Lung Ultrasound in Predicting Outcomes in Patients with COVID-19 Treated with Extracorporeal Membrane Oxygenation
Abstract
:1. Background
2. Materials and Methods
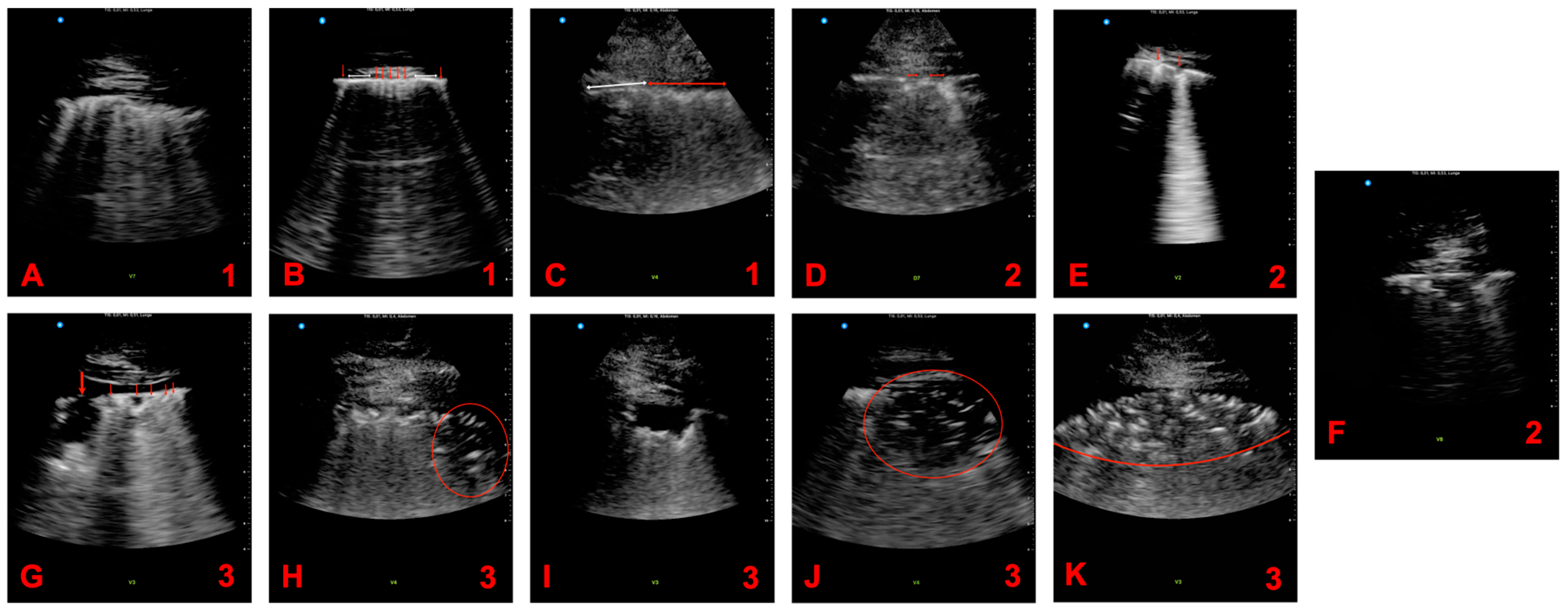
2.1. Ultrasound Examination
2.2. Scoring System
2.3. Clinical Assessement
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Patients’ Characteristics
3.2. Pathologies in Lung Ultrasound
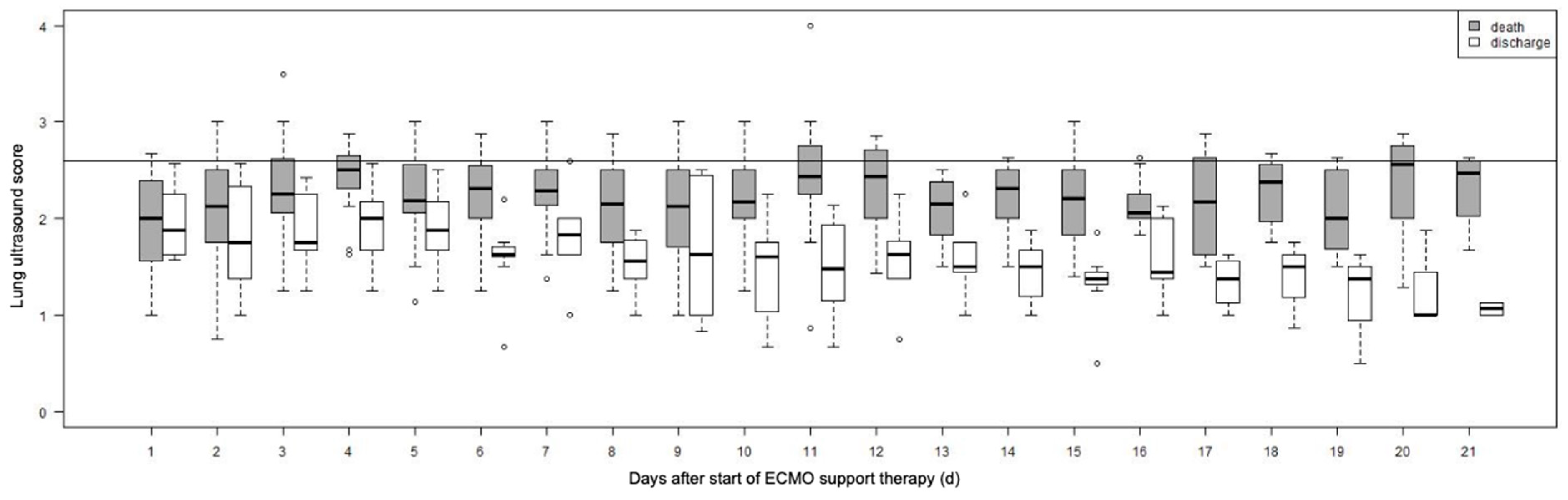
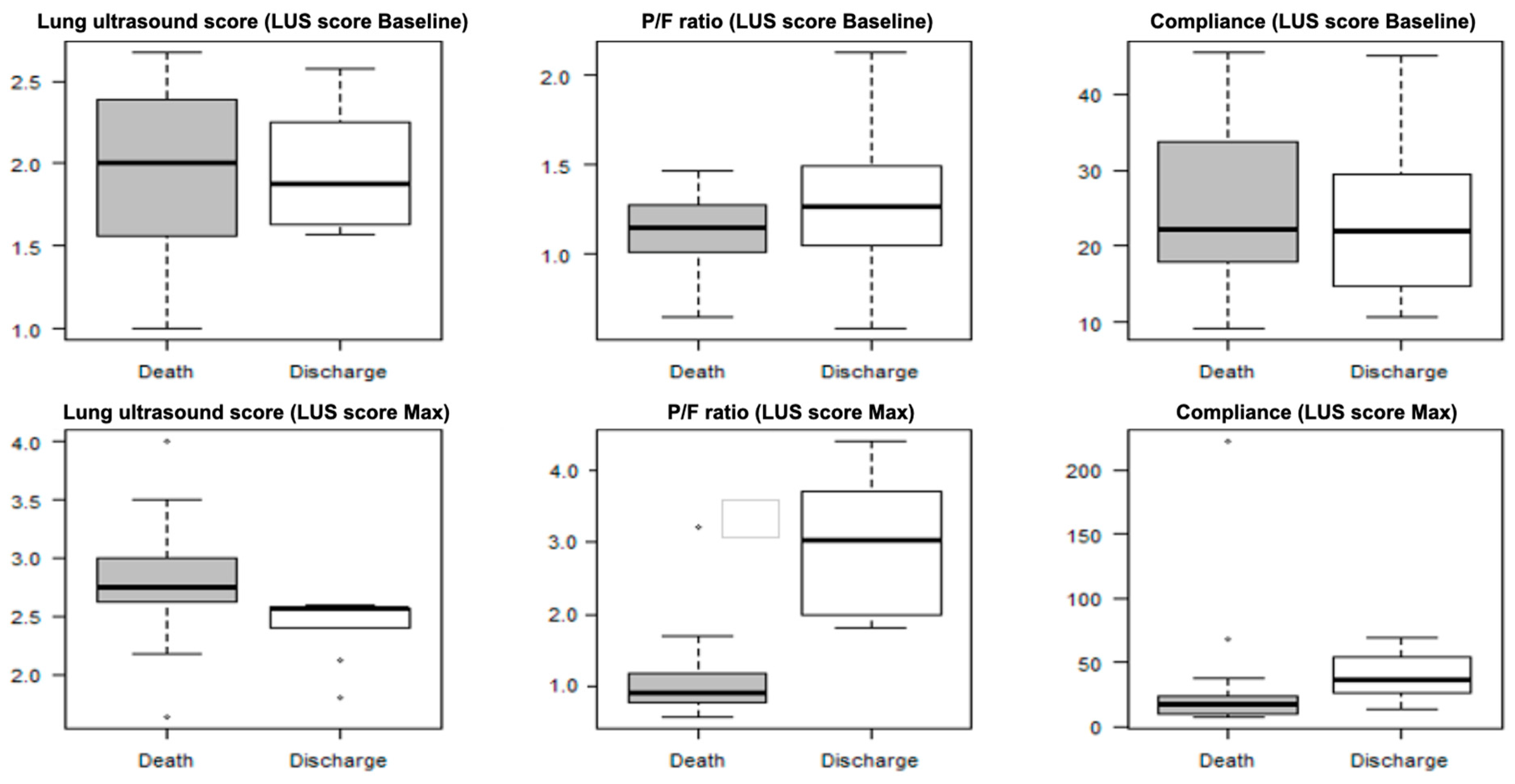
3.3. LUS Score
3.4. LUS Score Max
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, K.H.; Lee, S.W.; Kim, T.S.; Huh, H.J.; Lee, J.; Kim, S.Y.; Park, J.-S.; Kim, G.J.; Sung, H.; Roh, K.H.; et al. Guidelines for Laboratory Diagnosis of Coronavirus Disease 2019 (COVID-19) in Korea. Ann. Lab. Med. 2020, 40, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Jarrom, D.; Elston, L.; Washington, J.; Prettyjohns, M.; Cann, K.; Myles, S.; Groves, P. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: A rapid systematic review. BMJ Evid. Based Med. 2022, 27, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Ebrahimzadeh, S.; Salameh, J.