Lichen or Associated Micro-Organism Compounds Are Active against Human Coronaviruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Natural Lichen Products
2.3. Cells and Culture Conditions
2.4. Viruses
2.5. Cell Toxicity Assay
2.6. HCoV-229E Infection Inhibition Assays
2.6.1. Luciferase Assay
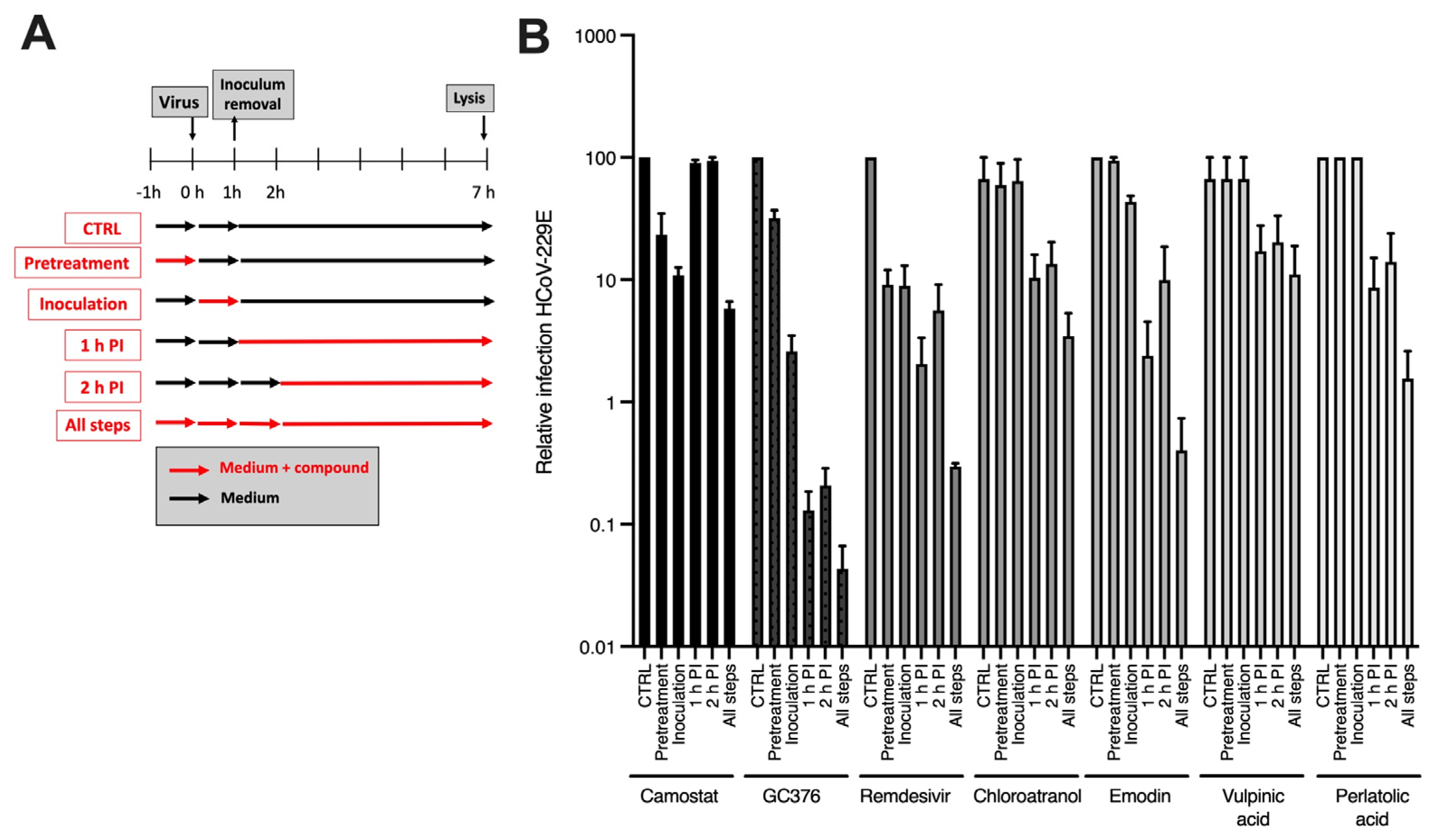
2.6.2. Time-of-Addition Assay
2.7. SARS-CoV-2 Infection Inhibition Assays
2.8. Statistical Analysis
3. Results
3.1. Screening of 42 Compounds Isolated from Lichens
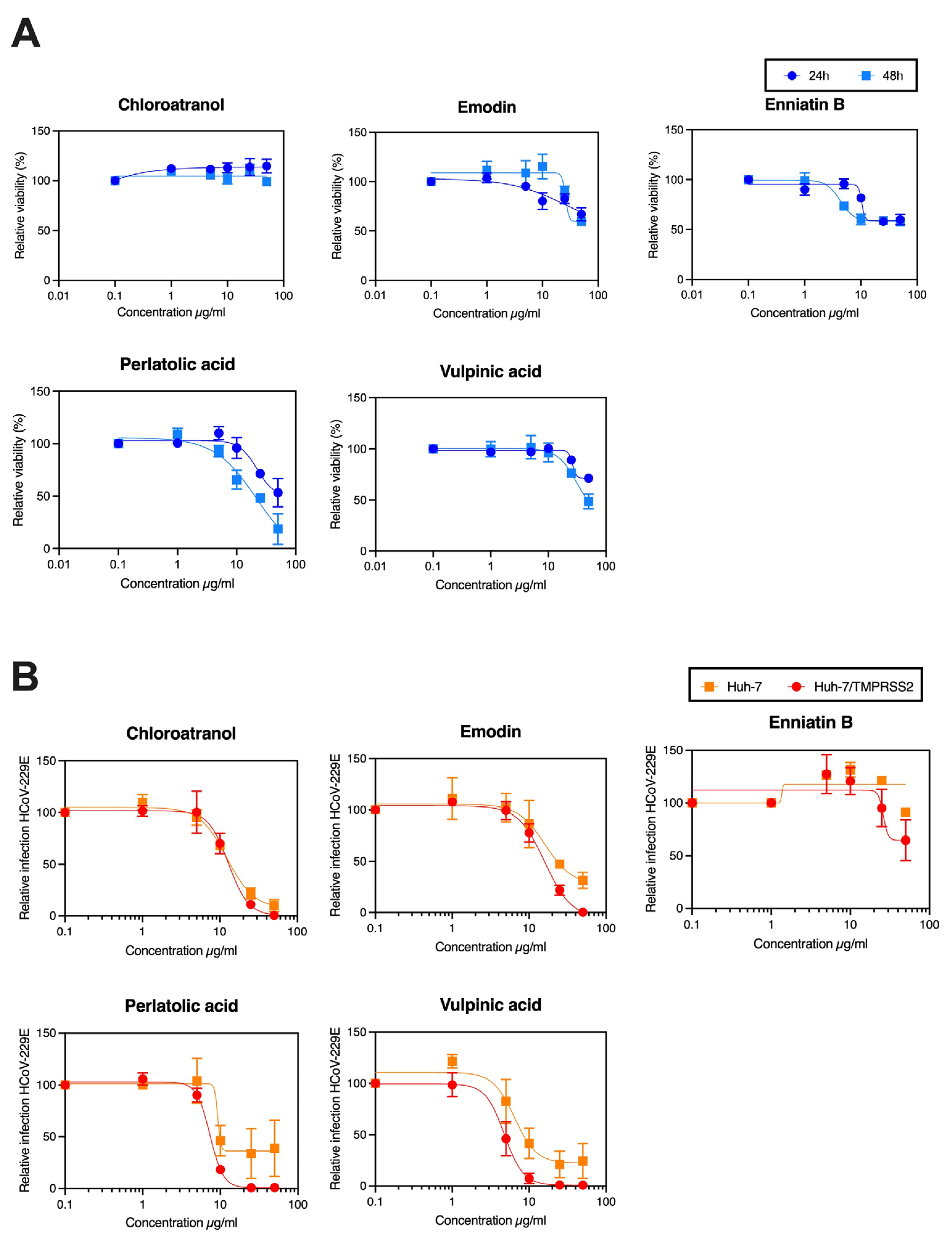
3.2. Determination of IC50 of the Active Compounds
3.3. Kinetic Study
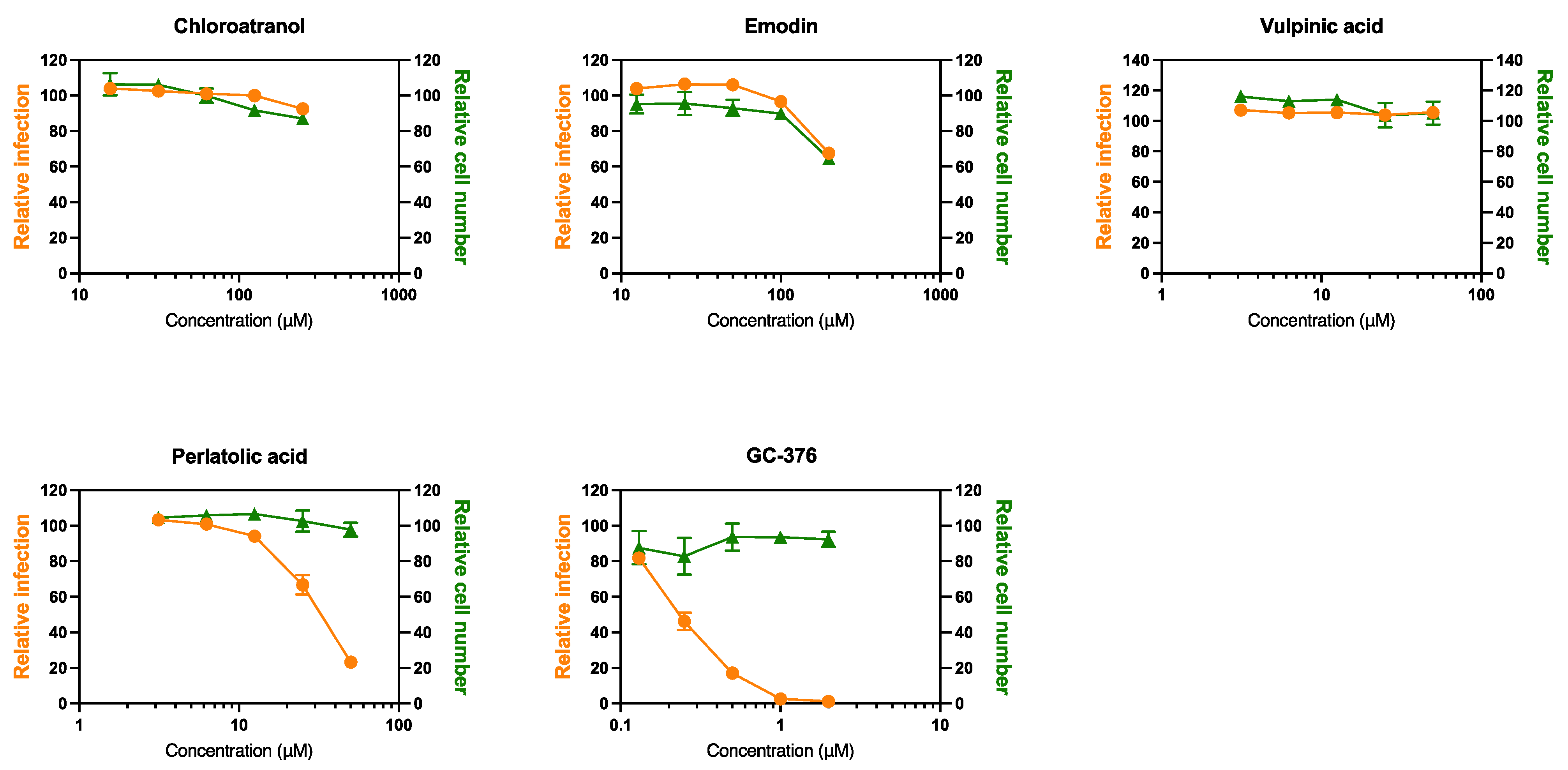
3.4. Anti-SARS-CoV-2 Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forni, D.; Cagliani, R.; Clerici, M.; Sironi, M. Molecular evolution of Human coronavirus genomes. Trends Microbiol. 2017, 25, 35–48. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Malone, B.; Urakova, N.; Snijder, E.J.; Campbell, E.A. Structures and functions of coronavirus replication-transcription complexes and their relevance for SARS-CoV-2 drug design. Nat. Rev. Mol. Cell Biol. 2022, 23, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on Ace2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zheng, Q.; Sun, L.; Ji, M.; Li, Y.; Deng, H.; Zhang, H. ORF3a of SARS-CoV-2 promotes lysosomal exocytosis-mediated viral egress. Dev. Cell 2021, 56, 3250–3263.e5. [Google Scholar] [CrossRef]
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.; Fehr, A.R.; Perlman, S.; et al. β-Coronaviruses use lysosomal organelles for cellular egress. bioRxiv 2020, 7, 192310. [Google Scholar] [CrossRef]
- Bruel, T.; Hadjadj, J.; Maes, P.; Planas, D.; Seve, A.; Staropoli, I.; Guivel-Benhassine, F.; Porrot, F.; Bolland, W.-H.; Nguyen, Y.; et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat. Med. 2022, 28, 1297–1302. [Google Scholar] [CrossRef]
- Takashita, E.; Yamayoshi, S.; Simon, V.; van Bakel, H.; Sordillo, E.M.; Pekosz, A.; Fukushi, S.; Suzuki, T.; Maeda, K.; Halfmann, P.; et al. Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. N. Engl. J. Med. 2022, 387, 468–470. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Pruijssers, A.J.; Agostini, M.L.; Leist, S.R.; Schäfer, A.; Dinnon, K.H.; Stevens, L.J.; et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020, 12, eabb5883. