, Sudan, reported an explosive epizootic emergence of LSD in 1971 [111,112]. Chad and Niger experienced introduction of LSD in 1973 and were largely responsible for the outbreak in Nigeria in 1974 [113,114]. In Ethiopia, LSD was first observed in 1983 [115] and it gradually spread to almost all regions and agroecological zones of this country [49]. Several sporadic occurrences of LSD in Tanzania, Kenya, Zimbabwe, Somalia and Cameroon were recorded in different studies in the period of 1950 to 1985 with spontaneous re-emergence in different African countries over that timeline [8,116].
Outside the Sub-Saharan continent, an LSD outbreak was first reported in 1988 in Egypt, followed by Israel in 1989 [44,69]. Although a smaller outbreak with cases not being confirmed, LSD was reported in Oman in 1984 with an epizootic re-emergence in 2009 [50]. The disease was for the first time detected in Kuwait in 1986 and again in 1991. Furthermore, prevalence of LSD for the first time was recoded in Lebanon in 1993, Yemen in 1995, United Arab Emirates in 2000, Bahrain in 1993 and again in 2002–2003, Oman in 2009 and finally in Saudi Arabia and Iraq in 2013 [117,118].
Sporadic occurrences of LSD in several European countries (Greece, Albania, Russian Federation) were first recorded in 2015. A massive outbreak was noted in Russia in 2017 and cases were recorded in several countries within the European continent, where it was mostly successfully controlled with mass vaccination and appropriate managerial practices [26]. Although Georgia started vaccination of cattle in 2014 with the outbreak of neighboring Azerbaijan, an outbreak was recorded in 2016 and re-emerged with 6 more outbreaks in 2018 [119]. At the same time, LSD outbreaks were reported in eight Balkan countries (Greece, Bulgaria, The Former Yugoslav Republic of Macedonia [FYROM], Serbia, Kosovo, and Albania) with higher incidence at the border areas [120].
Since 2019, LSD has become a major concern for Asian countries and presents one of the greatest threats for animal health and food security on the Asian continent. The reports of the devastating LSD outbreak in Bangladesh in July 2019 emphasized LSD’s importance [23,121] and this was again evident with a subsequent outbreak in 2020 [76]. Subsequently, many outbreaks have been reported across Asia, including simultaneous outbreaks in China and India [13,44]. The intra-country spread of LSD has been extensive and rapid with frequent outbreaks now occurring on a regular basis [122]. Outbreaks have been reported in Nepal, Bhutan, Sri Lanka, Vietnam and Malaysia in 2020 at the month of June, July, September, October and November, respectively [44,81]. An outbreak in Thailand was reported to WOAH in April 2021, subsequently also in Lao People’s Democratic Republic in May and in Pakistan in November of the same year [11,13,123]. Very recently, LSD was confirmed in Indonesia and Singapore in March 2022, [124]. The emergence and re-emergence of LSD is a matter of great concern, especially since recent outbreaks have not followed distinct patterns previously reported [2]. Table 1 and Figure 2 present the historical occurrences of LSD and an indication of countries at threat of a possible incursion of this disease.

Table 1.
Historical outbreak of natural lumpy skin disease (LSD).
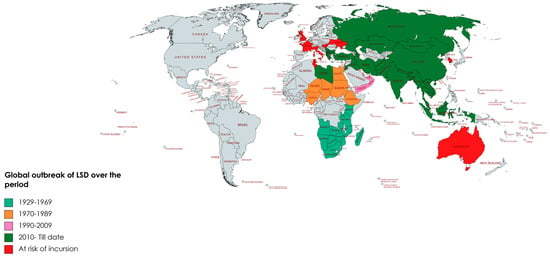
Figure 2.
Global outbreak of natural lumpy skin disease (LSD) starting from 1929 to 2023?
4. Diagnosis and Management
4.1. Presumptive Diagnosis
Primarily the clinical history, clinical signs, morbidity and mortality rate can provide some basis for the presumption of LSD infection. A confirmatory diagnosis of LSD is indicated in all cases where owners report cattle with characteristic necrotic skin nodules (sit-fast) observed on face, eyelid, neck, muzzle, nostrils, udder, limbs, enlarged lymph nodes, persistent high fever and gradual emaciation [13,164].
The clinical signs can occasionally be confused with other skin lesion producing diseases, requiring laboratory confirmation. Postmortem diagnosis is also important. In sum, pock-like lesions are found on the mucous membrane of the mouth and different parts of the alimentary tract, the nasal cavity, trachea and lungs [13,99]. The lesions may also be present in the testes and urinary bladder [13,70]. Severe oedema is found in the dependent parts of the body with enlarged mediastinal lymph nodes. Synovitis and tenosynovitis with fibrin formation are detected in the synovial fluid [165]. Skin sample from affected animals may be useful for diagnosis on histopathology [37]. Eosinophilic intracytoplasmic inclusion bodies marked in the keratinocytes, macrophages, endothelial cells and pericytes from skin nodules is considered a pathognomonic lesion, along with ballooning degeneration of the epidermal cell layers [166]. Other inflammatory cells such as macrophages, lymphocytes and eosinophils are also infiltrated in the affected area. Widespread vasculitis, thrombosis, infarction, perivascular fibroplasia are seen histologically [104]. Due to muscular involvement, sever coagulative necrosis (Zenker’s necrosis) in subcutaneous muscle may be observed histologically. Lymph node enlargement is associated with lymphoid proliferation, oedema, congestion, and hemorrhage [9,102].
4.2. Confirmatory Diagnosis
The skin biopsy sample is an excellent source of virus for confirmatory test including virus isolation and identification. Where available, the samples should be collected and transported to the laboratory by using virus transport medium (VTM) such as 20 to 50% glycerol in phosphate buffer saline. Several laboratory diagnostic tests are recommended for the viral agent diagnosis such as, virus isolation, conventional or real-time polymerase chain reaction (PCR) and electron microscopy. Several serological tests such as virus neutralization (VN), agar gel immunodiffusion (AGID), indirect fluorescent antibody test (IFAT), Western blot analysis and enzyme-linked immunosorbent assay (ELISA) are available for detection of immune response of infected animal specific to LSDV [13,37].
Both conventional gel-based PCR methods and quantitative real-time PCR methods are considered as rapid, simple and sensitive tests, whereas RT-PCR is considered more sensitive, faster and labor-saving than conventional PCR [160,167]. PCR is the most popular testing method for the detection of viral genome from skin lesions (scabs or nodules), un-clotted blood, saliva, nasal swabs, semen, milk, and tissue culture samples following the standard protocol specific for capripoxvirus [37,168].
LSDV isolation and identification in cell/tissue culture is well established. Tissue cultures of bovine, ovine or caprine origin, especially primary or secondary culture of bovine dermis cells, kidney cells (Madin–Darby Bovine Kidney, MDBK) cells, lamb testis (LT) cells, etc., are considered to be the most susceptible cells for LSD viral growth. The chorioallantoic membrane of embryonated chicken eggs and African green monkey kidney (Vero) cells are also suitable for LSDV growth [37,169].
Electron microscopy can be used to identify the classic poxvirus virion by using a negative staining preparation technique. Brick shaped, covered in short tubular elements and approximately 290 × 270 nm in sized capripox virion are well observed with pioloform-carbon substrate under electron microscope. This test method is, however, limited in its ability to distinguish LSDV from other orthopox species or varieties [13,37].
Immune responses of either infected or recovered animals can be detected by different serological tests; however, some tests are unable to distinguish LSDV from other species of capripoxvirus [37]. Detection of antibody in infected or recovered cattle, using virus neutralization test (VNT), is successful between 2 days post-infection and approximately 7 months after infection. VNT has a limitation of antibody detection with low titers and its sensitivity has been reported to be approximately 70% [48]. An indirect fluorescence antibody test (IFAT), using the capripoxvirus antigen fixed in the tissue culture plate, can be used to detect antibody titers of up to 1/5000 in the serum of convalescent animals [26]. It has the capacity to test a larger number of samples than VNT. However, it has a limitation of cross-reaction with cowpox virus, but not with parapox viruses [8]. Agar gel immunodiffusion (AGID) test is a simple and cost effective procedure used for detection of precipitated antigenic particle but this method is not recommended for LSDV [37] due to cross-reaction of LSDV with antibodies to bovine papular stomatitis and pseudocowpox virus resulting in false-positive results [64].
Western blot analysis is designed with sensitive and more specific configuration for detection of capripoxvirus antibody but this method is time-consuming and expensive due to its requirement of pure antigen [37]. A new commercial and WOAH recommended enzyme-linked immunosorbent assay (ELISA) kit is commercially available for the detection of blood antibody against LSDV. Experimentally, ELISA has high specificity and does not cross-react with parapox viruses, but it cannot differentiate between antibodies against LSDV, SPPV and GTPV virus in serum and plasma [164]. Immunohistochemistry investigations can demonstrate LSDV antigen particles within the cytoplasm of epidermal basal cell layer, especially in prickle cells; the reactions demonstrated as a granular golden brown immunoperoxidase staining of viral antigen [170].
4.3. Differential Diagnosis
Since there are couple of other diseases with similar skin lesions to LSD, the differential diagnosis is important [99]. Pseudo lumpy skin disease is caused by bovid herpesvirus 2 (BHV2), which causes superficial skin lesion and is characterized by a short course of disease. Pseudo cowpox (Para poxvirus) is differentiated from LSD lesions by its sites of infection, which is mainly on the teat and udder. Vaccinia virus and Cowpox virus (Ortho poxviruses) also cause site-specific lesions on the teat, udder and muzzle and are zoonotic in nature, unlike LSD. Dermatophilosis caused by Dermatophillus congolensis and Hypoderma bovis infection may cause confusion due to its similarities with LSD. However, the lesions are severely swollen and eroded, with exposure of larvae in the skin of the back of the animal. There may be paralysis of the lower body and legs when the spinal cord is involved. Onchocercosis and demidocosis are parasitic skin problems often characterized by the presence of parasites and they are usually site specific. There are other hypersensitivity reactions (photosensitization, insects, and arachnid biting), which may create confusion with LSD lesions. The differentiation of these diseases can be done through PCR and other antigen or antibody specific tests [39,171].