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.; Hobbs, R.; Gbinigie, O.; Rahman, N.M.; Hayward, G.; Richards, D.; Dorward, J.; Lowe, D.; Standing, J.F.; Breuer, J.; et al. Molnupiravir Plus Usual Care Versus Usual Care Alone as Early Treatment for Adults with COVID-19 at Increased Risk of Adverse Outcomes (PANORAMIC): Preliminary Analysis from the United Kingdom Randomised, Controlled Open-Label, Platform Adaptive Trial. Lancet 2022, 401, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V.; Muzamil Bashir, S.; Nabi, S.U. Medicinal plants: Treasure for antiviral drug discovery. Phytother. Res. 2021, 35, 3447–3483. [Google Scholar] [CrossRef]
- Vougogiannopoulou, K.; Corona, A.; Tramontano, E.; Alexis, M.N.; Skaltsounis, A.-L. Natural and Nature-Derived Products Targeting Human Coronaviruses. Molecules 2021, 26, 448. [Google Scholar] [CrossRef]
- Christy, M.P.; Uekusa, Y.; Gerwick, L.; Gerwick, W.H. Natural Products with Potential to Treat RNA Virus Pathogens Including SARS-CoV-2. J. Nat. Prod. 2021, 84, 161–182. [Google Scholar] [CrossRef]
- Müller, C.; Schulte, F.W.; Lange-Grünweller, K.; Obermann, W.; Madhugiri, R.; Pleschka, S.; Ziebuhr, J.; Hartmann, R.K.; Grünweller, A. Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses. Antivir. Res. 2018, 150, 123–129. [Google Scholar] [CrossRef]
- Kim, D.E.; Min, J.S.; Jang, M.S.; Lee, J.Y.; Shin, Y.S.; Song, J.H.; Kim, H.R.; Kim, S.; Jin, Y.-H.; Kwon, S. Natural Bis-Benzylisoquinoline Alkaloids-Tetrandrine, Fangchinoline, and Cepharanthine, Inhibit Human Coronavirus OC43 Infection of MRC-5 Human Lung Cells. Biomolecules 2019, 9, 696. [Google Scholar] [CrossRef]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Meunier, T.; Desmarets, L.; Bordage, S.; Bamba, M.; Hervouet, K.; Rouillé, Y.; François, N.; Decossas, M.; Sencio, V.; Trottein, F.; et al. A Photoactivable Natural Product with Broad Antiviral Activity against Enveloped Viruses, Including Highly Pathogenic Coronaviruses. Antimicrob. Agents Chemother. 2022, 66, e01581-21. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.-S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020, 35, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Sharma, P.; Joshi, T.; Pundir, H.; Mathpal, S.; Chandra, S. Structure-based screening of novel lichen compounds against SARS Coronavirus main protease (Mpro) as potentials inhibitors of COVID-19. Mol. Divers. 2021, 25, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hamdoun, S.; Chen, R.; Yang, L.; Ip, C.K.; Qu, Y.; Li, R.; Jiang, H.; Yang, Z.; Chung, S.K.; et al. Identification of natural compounds as SARS-CoV-2 entry inhibitors by molecular docking-based virtual screening with bio-layer interferometry. Pharmacol. Res. 2021, 172, 105820. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Zambare, V.P.; Christopher, L.P. Biopharmaceutical potential of lichens. Pharm. Biol. 2012, 50, 778–798. [Google Scholar] [CrossRef]
- Honegger, R. Lichens and Their Allies Past and Present. Mycota 2022, 5, 133–183. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef]
- Huneck, S.; Yoshimura, I. Identification of Lichen Substances. In Identification of Lichen Substances; Huneck, S., Yoshimura, I., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 11–123. ISBN 978-3-642-85243-5. [Google Scholar]
- Parrot, D.; Antony-Babu, S.; Intertaglia, L.; Grube, M.; Tomasi, S.; Suzuki, M.T. Littoral lichens as a novel source of potentially bioactive Actinobacteria. Sci. Rep. 2015, 5, 15839. [Google Scholar] [CrossRef]
- Lagarde, A.; Jargeat, P.; Roy, M.; Girardot, M.; Imbert, C.; Millot, M.; Mambu, L. Fungal communities associated with Evernia prunastri, Ramalina fastigiata and Pleurosticta acetabulum: Three epiphytic lichens potentially active against Candida biofilms. Microbiol. Res. 2018, 211, 1–12. [Google Scholar] [CrossRef]
- Desmarets, L.; Callens, N.; Hoffmann, E.; Danneels, A.; Lavie, M.; Couturier, C.; Dubuisson, J.; Belouzard, S.; Rouillé, Y. A reporter cell line for the automated quantification of SARS-CoV-2 infection in living cells. Front. Microbiol. 2022, 13, 1031204. [Google Scholar] [CrossRef] [PubMed]
- Belouzard, S.; Machelart, A.; Sencio, V.; Vausselin, T.; Hoffmann, E.; Deboosere, N.; Rouillé, Y.; Desmarets, L.; Séron, K.; Danneels, A.; et al. Clofoctol inhibits SARS-CoV-2 replication and reduces lung pathology in mice. PLoS Pathog. 2022, 18, e1010498. [Google Scholar] [CrossRef] [PubMed]