4.4. Treatment Strategies
Similar to other viral diseases, there is no definite treatment protocol for LSD apart from symptomatic [84] and supportive treatment. Treatment may include antibiotics for the secondary bacterial infection, anti-inflammatory pain-killer therapy to reduce pain [60] and local application of ointment or antiseptic spray for wound healing [72]. Often intravenous fluid therapy is used to address dehydration and vitamin supplementation is given to reduce the weakness and increase the appetite [110]. It is worth mentioning that autohemotherapy and autogenous serum therapy from a recently recovered animal to the infected animal is being practiced in different areas of Bangladesh following Bangladesh Livestock Research Institution (BLRI) guidelines as a trial (Mahmud T., Personal communication, 7 July 2023). However, proper scientific research is yet to be performed, and no further data are available.
5. Vaccines and Vaccine Controversies
According to the WOAH, appropriate vaccination can control LSD by providing good immunity to cattle against LSDV. The vaccines should be safe to use for all cattle breeds, ages and pregnant animals and the vaccines should be well prepared with an appropriate label. Recently, the use of live attenuated vaccines of both homologous (vaccinating cattle with a LSDV-based vaccine) and heterologous (vaccinating cattle using a sheep pox/goat pox virus-based vaccine) has been used to control the outbreak in endemic regions [18]. The heterologous vaccines are used as alternative options in those areas, where both LSD and sheep and goat pox occur simultaneously, and the countries have manufacturing capacity for these vaccines [10,18]. However, the heterologous vaccines should be well characterized, adjusted, and evaluated using a vaccine challenge trial.
The available attenuated vaccine consists of either the well-known South African Neethling strain or the Kenyan sheep and goat pox (KSGP) O-240 and O-180 strains. The vaccine-producing viruses are derived from 61 serial passages in lamb kidney (LK) cells culture, followed by 20 passages in the chorioallantoic membrane of embryonated chicken eggs, and three passages again in LK cells [172,173]. The South African Neethling strain is the vaccine most commonly used in endemic countries to protect cattle against LSD. It has been shown to be effective; however, it can cause some adverse effects. It has been reported that vaccination with Neethling strain may cause local skin reaction at the vaccination site or generalized small sized skin nodules with a reduction in the milk yield from lactating cows, often referred to as “Neethling response” [63,99,172]. Several studies have reported that vaccine viruses were detected from skin nodules, blood, and milk of cattle vaccinated with Neethling vaccines [174]. The KSGP O-240 and O-180 have been used against LSD in the horn of Africa, Israel and Egypt, and reported to give protection with post-vaccination complications such as fever and skin lesions [100,175,176]. Inadequate protection with comparatively higher morbidity rate were reported in dairy cattle vaccinated with both KSGP O-240 and KSGP O-80 strains [174]. Another example of attenuation of a virulent field strain is the Madagascan LSD strain. The original LSDV strain is also reported to be used for controlling LSDV [177].
The Gorgan goatpox strain (a goatpox virus-based vaccine), Romanian SPPV strain (a sheeppox virus-based vaccine), and the Yugoslavian SPPV RM65 strain (Ramyar) are used as heterologous vaccines against LSDV. Gorgan goatpox strain vaccine was first used against LSD Ethiopian cattle and reported to give sufficient protection and seroconversion in cattle against highly virulent LSDV [17,178]. The immunogenicity and immune response of heterologous vaccines are well studied compared to that of the homologous formation [17,110,179]. A minimal, or in most cases, no adverse reaction was found at the injection site [18]. Varying degrees of success was reported with the use of different heterogenous-type sheeppox based vaccine in the Russian Federation, parts of the Middle East, and Africa [180]. These vaccines were demonstrated to afford lower protection in cattle against LSDV and some effects such as fever, reduced milk production, the development of nodules in vaccinated cattle, and sometimes re-emergence were also recorded in the vaccinated herd [18,134]. Despite minimal adverse reactions, the heterologous vaccines usually require a lower level of attenuation for safe use in cattle than homologous vaccines [81]. The production cost and price of homologous vaccines are also much higher because of the requirement of a higher number of passages for attenuation in comparison to the heterologous products [168].
Inactivated LSDV vaccines are not yet described in the WOAH Manual, but some manufacturers have developed inactivated vaccines against LSD for countries that may be willing to use it [18]. Inactivated vaccines generate a shorter duration of immunity and may be preferred as a prophylactic vaccine alternative in disease free, at-risk countries [18,181]. Nevertheless, recombination might be possible due to improper safety maintenance during the manufacturing process. Recombinant LSDV could be developed when under-attenuated vaccines are introduced in animals previously infected with virulent field strain [182,183]. The risk of cross-contamination is another threat to consider when using vaccines of different strains or multivalent vaccines of the same species [180,184,185]. Sometimes emergence of multi strain LSDV could result from improper safety measures during the propagation of viruses [186]; recombination may also occur [187]. Appropriate molecular tools should be available for screening of viral strains present in the LSDV vaccine, which could minimize the risk of recombination and cross-contamination [18]. Some studies have suggested the need to distinguish between the recombinant wild-type LSDV strains and the Neethling-based vaccine strains which are used in most live attenuated commercial vaccines [188].
Vaccination should be done as per manufacturer instructions, but some common instruction must be maintained for effective vaccination programs. Annual vaccination of adult cattle, calves from vaccinated animals, or newly purchased animals before entering a property, is recommended [177]. However, animals which might be in their incubation period or actively infected, should strictly not be vaccinated with live attenuated vaccine as there is a risk of recombination of virus and vaccine strains [189]. In the case of inactivated vaccination, initial vaccinations comprise two vaccinations one month apart and then re-vaccination every six months is required to maintain immunity [182].
6. Economic Impact
LSD has both direct (related to mortality) and indirect (related to impacts of the disease on animal health and production) impacts on countries’ economies, with indirect effects outweighing direct effects [16]. The mortality rate of LSD is low and usually considered as 1–3%, whereas the morbidity rate varies from 3% to 85% worldwide [44]. The major economic losses are followed by the high morbidity rate along with subsequent disability of the infected animals [15], costly treatment and control measures, restricted global animal trade and higher cost of maintenance of proper biosecurity measures [44]. Chronic effects found in both dairy and beef cattle include emaciation, cachexia, permanent scar formation in the skin lesion resulting in decreased hide and meat quality [14,15] and reduced draft power of oxen used in mixed farming systems [16] in some parts of the world. Loss of production in cows include a decline in milk yield due to high fever, development of secondary bacterial mastitis and abortion [48,100]. In bulls, severe orchitis cause temporary infertility that may lead to permanent infertility, if severe [17,51]. To mitigate the clinical signs, symptomatic treatment measures should be evaluated from a financial perspective. Often, infected or in-contact animals are slaughtered for eradication and control of the disease. Strict biosecurity measures are also adopted, which become an economic burden [44,190]. All these factors lead to reduced quantity and quality of production and are associated with the trade restriction of animals and animal products resulting in economic losses for the industries and countries involved with livestock [10,104]. Lumpy skin disease is currently exerting a huge economic impact on the livestock industry as the disease is wiping out small scale marginal farmers. For example, a total annual loss of approximately 31.37 and 59.97 million USD were recorded from two districts (Gaibandha and Mymensingh) of Bangladesh [191]. The incursion of LSD in LSD-free countries could have devastating economic consequences. It has been estimated that LSD incursion into Australia could cost millions of dollars [192].
7. Transboundary Biosecurity Threat
Lumpy skin disease is categorized as an important transboundary disease and several factors are associated with the rapid cross-border spread of LSD [189,193]. Legal or illegal transportation of both live domestic and wild animals, including their products, (milk, meat, hides and skin and biomaterials such as embryo, semen, blood and bone), all present threats for both short and long-distance transmission of LSD [182,189]. Vectors are an important factor to consider in the transmission of LSDV, especially over longer distances. Vector abundance in the border area is also associated with favorable.
Environmental conditions such as temperature, humidity, wind and season of the particular area [64]. Live animal movement from the infected area to the at-risk area pose a significant threat [194], as does unpasteurized milk from infected and carrier animals imported from the threatened zone to free or at-risk zones [195,196]. Trade of hides, skin, wool and other fiber, different animal byproducts, animal wastage and effluents are also important considerations in transmission of LSDV [36]. Some biomedical components from infected animals such as embryos, semen, dried blood, bone and even whole carcass may also be implicated in transmitting LSDV [64,70,189]. Equipment which are involved in the transportation of animals and their products from contaminated areas, as well as animal handlers, are also considered to be threats for the transboundary transmission [195,197]. Vaccinated animals may sometimes pose a threat if they experience activation of live attenuated virus, so the probability of infection by the transportation of vaccinated animals should not be ignored [182,189].
8. Biosecurity Policies
Robust biosecurity policies are pivotal to control and prevent LSD. Control measures in endemic areas are largely dependent upon vaccination, movement restriction of infected animals and vector control, but incursion of LSD can be avoided by strict animal movement control from the infected countries or areas and through maintenance of proper biosecurity measures at the farm level [20]. Biosecurity policies for controlling and eradicating the incursion include proper management of input products, management of production practices, movement management of humans, vehicles and other equipment and finally, through management of vectors [198]. The main input materials of a farm include the cattle, feed and water and the bedding material. Proper biosecurity measures should be maintained during entrance of such products to minimize the risk of infection. Cattle purchases should be done from preferred suppliers and quarantine should be maintained before introduction to the feedlot [135,198]. Ensuring the quality of feed materials and safe and suitable water supply is a key point in mitigating against any kind of infection and essential to improve immunity. Production practices include monitoring of the infected animals, management of manure, effluents and carcasses which could be a further source of contamination [20]. Routine monitoring of sick cattle and disposal of different materials and carcasses should be mandatory with proper hygienic measures for effective control of this disease [171]. To minimize the risk of introduction and spread of disease or contaminants, personnel including employees, family, visitors, service personnel and veterinarians should use personal protective equipment (PPE) when attending the animals or for the disposal of carcasses [145]. There should be minimal movement of necessary equipment and vehicles to prevent the introduction of LSDV into the shed. Strict and well-defined policies for proper cleaning of vehicles, shoes, farm machineries and equipment should be in place [99]. Control of insect vectors is of outmost importance to control an outbreak of LSD because insect vector is the mechanical transmitter of LSDV. Limiting vector breeding sites such as standing water, slurry, or manure by improving drainage system is important. Various insecticides can control breeding of LSDV vectors such as flies, mosquitoes, ticks and midges. Use of chemical agents and insect repellents in the infected premises and on the skin of livestock will reduce mechanical transmission of LSD [145]. Apart from biosecurity measures, proper tracing and surveillance is required to map and zone the possible risk area. Regular awareness campaigns among veterinarians, students, farmers, herdsmen, cattle traders, cattle truck drivers and artificial inseminators, and vaccination with inactivated vaccines could be adopted to minimize possible threats of an LSD outbreak [195]. In addition to farm biosecurity measures, a coordinated biosecurity and one health effort of Local Government, state and Federal Government and, most importantly, regional and global Government, non-governmental organizations (NGO) and industry efforts are warranted.