- Dieu, A.; Mambu, L.; Champavier, Y.; Chaleix, V.; Sol, V.; Gloaguen, V.; Millot, M. Antibacterial activity of the lichens Usnea Florida and Flavoparmelia caperata (Parmeliaceae). Nat. Prod. Res. 2020, 34, 3358–3362. [Google Scholar] [CrossRef]
- Le Pogam, P.; Boustie, J.; Richomme, P.; Denis, A.; Schinkovitz, A. The inherent matrix properties of lichen metabolites in matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Parrot, D.; Intertaglia, L.; Jehan, P.; Grube, M.; Suzuki, M.T.; Tomasi, S. Chemical analysis of the Alphaproteobacterium strain MOLA1416 associated with the marine lichen Lichina pygmaea. Phytochemistry 2018, 145, 57–67. [Google Scholar] [CrossRef]
- Noël, A.; Ferron, S.; Rouaud, I.; Gouault, N.; Hurvois, J.-P.; Tomasi, S. Isolation and Structure Identification of Novel Brominated Diketopiperazines from Nocardia ignorata—A Lichen-Associated Actinobacterium. Molecules 2017, 22, 371. [Google Scholar] [CrossRef]
- Parrot, D.; Legrave, N.; Intertaglia, L.; Rouaud, I.; Legembre, P.; Grube, M.; Suzuki, M.T.; Tomasi, S. Cyaneodimycin, a Bioactive Compound Isolated from the Culture of Streptomyces cyaneofuscatus Associated with Lichina confinis. Eur. J. Org. Chem. 2016, 2016, 3977–3982. [Google Scholar] [CrossRef]
- Lagarde, A.; Mambu, L.; Mai, P.-Y.; Champavier, Y.; Stigliani, J.-L.; Beniddir, M.A.; Millot, M. Chlorinated bianthrones from the cyanolichen Nephroma laevigatum. Fitoterapia 2021, 149, 104811. [Google Scholar] [CrossRef]
- Toure, S.; Millot, M.; Ory, L.; Roullier, C.; Khaldi, Z.; Pichon, V.; Girardot, M.; Imbert, C.; Mambu, L. Access to Anti-Biofilm Compounds from Endolichenic Fungi Using a Bioguided Networking Screening. J. Fungi 2022, 8, 1012. [Google Scholar] [CrossRef]
- Parrot, D.; Peresse, T.; Hitti, E.; Carrie, D.; Grube, M.; Tomasi, S. Qualitative and spatial metabolite profiling of lichens by a LC-MS approach combined with optimised extraction. Phytochem. Anal. 2015, 26, 23–33. [Google Scholar] [CrossRef]
- Bauer, J.; Waltenberger, B.; Noha, S.M.; Schuster, D.; Rollinger, J.M.; Boustie, J.; Chollet, M.; Stuppner, H.; Werz, O. Discovery of depsides and depsidones from lichen as potent inhibitors of microsomal prostaglandin E2 synthase-1 using pharmacophore models. ChemMedChem 2012, 7, 2077–2081. [Google Scholar] [CrossRef]