9. Incursion Threat and Global Chaos
Continuous emergence and re-emergence of LSD in different countries makes it a great livestock health concern. Since LSD is a transboundary disease, due to increased trade demand of animal and products, a possible threat of intrusion is becoming alarming for LSD-free countries. Countries with no record of LSD infection such as Australia, New Zealand, United Kingdom (UK), USA, France, Italy, Belgium, Hungary, Spain, Portugal, Slovenia, Slovakia, Croatia, Bosnia and Ukraine [199] are bearing a high risk of disease incursion associated with shared borders with countries with outbreaks. In countries where outbreaks have been managed, recurrent emergence is a risk should control and prevention methods be less stringently applied. Kenya, Romania, Russia, Moldova, Georgia, Armenia, Azerbaijan, Tajikistan, Mongolia, Turkey, Israel, Iraq, Syria, Jordan, Afghanistan, Pakistan, India, Nepal, Bangladesh, Sri Lanka, Myanmar, Thailand, Malaysia, Singapore, China and Indonesia have all recently been faced with re-emergence of LSD with severe animal health and economic effects [196].
A recent outbreak of LSD in Libya is an obvious example of incursive nature of LSDV. It is likely that the source of this outbreak was the importation of infected animals, indicating transboundary transmission [155]. Transboundary incursion may be resulted from either the mechanical vector borne transmission for short distance spread or by the movement of infected animal and infected vehicle for long distance spread [200]. Latest outbreak of LSDV is reported by the Indonesian Government in the Sumatra Island and Bali of Indonesia which increases the risk for introduction of LSD in Australia because of Australian tourists regularly visiting Bali [201]. Similarly, the outbreaks in Turkey represent a threat for the neighboring EU countries, especially Greece and Bulgaria, which can be at risk of new incursion. The cost of an incursion would produce severe economic losses due to stock losses and reduced production, including reduced milk yield, loss of animal body condition and rejection or reduced value of the hide. Hence, lack of regional and international collaboration could lead to a worldwide chaos in the livestock industry. LSD-free countries should be in high alert and take necessary coordinated actions to protect the livestock industry. For example, Australia does not import live cattle or their germplasm from LSD-infected countries. The chance of arthropod vectors entering via aircraft is low, but strong border managements are in place for in bound travelers in Australia [200]. Furthermore, a National Lumpy Skin Disease Action Plan aimed to improve Australia’s preparedness for a potential incursion of LSD is designed by the Minister for Agriculture, Fisheries and Forestry, Australia [12]. Minimizing the spread of the disease through early detection and reporting will reduce the economic and social costs of an outbreak to livestock producers, regional industries and national economy of possible threatened countries [183]. A systemic review has been recently published on LSD transmission and risk of emerging [202]. This review has extensively data mined and listed research articles published on LSD and has summarized different experimental studies. However, this review is critically discussing the historical outbreaks, genomic resources, limitations of vaccinations and diagnosis, and importance of international collaborations to mitigate the global threats of LSDV.
10. Conclusions
LSD is a global concern to the livestock industry due to its rapid spread in recent years accompanied by the reported huge economic impacts. There is a global threat of continued spread of the disease as observed in Africa, the Middle East and Asia, and it currently threatens the Western Europe and Australian livestock industries. Due to its fast-expanding nature, huge livestock losses would be inexorable. Proper vaccination, vector control, restriction on importing animals, and animal products from affected countries, quarantine measures, disease surveillance programs, quick and early diagnosis of infectious agent with typing, and stamping-out of infection would be possible measures for controlling the emergence and preventing the re-emergence and incursion of LSD. Moreover, proper disposal of carcasses and infected materials, disinfection of affected sites and strict movement control should also be followed for complete protection. There is a need for collaborative and global action towards the control and eradication of LSD in different parts of the world. As vaccination is the only way to prevent the emergence, re-emergence as well as incursion of this disease, strategies such as vaccine development are deemed important, informed by high-throughput LSD surveillance and LSDV genomic data across the incursions. LSDV genomic data will allow for accurate identification of the potential incursion pathways, which is crucial for devising effective biosecurity measures to keep LSDV at the bay. Furthermore, the genomic data will pinpoint any emerging LSDV variants, thus informing the efficacy of the existing LSDV vaccines and revealing new target sites for vaccine optimization if required.
Author Contributions
Conceptualization, M.A., J.M.U., S.A. and H.A.; methodology, M.A. and J.M.U.; data curation, M.A., S.H.A. and J.M.U.; writing—original draft preparation, M.A. and J.M.U.; writing—review and editing, M.A., J.M.U., J.W.A., S.A., S.S., H.A. and S.H.A.; visualization, M.A.; supervision, J.M.U. All authors have read and agreed to the published version of the manuscript.
Funding
Subir Sarker is a recipient of an Australian Research Council Discovery Early Career Researcher Award (grant no. DE200100367) funded by the Australian Government. The Australian Government had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clemmons, E.A.; Alfson, K.J.; Dutton, J.W. Transboundary Animal Diseases, an Overview of 17 Diseases with Potential for Global Spread and Serious Consequences. Animals 2021, 11, 2039. [Google Scholar] [CrossRef]
- Das, M.; Chowdhury, M.S.R.; Akter, S.; Mondal, A.K.; Uddin, M.J.; Rahman, M.M.; Rahman, M.M. An Updated Review on Lumpy Skin Disease: Perspective of Southeast Asian Countries. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 322–333. [Google Scholar] [CrossRef]
- Yilmaz, H. Lumpy Skin Disease: Global and Turkish Perspectives. Approaches Poult. Dairy Vet. Sci. 2017, 1, 11–15. [Google Scholar] [CrossRef]
- Organização Mundial de Saúde. World Health Statistics 2022 (Monitoring Health of the SDGs); World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240051140. [Google Scholar]
- Pandey, N.; Hopker, A.; Prajapati, G.; Rahangdale, N.; Gore, K.; Sargison, N. Observations on Presumptive Lumpy Skin Disease in Native Cattle and Asian Water Buffaloes around the Tiger Reserves of the Central Indian Highlands. New Zealand Vet. J. 2022, 70, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Amenu, A. Review on Epidemiological Aspects and Economic Impact of Lumpy Skin Disease. J. Dairy Vet. Sci. 2018, 7, 555716. [Google Scholar] [CrossRef][Green Version]
- Al-Salihi, K.A.; Hassan, I.Q. Lumpy Skin Disease in Iraq: Study of the Disease Emergence. Transbound. Emerg. Dis. 2015, 62, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.G. Special Review Series Lumpy Skin Disease, an African Capripox Virus Disease of Cattle. Br. Vet. J. 1991, 147, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Mulatu, E.; Feyisa, A. Review: Lumpy Skin Disease. J. Vet. Sci. Technol. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Khan, Y.R.; Ali, A.; Hussain, K.; Ijaz, M.; Rabbani, A.H.; Khan, R.L.; Abbas, S.N.; Aziz, M.U.; Ghaffar, A.; Sajid, H.A. A Review: Surveillance of Lumpy Skin Disease (LSD) a Growing Problem in Asia. Microb. Pathog. 2021, 158, 105050. [Google Scholar] [CrossRef]
- Saltykov, Y.V.; Kolosova, A.A.; Feodorova, V.A. Update of Lumpy Skin Disease: Emergence in Asian Part of Eurasia. Acta Vet. 2022, 72, 287–299. [Google Scholar] [CrossRef]
- National Lumpy Skin Disease Action Plan 2022. Available online: https://www.agriculture.gov.au/sites/default/files/documents/lsd-national-action-plan.pdf (accessed on 27 July 2023).
- Moudgil, G.; Chadha, J.; Khullar, L.; Chhibber, S.; Harjai, K. Lumpy Skin Disease: A Comprehensive Review on Virus Biology, Pathogenesis, and Sudden Global Emergence. Preprints 2023, 2023020074. [Google Scholar] [CrossRef]
- OIE WOAH. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Lumpy Skin Disease. 2010. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/A_summry.htm (accessed on 27 July 2023).
- Tamire, M. Current Status of Lumpy Skin Disease and Its Economic Impacts in Ethiopia. J. Vaccine Res. 2022, 1, 103. [Google Scholar]
- Degu, T. Epidemiological Status and Economic Impact of Lumpy Skin Disease-Review. Int. J. Recent Biotechnol. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Abera, Z.; Degefu, H.; Gari, G.; Ayana, Z. Review on Epidemiology and Economic Importance of Lumpy Skin Disease. Int. J. Basic Appl. Virol. 2015, 4, 8–21. [Google Scholar]
- Tuppurainen, E.; Dietze, K.; Wolff, J.; Bergmann, H.; Beltran-alcrudo, D.; Fahrion, A.; Lamien, C.E.; Busch, F.; Sauter-louis, C.; Conraths, F.J.; et al. Review: Vaccines and Vaccination against Lumpy Skin Disease. Vaccines 2021, 9, 1136. [Google Scholar] [CrossRef]
- Roth, J.A. Animal Disease Information and Prevention Materials Developed by the Center for Food Security and Public Health. Iowa State Univ. Anim. Ind. Rep. 2007, 4. Available online: https://www.iastatedigitalpress.com/air/article/id/6062/ (accessed on 27 July 2023).
- Mutua, E.N.; Bett, B.K.; Bukachi, S.A.; Estambale, B.A.; Nyamongo, I.K. From Policy to Practice: An Assessment of Biosecurity Practices in Cattle, Sheep and Goats Production, Marketing and Slaughter in Baringo County, Kenya. PLoS ONE 2022, 17, e0266449. [Google Scholar] [CrossRef]
- Charlier, J.; Barkema, H.W.; Becher, P.; De Benedictis, P.; Hansson, I.; Hennig-Pauka, I.; La Ragione, R.; Larsen, L.E.; Madoroba, E.; Maes, D.; et al. Disease Control Tools to Secure Animal and Public Health in a Densely Populated World. Lancet Planet. Health 2022, 6, e812–e824. [Google Scholar] [CrossRef]
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Kutish, G.F.; Rock, D.L. Genome of Lumpy Skin Disease Virus. J. Virol. 2001, 75, 7122–7130. [Google Scholar] [CrossRef]
- Parvin, R.; Chowdhury, E.H.; Islam, M.T.; Begum, J.A.; Nooruzzaman, M.; Globig, A.; Dietze, K.; Hoffmann, B.; Tuppurainen, E. Clinical Epidemiology, Pathology, and Molecular Investigation of Lumpy Skin Disease Outbreaks in Bangladesh during 2020–2021 Indicate the Re-Emergence of an Old African Strain. Viruses 2022, 14, 2529. [Google Scholar] [CrossRef]
- Bakar, L.; Hussein, A.; Tamam, S.; Madbouly, H. Isolation of Lumpy Skin Disease Virus Isolated from SPPV Vaccinated Cattle. J. Vet. Med. Res. 2021, 28, 38–44. [Google Scholar] [CrossRef]
- Lojkić, I.; Šimić, I.; Krešić, N.; Bedeković, T. Complete Genome Sequence of a Lumpy Skin Disease Virus Strain Isolated from the Skin of a Vaccinated Animal. Genome Announc. 2018, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, E.S.M.; Babiuk, S.; Klement, E. Lumpy Skin Disease; Springer: Cham, Switzerland, 2018; pp. 1–109. [Google Scholar] [CrossRef]
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Sur, J.-H.; Sandybaev, N.T.; Kerembekova, U.Z.; Zaitsev, V.L.; Kutish, G.F.; Rock, D.L. The Genomes of Sheeppox and Goatpox Viruses. J. Virol. 2002, 76, 6054–6061. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.A. Lumpy Skin Disease-A Review. Trop. Anim. Health Prod. 1988, 20, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, C.; Lamien, C.E.; Fakhfakh, E.; Chadeyras, A.; Aba-Adulugba, E.; Libeau, G.; Tuppurainen, E.; Wallace, D.B.; Adam, T.; Silber, R.; et al. Capripoxvirus G-Protein-Coupled Chemokine Receptor: A Host-Range Gene Suitable for Virus Animal Origin Discrimination. J. Gen. Virol. 2009, 90, 1967–1977. [Google Scholar] [CrossRef]
- Bhatt, L.; Bhoyar, R.C.; Jolly, B.; Israni, R.; Vignesh, H.; Scaria, V.; Sivasubbu, S. The Genome Sequence of Lumpy Skin Disease Virus from an Outbreak in India Suggests a Distinct Lineage of the Virus. Arch. Virol. 2023, 168, 81. [Google Scholar] [CrossRef]
- Söllner, J.H.; Mettenleiter, T.C.; Petersen, B. Genome Editing Strategies to Protect Livestock from Viral Infections. Viruses 2021, 13, 1996. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, Y.; Shao, J.; Sun, M.; He, W.; Chen, J.; Liu, Q. Genomic characterization of lumpy skin disease virus in southern China. Transbound. Emerg. Dis. 2021, 69, 2788–2799. [Google Scholar] [CrossRef]
- Grant, K.; Jenkins, C.; Arnold, C.; Green, J.; Zambon, M. Implementing Pathogen Genomics–A case study. Public Health England. 2018. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/731057/implementing_pathogen_genomics_a_case_study.pdf (accessed on 27 July 2023).
- Singh, J.; Kumar, M.; Sharma, A.; Pandey, G.; Chae, K.; Lee, S. We Are IntechOpen, the World’ s Leading Publisher of Open Access Books Built by Scientists, for Scientists TOP 1%. Intech 2016, 11, 13. [Google Scholar]
- Zewdie, G.A. Review on: Lumpy Skin Disease: Enhance Awareness on the Epidemiological Situation and Diagnosis; Prevention and Control Measures in Ethiopia. Virol. Immunol. J. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Biosecurity New Zealand. Technical Advice, Risk of Lumpy Skin Disease via Import of Cattle and Buffalo Meat and Meat Products for Human and Animal Consumption. Import Risk Analysis | NZ Government. 2022. Available online: https://www.mpi.govt.nz/dmsdocument/51352-Technical-Advice-Risk-of-lumpy-skin-disease-via-import-of-cattle-and-buffalo-meat-and-meat-products-for-human-and-animal-consumption (accessed on 27 July 2023).
- World Organisation for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2017; Available online: http://www.oie.int/en/international-standard-setting/terrestrial-manual/access-online/ (accessed on 27 July 2023).
- Azeem, S.; Sharma, B.; Shabir, S.; Akbar, H.; Venter, E. Lumpy Skin Disease Is Expanding Its Geographic Range: A Challenge for Asian Livestock Management and Food Security. Vet. J. 2022, 279, 105785. [Google Scholar] [CrossRef] [PubMed]
- Al-Salihi, K. Lumpy Skin Disease: Review of Literature. Mirror Res. Vet. Sci. Anim. 2014, 3, 6–23. [Google Scholar]
- Kumar, P.; Kumari, R.R.; Devi, S.; Tripathi, M.K.; Singh, J.; Kumar, R.; Kumar, M. Emergence and Transboundary Spread of Lumpy Skin Disease in South Asia. Indian J. Anim. Sci. 2021, 91, 507–517. [Google Scholar] [CrossRef]
- USDA. Lumpy Skin Disease Standard Operating Procedures. The Foreign Animal Disease Preparedness and Response Plan (FAD PReP). 2016; pp. 1–10. Available online: https://www.aphis.usda.gov/animal_health/emergency_management/downloads/sop/lsdv_fadprep_ee.pdf (accessed on 27 July 2023).
- Pal, M.; Paulos Gutama, K. Can Lumpy Skin Disease Be Considered a Zoonosis? Am. J. Infect. Dis. Microbiol. 2023, 11, 13–17. [Google Scholar] [CrossRef]
- Fagbo, S.; Coetzer, J.A.W.; Venter, E.H.; Venter, E. Seroprevalence of Rift Valley Fever and Lumpy Skin Disease in African Buffalo (Syncerus Caffer) in the Kruger National Park and Hluhluwe-IMfolozi Park, South Africa. J. South Afr. Vet. Assoc. 2014, 85, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, E.S.M.; Oura, C.A.L. Review: Lumpy Skin Disease: An Emerging Threat to Europe, the Middle East and Asia. Transbound. Emerg. Dis. 2012, 59, 40–48. [Google Scholar] [CrossRef]
- OIE (WOAH). Technical Meeting on Lumpy Skin Disease (LSD). LSD Situation in Viet Nam. 21 December 2020. Available online: https://rr-asia.woah.org/wp-content/uploads/2021/01/4-201221_lsd_vietnam_update_oie_meeting.pdf (accessed on 27 July 2023).
- Elhaig, M.M.; Selim, A.; Mahmoud, M. Lumpy Skin Disease in Cattle: Frequency of Occurrence in a Dairy Farm and a Preliminary Assessment of Its Possible Impact on Egyptian Buffaloes. Onderstepoort J. Vet. Res. 2017, 84, 1–6. [Google Scholar] [CrossRef]
- Sudhakar, S.B.; Mishra, N.; Kalaiyarasu, S.; Jhade, S.K.; Hemadri, D.; Sood, R.; Bal, G.C.; Nayak, M.K.; Pradhan, S.K.; Singh, V.P. Lumpy Skin Disease (LSD) Outbreaks in Cattle in Odisha State, India in August 2019: Epidemiological Features and Molecular Studies. Transbound. Emerg. Dis. 2020, 67, 2408–2422. [Google Scholar] [CrossRef]
- Babiuk, S.; Bowden, T.R.; Boyle, D.B.; Wallace, D.B.; Kitching, R.P. Capripoxviruses: An Emerging Worldwide Threat to Sheep, Goats and Cattle. Transbound. Emerg. Dis. 2008, 55, 263–272. [Google Scholar] [CrossRef]
- Farah Gumbe, A.A. Review on Lumpy Skin Disease and Its Economic Impacts in Ethiopia. J. Dairy Vet. Anim. Res. 2018, 7, 39–46. [Google Scholar] [CrossRef]
- Tageldin, M.H.; Wallace, D.B.; Gerdes, G.H.; Putterill, J.F.; Greyling, R.R.; Phosiwa, M.N.; Al Busaidy, R.M.; Al Ismaaily, S.I. Lumpy Skin Disease of Cattle: An Emerging Problem in the Sultanate of Oman. Trop. Anim. Health Prod. 2014, 46, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Gari, G.; Waret-Szkuta, A.; Grosbois, V.; Jacquiet, P.; Roger, F. Risk Factors Associated with Observed Clinical Lumpy Skin Disease in Ethiopia. Epidemiol. Infect. 2010, 138, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.E. Lumpy Skin Disease Virus; Springer: Berlin/Heidelberg, Germany, 1968; pp. 111–131. [Google Scholar] [CrossRef]
- Sudhakar, S.B.; Mishra, N.; Kalaiyarasu, S.; Ahirwar, K.; Chatterji, S.; Parihar, O.; Singh, V.P.; Sanyal, A. Lumpy Skin Disease Virus Infection in Free-Ranging Indian Gazelles (Gazella Bennettii). Emerg. Infect. Dis. 2023, 29, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Gibbs, E.; Horzinek, M.S.M. Veterinary Virology, 3rd ed.; Academic Press: San Diego, CA, USA, 1999; ISBN 9780080552033. [Google Scholar]