- Delebassée, S.; Mambu, L.; Pinault, E.; Champavier, Y.; Liagre, B.; Millot, M. Cytochalasin E in the lichen Pleurosticta acetabulum. Anti-proliferative activity against human HT-29 colorectal cancer cells and quantitative variability. Fitoterapia 2017, 121, 146–151. [Google Scholar] [CrossRef]
- Millot, M.; Tomasi, S.; Articus, K.; Rouaud, I.; Bernard, A.; Boustie, J. Metabolites from the Lichen Ochrolechia parella growing under two different heliotropic conditions. J. Nat. Prod. 2007, 70, 316–318. [Google Scholar] [CrossRef]
- Dieu, A.; Millot, M.; Champavier, Y.; Mambu, L.; Chaleix, V.; Sol, V.; Gloaguen, V. Uncommon Chlorinated Xanthone and Other Antibacterial Compounds from the Lichen Cladonia incrassata. Planta Med. 2014, 80, 931–935. [Google Scholar] [CrossRef]
- Millot, M.; Martin-de-Lassalle, M.; Chollet-Krugler, M.; Champavier, Y.; Mambu, L.; Chulia, J.-A.; Lacaille-Dubois, M.-A. Two New Retigerane-Type Sesterterpenoids from the Lichen Leprocaulon microscopicum. Helv. Chim. Acta 2016, 99, 169–173. [Google Scholar] [CrossRef]
- Horhant, D.; Lamer, A.-C.L.; Boustie, J.; Uriac, P.; Gouault, N. Separation of a mixture of paraconic acids from Cetraria islandica (L.) Ach. employing a fluorous tag—Catch and release strategy. Tetrahedron Lett. 2007, 48, 6031–6033. [Google Scholar] [CrossRef]
- Sweidan, A.; Chollet-Krugler, M.; van de Weghe, P.; Chokr, A.; Tomasi, S.; Bonnaure-Mallet, M.; Bousarghin, L. Design, synthesis and biological evaluation of potential antibacterial butyrolactones. Bioorg. Med. Chem. 2016, 24, 5823–5833. [Google Scholar] [CrossRef]
- Huneck, S.; Preiss, A.; Schmidt, J.; Mendez, A.M. 3β-Acetoxyhopan-1β,22-diol, a triterpene from the lichen Pseudoparmelia texana. Phytochemistry 1983, 22, 2027–2030. [Google Scholar] [CrossRef]
- Lagarde, A. Études Phytochimiques du Lichen Nephroma laevigatum et de ses Champignons Endolichéniques. Évaluation des Activités Antiprolifératives et Anti-Biofilms. Ph.D. Thesis, Université de Limoges, Limoges, France, 2017. [Google Scholar]
- Prosperini, A.; Berrada, H.; Ruiz, M.J.; Caloni, F.; Coccini, T.; Spicer, L.J.; Perego, M.C.; Lafranconi, A. A Review of the Mycotoxin Enniatin B. Front. Public Health 2017, 5, 304. [Google Scholar] [CrossRef]
- Bendz, G.; Bohman, G.; Santesson, J. Chlorinated Anthraquinones from Nephroma laevigatum. Acta Chem. Scand. 1967, 21, 2889–2890. [Google Scholar] [CrossRef][Green Version]
- Dwivedi, S.P.D.; Pandey, V.B.; Shah, A.H.; Rao, Y.B. Chemical constituents of Rhamnus procumbens and pharmacological actions of emodin. Phytother. Res. 1988, 2, 51–53. [Google Scholar] [CrossRef]
- Ho, T.-Y.; Wu, S.-L.; Chen, J.-C.; Li, C.-C.; Hsiang, C.-Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007, 74, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Fang, M.-C.; Chen, Y.-Z.; Huang, S.-C.; Wang, D.-Y. Quantitative analysis of fragrance allergens in various matrixes of cosmetics by liquid-liquid extraction and GC-MS. J. Food Drug Anal. 2021, 29, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, D.N.; Zarubaev, V.V.; Shtro, A.A.; Polovinka, M.P.; Luzina, O.A.; Komarova, N.I.; Salakhutdinov, N.F.; Kiselev, O.I. Anti-viral activity of (−)- and (+)-usnic acids and their derivatives against influenza virus A(H1N1)2009. Bioorg. Med. Chem. Lett. 2012, 22, 7060–7064. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sahu, N.; Singh, A.P.; Singh, V.K.; Singh, S.C.; Upadhye, V.J.; Mathew, A.T.; Kumar, R.; Sinha, R.P. Exploration of Novel Lichen Compounds as Inhibitors of SARS-CoV-2 Mpro: Ligand-Based Design, Molecular Dynamics, and ADMET Analyses. Appl. Biochem. Biotechnol. 2022, 194, 6386–6406. [Google Scholar] [CrossRef] [PubMed]
- Prateeksha, G.; Rana, T.S.; Ashthana, A.K.; Barik, S.K.; Singh, B.N. Screening of cryptogamic secondary metabolites as putative inhibitors of SARS-CoV-2 main protease and ribosomal binding domain of spike glycoprotein by molecular docking and molecular dynamics approaches. J. Mol. Struct. 2021, 1240, 130506. [Google Scholar] [CrossRef]
- Oettl, S.K.; Gerstmeier, J.; Khan, S.Y.; Wiechmann, K.; Bauer, J.; Atanasov, A.G.; Malainer, C.; Awad, E.M.; Uhrin, P.; Heiss, E.H.; et al. Imbricaric acid and perlatolic acid: Multi-targeting anti-inflammatory depsides from Cetrelia monachorum. PLoS ONE 2013, 8, e76929. [Google Scholar] [CrossRef]
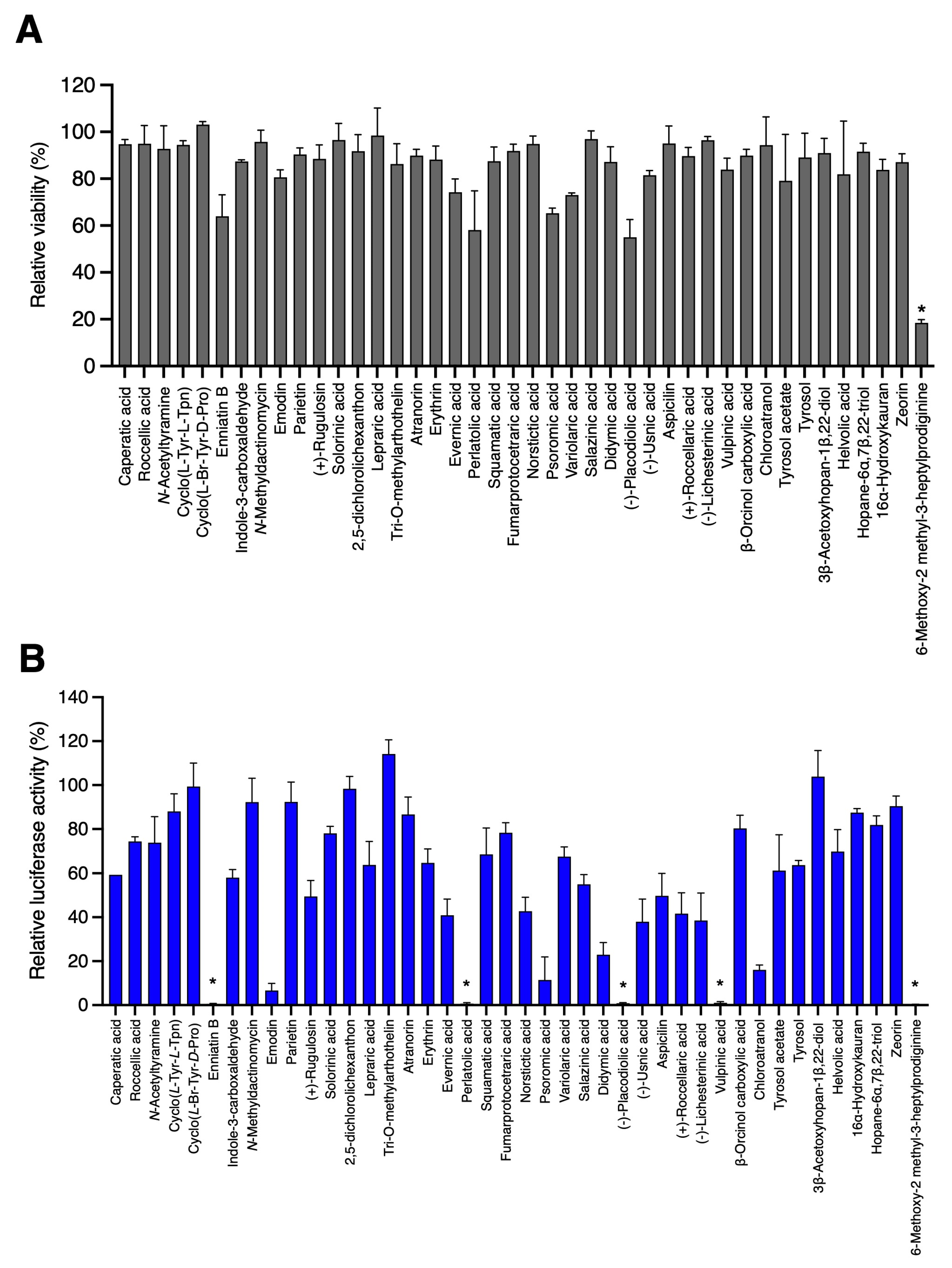

| N° | Name of Compound | Class of Compound | Source | Reference |
|---|---|---|---|---|
| Aliphatic acids | ||||
| 1 | Caperatic acid | Flavoparmelia caperata | [34] | |
| 2 | Roccellic acid | Lepraria membranacea | [35] | |
| Amino-acid derivatives | ||||