- Lefèvre, P.; Blancou, J.; Chermette, R.; Uilenberg, G. Infectious and Parasitic Diseases of Livestock (2 Volume Set); CABI: Wallingford, UK, 2010; ISBN 13: 9782743008727. [Google Scholar]
- Zhang, J.H.; Huang, Y.G. The Immune System: A New Look at Pain. Chin. Med. J. 2006, 119, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Boulter, E.A.; Appleyard, G. Differences between Extracellular and Intracellular Forms of Poxvirus and Their Implications. Prog. Med. Virol. Fortschritte Med. Virusforsch. Prog. Virol. Medicale 1973, 16, 86–108. [Google Scholar]
- Coetzer, J.A.W.; Tustin, R.C. Infectious Diseases of Livestock, 2nd ed.; Oxford University Press: Oxford, UK, 2004; ISBN 0195761715. [Google Scholar]
- Milovanović, M.; Dietze, K.; Milicévić, V.; Radojičić, S.; Valčić, M.; Moritz, T.; Hoffmann, B. Humoral Immune Response to Repeated Lumpy Skin Disease Virus Vaccination and Performance of Serological Tests. BMC Vet. Res. 2019, 15, 1–9. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.M.; Venter, E.H.; Coetzer, J.A.W. The Detection of Lumpy Skin Disease Virus in Samples of Experimentally Infected Cattle Using Different Diagnostic Techniques. Onderstepoort J. Vet. Res. 2005, 72, 153–164. [Google Scholar] [CrossRef]
- Ratyotha, K.; Prakobwong, S.; Piratae, S. Lumpy Skin Disease: A Newly Emerging Disease in Southeast Asia. Vet. World 2022, 15, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Sprygin, A.; Artyuchova, E.; Babin, Y.; Prutnikov, P.; Kostrova, E.; Byadovskaya, O.; Kononov, A. Epidemiological Characterization of Lumpy Skin Disease Outbreaks in Russia in 2016. Transbound. Emerg. Dis. 2018, 65, 1514–1521. [Google Scholar] [CrossRef]
- Chihota, C.M.; Rennie, L.F.; Kitching, R.P.; Mellor, P.S. Mechanical Transmission of Lumpy Skin Disease Virus by Aedes Aegypti (Diptera: Culicidae). Epidemiol. Infect. 2001, 126, 317–321. [Google Scholar] [CrossRef]
- Sprygin, A.; Pestova, Y.; Wallace, D.B.; Tuppurainen, E.; Kononov, A.V. Transmission of Lumpy Skin Disease Virus: A Short Review. Virus Res. 2019, 269, 197637. [Google Scholar] [CrossRef] [PubMed]
- Kasem, S.; Saleh, M.; Qasim, I.; Hashim, O.; Alkarar, A.; Abu-Obeida, A.; Gaafer, A.; Hussien, R.; AL-Sahaf, A.; Al-Doweriej, A.; et al. Outbreak Investigation and Molecular Diagnosis of Lumpy Skin Disease among Livestock in Saudi Arabia 2016. Transbound. Emerg. Dis. 2018, 65, e494–e500. [Google Scholar] [CrossRef] [PubMed]
- Carn, V.M.; Kitching, R.P. An Investigation of Possible Routes of Transmission of Lumpy Skin Disease Virus (Neethling). Epidemiol. Infect. 1995, 114, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Magori-Cohen, R.; Louzoun, Y.; Herziger, Y.; Oron, E.; Arazi, A.; Tuppurainen, E.; Shpigel, N.Y.; Klement, E. Mathematical Modelling and Evaluation of the Different Routes of Transmission of Lumpy Skin Disease Virus. Vet. Res. 2012, 43, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A. A Review on Current Epidemiology and Molecular Studies of Lumpy Skin Disease Virus-an Emerging Worldwide Threat to Domestic Animals. J. Med. Pharm. Allied Sci. 2023, 12, 5635–5643. [Google Scholar] [CrossRef]
- Ali, H.; Ali, A.A.; Atta, M.S.; Cepica, A. Common, Emerging, Vector-Borne and Infrequent Abortogenic Virus Infections of Cattle. Transbound. Emerg. Dis. 2012, 59, 11–25. [Google Scholar] [CrossRef]
- Annandale, C.H.; Holm, D.E.; Ebersohn, K.; Venter, E.H. Seminal Transmission of Lumpy Skin Disease Virus in Heifers. Transbound. Emerg. Dis. 2014, 61, 443–448. [Google Scholar] [CrossRef]
- Rouby, S.; Aboulsoud, E. Evidence of Intrauterine Transmission of Lumpy Skin Disease Virus. Vet. J. 2016, 209, 193–195. [Google Scholar] [CrossRef]
- Salib, A.; Osman, F.H. Incidence of Lumpy Skin Disease among Egyptian Cattle in Giza Governorate, Egypt. Vet. World 2011, 4, 162–167. [Google Scholar]
- Lu, G.; Xie, J.; Luo, J.; Shao, R.; Jia, K.; Li, S. Lumpy Skin Disease Outbreaks in China, since 3 August 2019. Transbound. Emerg. Dis. 2021, 68, 216–219. [Google Scholar] [CrossRef]
- Lubinga, J.C.; Tuppurainen, E.S.M.; Mahlare, R.; Coetzer, J.A.W.; Stoltsz, W.H.; Venter, E.H. Evidence of Transstadial and Mechanical Transmission of Lumpy Skin Disease Virus by Amblyomma Hebraeum Ticks. Transbound. Emerg. Dis. 2015, 62, 174–182. [Google Scholar] [CrossRef] [PubMed]
- El-Kenawy, A.A.; El-Tholoth, M.S. Lumpy Skin Disease Virus Identification in Different Tissues of Naturally Infected Cattle and Chorioallantoic Membrane of Emberyonated Chicken Eggs Using Immunofluorescence, Immunoperoxidase Techniques and Polymerase Chain Reaction. Int. J. Virol. 2011, 7, 158–166. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Hussan, M.T.; Hashem, M.A.; Eliyas, M.; Anower, A.K.M.M. Lumpy Skin Disease Virus Infection: An Emerging Threat to Cattle Health in Bangladesh. Hosts Viruses 2020, 7, 97. [Google Scholar] [CrossRef]
- Fenner, F.; Bachmann, P.A.; Gibbs, E.P.J.; Murphy, F.A.; Studdert, M.J.; White, D.O. Veterinary Virology, 1st ed.; Academic Press: Cambridge, MA, USA, 1987; ISBN 9781483257815. [Google Scholar]
- Coetzer, J.A.W.; Tustin, R.C. Infectious Diseases of Livestock, 1st ed.; Oxford University Press: New York, NY, USA, 2004; Volume 2, ISBN 019578202X. [Google Scholar]
- Lumpy Skin Disease–CFSPH. Available online: https://www.cfsph.iastate.edu/diseaseinfo/disease/?disease=lumpy-skin-disease&lang=en (accessed on 25 May 2023).
- Lumpy Skin Disease: Pathogenesis of an African Capripox Virus Disease | Pashudhan Praharee. Available online: https://www.pashudhanpraharee.com/lumpy-skin-disease-pathogenesis-of-an-african-capripox-virus-disease/ (accessed on 25 May 2023).
- Gupta, T.; Patial, V.; Bali, D.; Angaria, S.; Sharma, M.; Chahota, R. A Review: Lumpy Skin Disease and Its Emergence in India. Vet. Res. Commun. 2020, 44, 111–118. [Google Scholar] [CrossRef]
- Abutarbush, S.M. Lumpy Skin Disease (Knopvelsiekte, Pseudo-Urticaria, Neethling Virus Disease, Exanthema Nodularis Bovis). In Emerging and Re-Emerging Infectious Diseases of Livestock; Bayry, J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 309–326. [Google Scholar] [CrossRef]
- Jameel, G.H. Determination of Complications Decrease the Risk Factor in Cattle infected by lumpy skin disease virus in Diyala province, Iraq. Int. J. Micro Biol. Genet. Monocular Biol. Res. 2016, 2, 1–9. [Google Scholar]
- Abutarbush, S.M. Hematological and Serum Biochemical Findings in Clinical Cases of Cattle Naturally Infected with Lumpy Skin Disease. J. Infect. Dev. Ctries. 2015, 9, 283–288. [Google Scholar] [CrossRef]
- Neamat-allah, A.N.F. Studies on Cows Naturally Infected with Lumpy Skin Disease. Vet. World 2015, 8, 8–13. [Google Scholar] [CrossRef]
- Brooks, M.B.; Harr, K.E.; Seelig, D.M.; Wardrop, K.J.; Weiss, D.J. Schalm’s Veterinary Hematology, 6th ed.; Wiley-Blackwell: Ames, IA, USA, 2020; ISBN 9781119500537. [Google Scholar]
- Latimer, K.S. (Ed.) Duncan and Prasse’s Veterinary Laboratory Medicine: Clinical Pathology, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-119-94616-8. [Google Scholar]
- Kumar, V.; Abbas, A.; Aster, J. Robbins Basic Pathology, 10th ed.; Kumar, V., Abbas, A., Aster, J., Eds.; Elsevier Health Sciences: Amsterdam, The Netherland, 2017; ISBN 9780323394130. [Google Scholar]
- Coles, E.H. Veterinary Clinical Pathology, 4th ed.; Coles, E.H., Saunders, W.B., Eds.; Saunders Company: Philadelphia, PA, USA, 1986; ISBN 0721618286. [Google Scholar]
- Smith, B.P. Large Animal Internal Medicine; Smith, B.P., Ed.; Elsevier Mosby: St. Louis, MO, USA, 2015; ISBN 9780323088398. [Google Scholar]
- El-mandrawy, S.A.M.; Alam, R.T.M. Hematological, Biochemical and Oxidative Stress Studies of Lumpy Skin Disease Virus Infection in Cattle. J. Appl. Anim. Res. 2018, 46, 1073–1077. [Google Scholar] [CrossRef]
- Hassan, H. Immunobiochemical Profile in Cattle Infected with Lumpy Skin Disease. J. Basic Appl. Chem. 2011, 1, 21–25. [Google Scholar]
- Şevik, M.; Avci, O.; Doǧan, M.; Ince, Ö.B. Serum Biochemistry of Lumpy Skin Disease Virus-Infected Cattle. BioMed. Res. Int. 2016, 2016, 6257984. [Google Scholar] [CrossRef]