| 3 | N-Acetyltyramine | Endolichenic fungus Nemania aena var aureolatum | * | |
| 4 | Cyclo(L-Tyr-L-Tpn) | α-Proteobacterium MOLA 1416 (lichen-associated bacterium) | [36] | |
| 5 | Cyclo(L-Br-Tyr-D-Pro) | Nocardia ignorata (lichen-associated bacterium) | [37] | |
| 6 | Enniatin B | Fusarium avenaceum (endolichenic fungus) | * | |
| 7 | Indole-3-carboxaldehyde | Nocardia ignorata (lichen-associated bacterium) | [37] | |
| 8 | N-Methyldactinomycin | Streptomyces cyaneofuscatus (lichen-associated bacterium) | [38] | |
| Anthraquinones | ||||
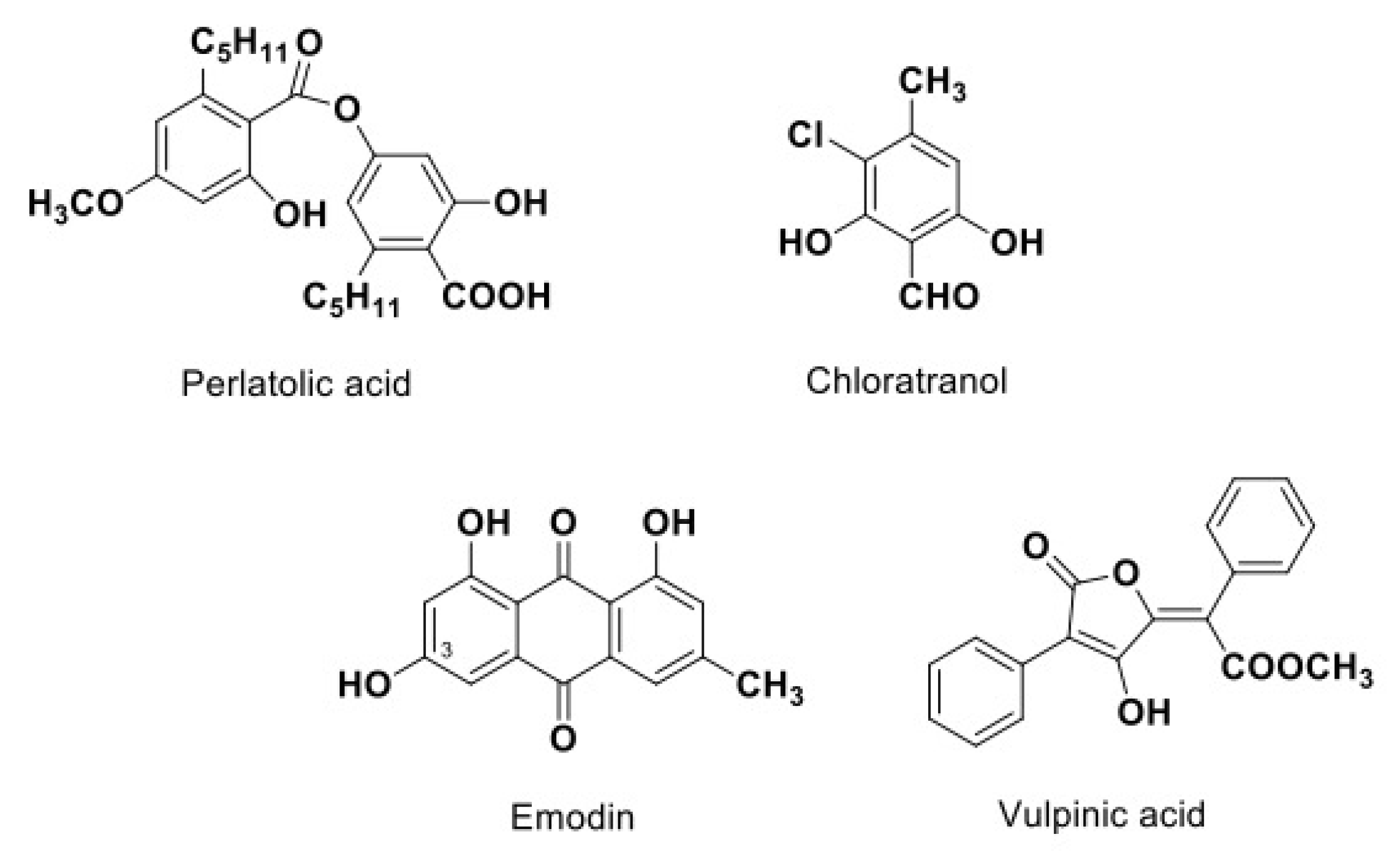
| 9 | Emodin | Nephroma laevigatum | [39] | |
| 10 | Parietin | Xanthoria parietina | [29] | |
| 11 | (+)-Rugulosin | Coniochaeta lignicola (endolichenic fungi) | [40] | |
| 12 | Solorinic acid | Solorina crocea | [29] | |
| Chromones and Xanthones | ||||
| 13 | 2,5-dichlorolichexanthon | Pertusaria aleianta | [29] | |
| 14 | Lepraric acid | Roccella fuciformis | [41] | |
| 15 | Tri-O-methylarthothelin | Dimelaena cf. australiensis | [29] | |
| Depsides | ||||
| 16 | Atranorin | Parmotrema tinctorum | [29] | |
| 17 | Erythrin | Roccella phycopsis | [36] | |
| 18 | Evernic acid | Evernia prunastri | [29] | |
| 19 | Perlatolic acid | Cladonia portentosa | [42] | |
| 20 | Squamatic acid | Cladonia squamosa | * | |
| Depsidones | ||||
| 21 | Fumarprotocetraric acid | Cetraria islandica | [29] | |
| 22 | Norstictic acid | Pleurosticta acetabulum | [43] | |
| 23 | Psoromic acid | Squamarina cartilaginea | [29] | |
| 24 | Variolaric acid | Ochrolechia parella | [44] | |
| 25 | Salazinic acid | Parmelia saxatilis | * | |
| Dibenzofurans | ||||
| 26 | Didymic acid | Cladonia incrassata | [45] | |
| 27 | (−)-Placodiolic acid | Leprocaulum microscopicum | [46] | |
| 28 | (−)-Usnic acid | Leprocaulum microscopicum | [46] | |
| Lactones and macrolides | ||||
| 29 | Aspicilin | Aspicilia caesiocinerea | [29] | |
| 30 | (+)-Roccellaric acid | Cetraria islandica | [47] | |
| 31 | (−)-Lichesterinic acid | Cetraria komarovii Synthetic compound | [48] | |
| Pulvinic acid derivative | ||||
| 32 | Vulpinic acid | Letharia vulpina | [29] | |
| Phenolic acid and derivatives | ||||
| 33 | β-Orcinol carboxylic acid | Pseudevernia furfuracea | [29] | |
| 34 | Chloroatranol | Evernia prunastri | [29] | |