- Ahmed, W.M.; Zaher, K.S. Observations on Lumpy Skin Disease in Local Egyptian Cows with Emphasis on Its Impact on Ovarian Function. Afr. J. Microbiol. Res. 2008, 2, 252–257. [Google Scholar]
- Jaffersab, A.; Ravindra, B.G.; Halmandge, S.; Mallinath, K.C.; Doddagoudar, V.; Kasaralikar, V.R. A Study on Hematobiochemical Alterations in Cattle Affected with Lumpy Skin Disease in and around Bidar. Pharma Innov. J. 2022, SP-11, 958–960. [Google Scholar]
- Helal, M.; Marawan, M.; Bahgy, H. Clinico-Biochemical and Electrocardiographic Changes in Cattle Naturally Infected with Lumpy Skin Disease. Alex. J. Vet. Sci. 2019, 60, 41. [Google Scholar] [CrossRef]
- Stockham, S.L.; Scott, M.A.; Michael, A. Fundamentals of Veterinary Clinical Pathology; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 908. [Google Scholar]
- Marmor, A.T.; Klein, R.; Plich, M.; Groshar, D.; Schneeweiss, A. Elevated CK-MB Isoenzyme after Exercise Stress Test and Atrial Pacing in Patients with Ischemic Heart Disease. Chest 1988, 94, 1216–1220. [Google Scholar] [CrossRef]
- Tuppurainen, E.; Alexandrov, T.; Beltrán-Alcrudo, D. Lumpy Skin Disease Field Manual—A Manual for Veterinarians; FAO Animal Production and Health Manual No. 20; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2017; ISBN 9789251097762. [Google Scholar]
- Awad, W.S.; Ibrahim, A.K.; Salib, F.A. Using indirect ELISA to assess different antigens for the serodiagnosis of Fasciola gigantica infection in cattle, sheep and donkeys. Res. Vet. Sci. 2009, 86, 466–471. [Google Scholar] [CrossRef] [PubMed]
- El-Neweshy, M.S.; El-Shemey, T.M.; Youssef, S.A. Pathologic and Immunohistochemical Findings of Natural Lumpy Skin Disease in Egyptian Cattle. Pak. Vet. J. 2013, 33, 60–64. [Google Scholar]
- Body, M.; Singh, P.K.; Hussain, H.M.; Al-rawahi, A.; Al-maawali, M.; Al-lamki, K.; Al-habsy, S. Clinico-Histopathological Findings and PCR Based Diagnosis of Lumpy Skin Disease in the Sultanate of Oman. Pak. Vet. J. 2012, 32, 206–210. [Google Scholar]
- Sanz-Bernardo, B.; Haga, I.R.; Wijesiriwardana, N.; Basu, S.; Larner, W.; Diaz, A.V.; Langlands, Z.; Denison, E.; Stoner, J.; White, M.; et al. Quantifying and Modeling the Acquisition and Retention of Lumpy Skin Disease Virus by Hematophagus Insects Reveals Clinically but Not Subclinically Affected Cattle Are Promoters of Viral Transmission and Key Targets for Control of Disease Outbreaks. J. Virol. 2021, 95, 10–1128. [Google Scholar] [CrossRef]
- Şevik, M.; Doğan, M. Epidemiological and Molecular Studies on Lumpy Skin Disease Outbreaks in Turkey during 2014–2015. Transbound. Emerg. Dis. 2017, 64, 1268–1279. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Amina, A.D. Abattoir-Based Survey and Histopathological Findings of Lumpy Skin Disease in Cattle at Ismailia Abattoir. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 372–375. [Google Scholar]
- Prozesky, L.; Barnard, B.J. A Study of the Pathology of Lumpy Skin Disease in Cattle. Onderstepoort J. Vet. Res. 1982, 49, 167–175. [Google Scholar] [PubMed]
- Gammada, I.; Morshed, M.; Rabby, T.R.; Hossain, I. The Prevalence of Lumpy Skin Disease in the Cattle Population: A Brief Study. Int. J. Agric. Vet. Sci. 2022, 4, 55–67. [Google Scholar] [CrossRef]
- Davies, F.G. Observations on the Epidemiology of Lumpy Skin Disease in Kenya. J. Hyg. 1982, 88, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Modise, B.M.; Settypalli, T.B.K.; Kgotlele, T.; Xue, D.; Ntesang, K.; Kumile, K.; Naletoski, I.; Nyange, J.F.; Thanda, C.; Macheng, K.N.; et al. First Molecular Characterization of Poxviruses in Cattle, Sheep, and Goats in Botswana. Virol. J. 2021, 18, 167. [Google Scholar] [CrossRef] [PubMed]
- Capstick, P.B.; Coackley, W. Protection of Cattle Against Lumpy Skin Disease. Res. Vet. Sci. 1961, 2, 362–368. [Google Scholar] [CrossRef]
- Khalafalla, A.I.; Gaffar Elamin, M.A.; Abbas, Z. Lumpy Skin Disease: Observations on the Recent Outbreaks of the Disease in the Sudan. Rev. D’Elevage Médecine Vétérinaire Pays Trop. 1993, 46, 548–550. [Google Scholar]
- Ali, B.H.; Obeid, H.M. Investigation of the First Outbreaks of Lumpy Skin Disease in the Sudan. Br. Vet. J. 1977, 133, 184–189. [Google Scholar] [CrossRef]
- Wolff, J.; Tuppurainen, E.; Adedeji, A.; Meseko, C.; Asala, O.; Adole, J.; Atai, R.; Dogonyaro, B.; Globig, A.; Hoffmann, D.; et al. Characterization of a Nigerian Lumpy Skin Disease Virus Isolate after Experimental Infection of Cattle. Pathogens 2022, 11, 16. [Google Scholar] [CrossRef]
- Nawathe, D.R.; Asagba, M.O.; Abegunde, A.; Ajayi, S.A.; Durkwa, L. Some Observations on the Occurrence of Lumpy Skin Disease in Nigeria. Zentralblatt Für Veterinärmedizin Reihe B 1982, 29, 31–36. [Google Scholar] [CrossRef]
- Mebratu, G.Y.; Kassa, B.; Fikre, Y.; Berhanu, B. Observation on the Outbreak of Lumpy Skin Disease in Ethiopia. Rev. D’Elevage Médecine Vétérinaire Pays Trop. 1984, 37, 395–399. [Google Scholar]
- Rozstalnyy, A.; Tago, D.; Kamata, A.; Claudia, P. Introduction and Spread of Lumpy Skin Disease in South, East and Southeast Asia; Paper 183; Fao Animal Production and Health: Rome, Italy, 2020; ISBN 9789251335635. [Google Scholar]
- Rouby, S.R.; Safwat, N.M.; Hussein, K.H.; Abdel-Ra’ouf, A.M.; Madkour, B.S.; Abdel-Moneim, A.S.; Hosein, H.I. Lumpy Skin Disease Outbreaks in Egypt during 2017-2018 among Sheeppox Vaccinated Cattle: Epidemiological, Pathological, and Molecular Findings. PLoS ONE 2021, 16, e0258755. [Google Scholar] [CrossRef] [PubMed]
- Shimshony, A.; Economides, P. Disease Prevention and Preparedness for Animal Health Emergencies in the Middle East. OIE Rev. Sci. Tech. 2006, 25, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Calistri, P.; De Clercq, K.; Gubbins, S.; Klement, E.; Stegeman, A.; Cortiñas Abrahantes, J.; Marojevic, D.; Antoniou, S.E.; Broglia, A. Lumpy Skin Disease Epidemiological Report IV: Data Collection and Analysis. EFSA J. 2020, 18, e06010. [Google Scholar] [CrossRef] [PubMed]
- Mercier, A.; Arsevska, E.; Bournez, L.; Bronner, A.; Calavas, D.; Cauchard, J.; Falala, S.; Caufour, P.; Tisseuil, C.; Lefrançois, T.; et al. Spread Rate of Lumpy Skin Disease in the Balkans, 2015–2016. Transbound. Emerg. Dis. 2018, 65, 240–243. [Google Scholar] [CrossRef]
- Hasib, F.M.Y.; Islam, M.S.; Das, T.; Rana, E.A.; Uddin, M.H.; Bayzid, M.; Nath, C.; Hossain, M.A.; Masuduzzaman, M.; Das, S.; et al. Lumpy Skin Disease Outbreak in Cattle Population of Chattogram, Bangladesh. Vet. Med. Sci. 2021, 7, 1616–1624. [Google Scholar] [CrossRef]
- Mathivanan, D.E.; Raju, D.; Kavitha, M.D.R. Watching Brief. Learn. Disabil. Today 2022, 11, 11. [Google Scholar]
- Sai, C.H.; Goud, K.; Maheshwari, K.; Vigneshwari, K. Overview of Lumpy Skin Disease. Int. J. Pharm. Sci. Rev. Res. 2023, 79, 15–22. [Google Scholar] [CrossRef]
- Kumar, N.; Tripathi, B.N. A Serious Skin Virus Epidemic Sweeping through the Indian Subcontinent Is a Threat to the Livelihood of Farmers. Virulence 2022, 13, 1943–1944. [Google Scholar] [CrossRef]
- FAO. Emergence of Lumpy Skin Disease in the Eastern Mediterranean Basin Countries. Empres Watch. 2013, Volume 29. Available online: https://www.fao.org/documents/card/en/c/2a27bb10-8de5-510c-8303-c4c9d6414b3c (accessed on 27 July 2023).
- Mweene, A.S.; Pandey, G.S.; Sinyangwe, P.; Nambota, A.; Samui, K.; Kida, H. Viral diseases of livestock in Zambia. Jpn. J. Vet. Res. 1996, 44, 89–105. [Google Scholar] [PubMed]
- Mafirakureva, P.; Saidi, B.; Mbanga, J. Incidence and Molecular Characterisation of Lumpy Skin Disease Virus in Zimbabwe Using the P32 Gene. Trop. Anim. Health Prod. 2017, 49, 47–54. [Google Scholar] [CrossRef]
- Swiswa, S.; Masocha, M.; Pfukenyi, D.M.; Dhliwayo, S.; Chikerema, S.M. Long-Term Changes in the Spatial Distribution of Lumpy Skin Disease Hotspots in Zimbabwe. Trop. Anim. Health Prod. 2017, 49, 195–199. [Google Scholar] [CrossRef] [PubMed]
- HANDISTATUS II Multiannual Animal Disease Status. Available online: https://web.oie.int/hs2/sit_pays_mald_pl.asp?c_pays=30&c_mald=8 (accessed on 1 June 2023).
- Hunter, P.; Wallace, D. Lumpy Skin Disease in Southern Africa: A Review of the Disease and Aspects of Control. J. S. Afr. Vet. Assoc. 2001, 72, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Elsaim, N.A.G.E. Prevalence of Lumpy Skin Disease in Cattle, in Red Sea State, Sudan. Master’s Thesis, University of Khartoum, Khartoum, Sudan, 2007. [Google Scholar]
- El-Ansary, R.E.; El-Dabae, W.H.; Bream, A.S.; El Wakil, A. Isolation and Molecular Characterization of Lumpy Skin Disease Virus from Hard Ticks, Rhipicephalus (Boophilus) Annulatus in Egypt. BMC Vet. Res. 2022, 18, 302. [Google Scholar] [CrossRef]
- Yeruham, O.N.; Braveman, M.D.H. Spread of Lumpy Skinn Disease in Israeli. Vet. Rec. 1995, 137, 91–93. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; Hananeh, W.M.; Ramadan, W.; Al Sheyab, O.M.; Alnajjar, A.R.; Al Zoubi, I.G.; Knowles, N.J.; Bachanek-Bankowska, K.; Tuppurainen, E.S.M. Adverse Reactions to Field Vaccination Against Lumpy Skin Disease in Jordan. Transbound. Emerg. Dis. 2016, 63, e213–e219. [Google Scholar] [CrossRef]
- Panel, E.; Ahaw, W. Scientific Opinion on Lumpy Skin Disease. EFSA J. 2015, 13, 3986. [Google Scholar] [CrossRef]
- Calistri, P.; DeClercq, K.; Gubbins, S.; Klement, E.; Stegeman, A.; Cortiñas Abrahantes, J.; Antoniou, S.E.; Broglia, A.; Gogin, A. Lumpy Skin Disease: III. Data Collection and Analysis. EFSA J. 2019, 17, e05638. [Google Scholar] [CrossRef] [PubMed]
- Bouchemla, F.; Agoltsov, V.A.; Larionov, S.V.; Popova, O.M.; Shvenk, E.V. Epizootiological Study on Spatiotemporal Clusters of Schmallenberg Virus and Lumpy Skin Diseases: The Case of Russia. Vet. World 2018, 11, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- EFSA, E.F.S.A. Lumpy Skin Disease: I. Data Collection and Analysis. EFSA J. 2017, 15, 1–54. [Google Scholar] [CrossRef]
- Ranjan Jena, B.; Kantale, R.A.; Dash, A.; Singh, P.; Basak, G.; Singh, S.; Jadhao, A. Emergence of Lumpy Skin Disease Virus (LSDV) Infection in Cattle and Buffaloes in India. Epidemiology 2022, 11, 1857–1861. [Google Scholar]
- Sudhakar, S.B.; Mishra, N.; Kalaiyarasu, S.; Jhade, S.K.; Singh, V.P. Genetic and Phylogenetic Analysis of Lumpy Skin Disease Viruses (LSDV) Isolated from the First and Subsequent Field Outbreaks in India during 2019 Reveals Close Proximity with Unique Signatures of Historical Kenyan NI-2490/Kenya/KSGP-like Field Strains. Transbound. Emerg. Dis. 2022, 69, e451–e462. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Doley, S.; Barlaskar, S.A.; Nath, A.J.; Yadav, S.N. Lumpy Skin Disease: An Emerging Bovine Viral Infection in India. Indian J. Anim. Health 2020, 59, 137–142. [Google Scholar] [CrossRef]
- Wei, Y.R.; Ma, W.G.; Wang, P.; Wang, W.; Su, X.H.; Yang, X.Y.; Mi, X.Y.; Wu, J.Y.; Huang, J. Retrospective Genomic Analysis of the First Lumpy Skin Disease Virus Outbreak in China (2019). Front. Vet. Sci. 2023, 9, 1073648. [Google Scholar] [CrossRef]
- Zan, X.; Huang, H.; Guo, Y.; Di, D.; Fu, C.; Wang, S.; Wu, Y.; Wang, J.; Wang, Y.; Ma, Y.; et al. Molecular Characterization of a Novel Subgenotype of Lumpy Skin Disease Virus Strain Isolated in Inner Mongolia of China. BMC Vet. Res. 2022, 18, 295. [Google Scholar] [CrossRef]
- An, Q.; Li, Y.; Sun, Z.; Gao, X.; Wang, H. Global Risk Assessment of the Occurrence of Bovine Lumpy Skin Disease: Based on an Ecological Niche Model. Transbound. Emerg. Dis. 2023, 2023, 2349173. [Google Scholar] [CrossRef]
- Koirala, P.; Meki, I.K.; Maharjan, M.; Settypalli, B.K.; Manandhar, S.; Yadav, S.K.; Cattoli, G.; Lamien, C.E. Molecular Characterization of the 2020 Outbreak of Lumpy Skin Disease in Nepal. Microorganisms 2022, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Kattel, P.; Kaphle, K. Review on Lumpy Skin Disease and Its Emerging Threat to Livestock in Nepal. Vet. Sci. Res. Rev. 2022, 8, 43–51. [Google Scholar] [CrossRef]
- Acharya, K.P.; Subedi, D. First Outbreak of Lumpy Skin Disease in Nepal. Transbound. Emerg. Dis. 2020, 67, 2280–2281. [Google Scholar] [CrossRef]
- Regmi, S. Lumpy Skin Disease (LSD) Outbreak in Nepal. Authorea Prepr. 2020, 1–4. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Truong, A.D.; Dang, A.K.; Ly, D.V.; Nguyen, C.T.; Chu, N.T.; Hoang, T.V.; Nguyen, H.T.; Nguyen, V.T.; Dang, H.V. Lumpy Skin Disease Outbreaks in Vietnam, 2020. Transbound. Emerg. Dis. 2021, 68, 977–980. [Google Scholar] [CrossRef]
- Mathijs, E.; Vandenbussche, F.; Nguyen, L.; Aerts, L.; Nguyen, T.; De Leeuw, I.; Quang, M.; Nguyen, H.D.; Philips, W.; Dam, T.V.; et al. Coding-Complete Sequences of Recombinant Lumpy Skin Disease Viruses Collected in 2020 from Four Outbreaks in Northern Vietnam. Microbiol. Resour. Announc. 2021, 10, 20–21. [Google Scholar] [CrossRef]
- Mehtani, Y. Study of Human Psychology for Introduction of Trult Intelligent. 2014, Volume 2021, pp. 1–2. Available online: http://data.conferenceworld.in/ICSTM-2015/101.pdf (accessed on 1 June 2023).
- Vinitchaikul, P.; Punyapornwithaya, V.; Seesupa, S.; Phuykhamsingha, S.; Arjkumpa, O.; Sansamur, C.; Jarassaeng, C. The First Study on the Impact of Lumpy Skin Disease Outbreaks on Monthly Milk Production on Dairy Farms in Khon Kaen, Thailand. Vet. World 2023, 16, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Arjkumpa, O.; Suwannaboon, M.; Boonrod, M.; Punyawan, I.; Liangchaisiri, S.; Laobannue, P.; Lapchareonwong, C.; Sansri, C.; Kuatako, N.; Panyasomboonying, P.; et al. The First Lumpy Skin Disease Outbreak in Thailand (2021): Epidemiological Features and Spatio-Temporal Analysis. Front. Vet. Sci. 2022, 8, 799065. [Google Scholar] [CrossRef] [PubMed]
- Sariya, L.; Paungpin, W.; Chaiwattanarungruengpaisan, S.; Thongdee, M.; Nakthong, C.; Jitwongwai, A.; Taksinoros, S.; Sutummaporn, K.; Boonmasawai, S.; Kornmatitsuk, B. Molecular Detection and Characterization of Lumpy Skin Disease Viruses from Outbreaks in Thailand in 2021. Transbound. Emerg. Dis. 2022, 69, e2145–e2152. [Google Scholar] [CrossRef]
- Manzoor, S.; Abubakar, M.; Rahman, A.U.; Syed, Z.; Ahmad, K.; Afzal, M. Molecular Characterization of Lumpy Skin Disease Virus from Recent Outbreaks in Pakistan. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Khatri, G.; Rai, A.; Aashish; Shahzaib; Hyder, S.; Priya; Hasan, M.M. Epidemic of Lumpy Skin Disease in Pakistan. Vet. Med. Sci. 2023, 9, 982–984. [Google Scholar] [CrossRef]
- Odonchimeg, M.; Erdenechimeg, D.; Tuvshinbayar, A.; Tsogtgerel, M.; Bazarragchaa, E.; Ulaankhuu, A.; Selenge, T.; Munkhgerel, D.; Munkhtsetseg, A.; Altanchimeg, A.; et al. Molecular Identification and Risk Factor Analysis of the First Lumpy Skin Disease Outbreak in Cattle in Mongolia. J. Vet. Med. Sci. 2022, 84, 1244–1252. [Google Scholar] [CrossRef]
- Lumpy Skin Disease (LSD) | Open Development Cambodia (ODC). Available online: https://opendevelopmentcambodia.net/tag/lumpy-skin-disease-lsd/#!/story=post-154955 (accessed on 17 July 2023).
- Lumpy Skin Disease Outbreak in Eastern Afghanistan Kills Hundreds of Livestock–Khaama Press. Available online: https://www.khaama.com/lumpy-skin-disease-outbreak-in-eastern-afghanistan-kills-hundreds-of-livestock-474833/ (accessed on 16 July 2023).
- Gongal, G.; Rahman, H.; Thakuri, K.C.; Vijayalakshmy, K. An Overview of Transboundary Animal Diseases of Viral Origin in South Asia: What Needs to Be Done? Vet. Sci. 2022, 9, 586. [Google Scholar] [CrossRef]
- Ko, Y.-S.; Oh, Y.; Lee, T.G.; Bae, D.-Y.; Tark, D.; Cho, H.-S. Serological and Molecular Prevalence of Lumpy Skin Disease Virus in Korean Water Deer, Native and Dairy Cattle in Korea. Korean J. Vet. Serv. 2022, 45, 133–137. [Google Scholar] [CrossRef]
- FAO Regional Office for Asia and the Pacific 2023. Indonesia Moves Another Step Closer to Ending Costly Livestock Diseases with Support from FAO and Australia. Available online: https://www.fao.org/asiapacific/news/detail-events/en/c/1638419/ (accessed on 27 July 2023).
- Home–ProMED–ProMED-Mail. Available online: https://promedmail.org/ (accessed on 28 June 2023).