| 35 | Tyrosol acetate | Endolichenic fungi Nemania aena var aureolatum | * | |
| 36 | Tyrosol | Endolichenic fungi Nemania aena var aureolatum | * | |
| Terpenes | ||||
| 37 | 3β-Acetoxyhopan-1β,22-diol | Pseudoparmelia texana | [49] | |
| 38 | Helvolic acid | Endolichenic fungi Nemania aena var aureolatum | [50] | |
| 39 | 16α-Hydroxykauran | Ramalina tumidula | [29] | |
| 40 | Hopane-6α,7β,22-triol | Nephroma laevigatum | * | |
| 41 | Zeorin | Leprocaulum microscopicum | [46] | |
| Others derivatives | ||||
| 42 | 6-Methoxy-2-methyl-3-heptylprodiginine | α-Proteobacterium MOLA 1416 (lichen-associated bacterium) | [36] |
| Huh-7 | Huh-7/TMPRSS2 | ||||
|---|---|---|---|---|---|
| CC50 (μM) * | IC50 (μM) | SI | IC50 (μM) | SI | |
| Perlatolic acid | 48.9 | 20.81 ± 7.44 | 2.3 | 16.42 ± 1.66 | 3.0 |
| Vulpinic acid | 155 | 19.86 ± 9.46 | 7.8 | 14.58 ± 5.55 | 10.6 |
| Emodin | >185 | 59.58 ± 1.62 | >3.1 | 59.25 ± 2.47 | >3.1 |
| Chloroatranol | >268 | 65.81 ± 6.27 | >4 | 68.86 ± 11.52 | >3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desmarets, L.; Millot, M.; Chollet-Krugler, M.; Boustie, J.; Camuzet, C.; François, N.; Rouillé, Y.; Belouzard, S.; Tomasi, S.; Mambu, L.; et al. Lichen or Associated Micro-Organism Compounds Are Active against Human Coronaviruses. Viruses 2023, 15, 1859. https://doi.org/10.3390/v15091859
Desmarets L, Millot M, Chollet-Krugler M, Boustie J, Camuzet C, François N, Rouillé Y, Belouzard S, Tomasi S, Mambu L, et al. Lichen or Associated Micro-Organism Compounds Are Active against Human Coronaviruses. Viruses. 2023; 15(9):1859. https://doi.org/10.3390/v15091859
Chicago/Turabian StyleDesmarets, Lowiese, Marion Millot, Marylène Chollet-Krugler, Joël Boustie, Charline Camuzet, Nathan François, Yves Rouillé, Sandrine Belouzard, Sophie Tomasi, Lengo Mambu, and et al. 2023. "Lichen or Associated Micro-Organism Compounds Are Active against Human Coronaviruses" Viruses 15, no. 9: 1859. https://doi.org/10.3390/v15091859
APA StyleDesmarets, L., Millot, M., Chollet-Krugler, M., Boustie, J., Camuzet, C., François, N., Rouillé, Y., Belouzard, S., Tomasi, S., Mambu, L., & Séron, K. (2023). Lichen or Associated Micro-Organism Compounds Are Active against Human Coronaviruses. Viruses, 15(9), 1859. https://doi.org/10.3390/v15091859