- Selim, A.; Manaa, E.; Khater, H. Seroprevalence and Risk Factors for Lumpy Skin Disease in Cattle in Northern Egypt. Trop. Anim. Health Prod. 2021, 53, 350. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Bernardo, B.; Haga, I.R.; Wijesiriwardana, N.; Hawes, P.C.; Simpson, J.; Morrison, L.R.; MacIntyre, N.; Brocchi, E.; Atkinson, J.; Haegeman, A.; et al. Lumpy Skin Disease Is Characterized by Severe Multifocal Dermatitis With Necrotizing Fibrinoid Vasculitis Following Experimental Infection. Vet. Pathol. 2020, 57, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Gruenberg, W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 11th ed.; Saunders Company: Philadelphia, PA, USA, 2016. [Google Scholar]
- Balinsky, C.A.; Delhon, G.; Smoliga, G.; Prarat, M.; French, R.A.; Geary, S.J.; Rock, D.L.; Rodriguez, L.L. Rapid Preclinical Detection of Sheeppox Virus by a Real-Time PCR Assay. J. Clin. Microbiol. 2008, 46, 438–442. [Google Scholar] [CrossRef]
- Lamien, C.E.; Lelenta, M.; Goger, W.; Silber, R.; Tuppurainen, E.; Matijevic, M.; Luckins, A.G.; Diallo, A. Real Time PCR Method for Simultaneous Detection, Quantitation and Differentiation of Capripoxviruses. J. Virol. Methods 2011, 171, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Leliso, S.A. Overview on Diagnostic Techniques and Economic Importance of Lumpy Skin Disease. J. Vet. Med. Res. 2021, 8, 1218. [Google Scholar]
- Amin, D.M.; Shehab, G.; Emran, R.; Hassanien, R.T.; Alagmy, G.N.; Hagag, N.M.; Abd-El-Moniem, M.I.I.; Habashi, A.R.; Ibraheem, E.M.; Shahein, M.A. Diagnosis of Naturally Occurring Lumpy Skin Disease Virus Infection in Cattle Using Virological, Molecular, and Immunohistopathological Assays. Vet. World 2021, 14, 2230–2237. [Google Scholar] [CrossRef]
- Choudhari, A.N.; Moregaonkar, S.D.; Gangane, G.R.; Markandeya, N.M.; Narladkar, B.W. Lumpy Skin Disease (LSD), an Emerging Disease in India: A Review. Agric. Rev. 2020, 41, 398–402. [Google Scholar] [CrossRef]
- Wallace, D.B.; Viljoen, G.J. Immune Responses to Recombinants of the South African Vaccine Strain of Lumpy Skin Disease Virus Generated by Using Thymidine Kinase Gene Insertion. Vaccine 2005, 23, 3061–3067. [Google Scholar] [CrossRef]
- Kitching, R.P. Vaccines for Lumpy Skin Disease, Sheep Pox and Goat Pox. Dev. Biol. 2003, 114, 161–167. [Google Scholar]
- Hamdi, J.; Munyanduki, H.; Tadlaoui, K.O.; Harrak, M.E.; Fihri, O.F. Capripoxvirus Infections in Ruminants: A Review. Microorganisms 2021, 9, 902. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.M.; Pearson, C.R.; Bachanek-Bankowska, K.; Knowles, N.J.; Amareen, S.; Frost, L.; Henstock, M.R.; Lamien, C.E.; Diallo, A.; Mertens, P.P.C. Characterization of Sheep Pox Virus Vaccine for Cattle against Lumpy Skin Disease Virus. Antivir. Res. 2014, 109, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ayelet, G.; Haftu, R.; Jemberie, S.; Belay, A.; Gelaye, E.; Sibhat, B.; Skjerve, E.; Asmare, K. Lumpy Skin Disease in Cattle in Central Ethiopia: Outbreak Investigation and Isolation and Molecular Detection of the Virus. OIE Rev. Sci. Tech. 2014, 33, 877–887. [Google Scholar] [CrossRef] [PubMed]
- OIE. Frequently Asked Questions (FAQ) on Lumpy Skin Disease (LSD) Vaccination. 2022. Available online: https://www.woah.org/app/uploads/2022/06/faq-lsd-faired-v2-4forpublication.pdf (accessed on 27 July 2023).
- Varshovi, H. Immune Response Characteristics of Capri Pox Virus Vaccines Following Emergency Vaccination of Cattle against Lumpy Skin Disease Virus. Iran. J. Vet. Sci. Technol. 2018, 9, 33–40. [Google Scholar] [CrossRef]
- Brenner, J.; Bellaiche, M.; Gross, E.; Elad, D.; Oved, Z.; Haimovitz, M.; Wasserman, A.; Friedgut, O.; Stram, Y.; Bumbarov, V.; et al. Appearance of Skin Lesions in Cattle Populations Vaccinated against Lumpy Skin Disease: Statutory Challenge. Vaccine 2009, 27, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Haegeman, A.; De Leeuw, I.; Saduakassova, M.; Campe, W.V.; Aerts, L.; Philips, W.; Sultanov, A.; Mostin, L.; De Clercq, K. The Importance of Quality Control of LSDV Live Attenuated Vaccines for Its Safe Application in the Field. Vaccines 2021, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Boumart, Z.; Daouam, S.; Belkourati, I.; Rafi, L.; Tuppurainen, E.; Tadlaoui, K.O.; El Harrak, M. Comparative Innocuity and Efficacy of Live and Inactivated Sheeppox Vaccines. BMC Vet. Res. 2016, 12, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Sprygin, A.; Pestova, Y.; Bjadovskaya, O.; Prutnikov, P.; Zinyakov, N.; Kononova, S.; Ruchnova, O.; Lozovoy, D.; Chvala, I.; Kononov, A. Evidence of Recombination of Vaccine Strains of Lumpy Skin Disease Virus with Field Strains, Causing Disease. PLoS ONE 2020, 15, e0232584. [Google Scholar] [CrossRef]
- Gershon, P.D.; Black, D.N. A capripoxvirus pseudogene whose only intact homologs are in other poxvirus genomes. Virology 1989, 172, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Favis, N.; Famulski, J.; Evans, D.H. Evolution of and Evolutionary Relationships between Extant Vaccinia Virus Strains. J. Virol. 2015, 89, 1809–1824. [Google Scholar] [CrossRef]
- Bedson, H.S.; Dumbell, K.R. Hybrids Derived from the Viruses of Variola Major and Cowpox. J. Hyg. 1964, 62, 147–158. [Google Scholar] [CrossRef]
- Saltykov, Y.V.; Kolosova, A.A.; Filonova, N.N.; Chichkin, A.N.; Feodorova, V.A. Genetic Evidence of Multiple Introductions of Lumpy Skin Disease Virus into Saratov Region, Russia. Pathogens 2021, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Mathijs, E.; Philips, W.; Saduakassova, M.; De Leeuw, I.; Sultanov, A.; Haegeman, A.; De Clercq, K. Recombinant LSDV Strains in Asia: Vaccine Spillover or Natural Emergence? Viruses 2022, 14, 1429. [Google Scholar] [CrossRef] [PubMed]
- Haegeman, A.; De Leeuw, I.; Philips, W.; De Regge, N. Development and Validation of a New DIVA Real-Time PCR Allowing to Differentiate Wild-Type Lumpy Skin Disease Virus Strains, Including the Asian Recombinant Strains, from Neethling-Based Vaccine Strains. Viruses 2023, 15, 870. [Google Scholar] [CrossRef] [PubMed]
- Farra, D.; De Nardi, M.; Lets, V.; Holopura, S.; Klymenok, O.; Stephan, R.; Boreiko, O. Qualitative Assessment of the Probability of Introduction and Onward Transmission of Lumpy Skin Disease in Ukraine. Microb. Risk Anal. 2022, 20, 100200. [Google Scholar] [CrossRef]
- Hailu, B. Economic Importance and Control Techniques of Lumpy Skin Diseases. Anim. Vet. Sci. 2015, 3, 58. [Google Scholar] [CrossRef]
- Chouhan, C.S.; Parvin, M.S.; Ali, M.Y.; Sadekuzzaman, M.; Chowdhury, M.G.A.; Ehsan, M.A.; Islam, M.T. Epidemiology and Economic Impact of Lumpy Skin Disease of Cattle in Mymensingh and Gaibandha Districts of Bangladesh. Transbound. Emerg. Dis. 2022, 69, 3405–3418. [Google Scholar] [CrossRef]
- Foot and Mouth Disease: Virus Now in Bali a “$100b” Timebomb for Aussie Farmers. Available online: https://www.9news.com.au/national/foot-and-mouth-disease-in-bali-risks-100-billion-time-bomb-for-australia-cattle-livestock-exports-indsutry/353895ba-ede5-4924-a1c3-710ea4e43844 (accessed on 18 July 2023).
- Kononov, A.; Prutnikov, P.; Shumilova, I.; Kononova, S.; Nesterov, A.; Byadovskaya, O.; Pestova, Y.; Diev, V.; Sprygin, A. Determination of Lumpy Skin Disease Virus in Bovine Meat and Offal Products Following Experimental Infection. Transbound. Emerg. Dis. 2019, 66, 1332–1340. [Google Scholar] [CrossRef]
- Saegerman, C.; Bertagnoli, S.; Meyer, G.; Ganière, J.P.; Caufour, P.; De Clercq, K.; Jacquiet, P.; Hautefeuille, C.; Etore, F.; Casal, J. Risk of Introduction of Lumpy Skin Disease into France through Imports of Cattle. Transbound. Emerg. Dis. 2019, 66, 957–967. [Google Scholar] [CrossRef]
- Animal Health Australia. Response Strategy: Lumpy Skin Disease (Version 5.0). Australian Veterinary Emergency Plan (AUSVETPLAN), 5th ed.; ACT: Canberra, Australia, 2022. [Google Scholar]
- Li, Y.; An, Q.; Sun, Z.; Gao, X.; Wang, H. Risk Factors and Spatiotemporal Distribution of Lumpy Skin Disease Occurrence in the Asian Continent during 2012–2022: An Ecological Niche Model. Transbound. Emerg. Dis. 2023, 2023, 1–10. [Google Scholar] [CrossRef]
- Alemayehu, G.; Zewde, G.; Admassu, B. Risk Assessments of Lumpy Skin Diseases in Borena Bull Market Chain and Its Implication for Livelihoods and International Trade. Trop. Anim. Health Prod. 2013, 45, 1153–1159. [Google Scholar] [CrossRef]
- Animal Health Australia. National Biosecurity Manual for Beef Cattle Feedlots; Animal Health Australia: Lyneham, Australia, 2013; ISBN 9781921958182. Available online: https://www.farmbiosecurity.com.au/wp-content/uploads/2019/03/National-Biosecurity-Manual-for-Beef-Cattle-Feedlots1.pdf (accessed on 27 July 2023).
- LSD-Free Country List, Department of Agriculture, Fisheries and Forestry, Australian Government. 2022. Available online: https://www.agriculture.gov.au/sites/default/files/documents/lsd-free-country-list.pdf (accessed on 18 July 2023).
- Tuppurainen, E.; Lumpy Skin Disease. CABI Compendium. 2022. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.76780 (accessed on 27 July 2023).
- New Modelling Shows Lumpy Skin Disease Risk Reduced for Australia, as Cattle Industry Warns against Complacency–ABC News. Available online: https://www.abc.net.au/news/2023-05-08/lumpy-skin-foot-and-mouth-disease-risk-lower-livestock-australia/102308890 (accessed on 18 July 2023).
- Bianchini, J.; Simons, X.; Humblet, M.-F.; Saegerman, C. Lumpy Skin Disease: A Systematic Review of Mode of Transmission, Risk of Emergence and Risk Entry Pathway. Viruses 2023, 15, 1622. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).