-P.; Kazi, S.; Fabiano, N.; Treanor, L.; Absi, M.; Hallgrimson, Z.; Leeflang, M.M.G.; Hooft, L.; et al. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst. Rev. 2021, 2021, CD013639. [Google Scholar] [CrossRef]
- Li, M.; Lei, P.; Zeng, B.; Li, Z.; Yu, P.; Fan, B.; Wang, C.; Li, Z.; Zhou, J.; Hu, S.; et al. Coronavirus Disease (COVID-19): Spectrum of CT Findings and Temporal Progression of the Disease. Acad. Radiol. 2020, 27, 603–608. [Google Scholar] [CrossRef]
- Zieleskiewicz, L.; Markarian, T.; Lopez, A.; Taguet, C.; Mohammedi, N.; Boucekine, M.; Baumstarck, K.; Besch, G.; Mathon, G.; Duclos, G.; et al. Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med. 2020, 46, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Barskova, T.; Gargani, L.; Guiducci, S.; Randone, S.B.; Bruni, C.; Carnesecchi, G.; Conforti, M.L.; Porta, F.; Pignone, A.; Caramella, D.; et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann. Rheum. Dis. 2013, 72, 390–395. [Google Scholar] [CrossRef]
- Wang, G.; Ji, X.; Xu, Y.; Xiang, X. Lung ultrasound: A promising tool to monitor ventilator-associated pneumonia in critically ill patients. Crit. Care 2016, 20, 320. [Google Scholar] [CrossRef] [PubMed]
- Ferré, A.; Guillot, M.; Lichtenstein, D.; Mezière, G.; Richard, C.; Teboul, J.-L.; Monnet, X. Lung ultrasound allows the diagnosis of weaning-induced pulmonary oedema. Intensive Care Med. 2019, 45, 601–608. [Google Scholar] [CrossRef]
- Mayo, P.H.; Copetti, R.; Feller-Kopman, D.; Mathis, G.; Maury, E.; Mongodi, S.; Mojoli, F.; Volpicelli, G.; Zanobetti, M. Thoracic ultrasonography: A narrative review. Intensive Care Med. 2019, 45, 1200–1211. [Google Scholar] [CrossRef]
- Rouby, J.-J.; Arbelot, C.; Gao, Y.; Zhang, M.; Lv, J.; An, Y.; Chunyao, W.; Du, B.; Valente Barbas, C.S.; Dexheimer Neto, F.L.; et al. Training for Lung Ultrasound Score Measurement in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 398–401. [Google Scholar] [CrossRef]
- Bouhemad, B.; Mojoli, F.; Nowobilski, N.; Hussain, A.; Rouquette, I.; Guinot, P.-G.; Mongodi, S. Use of combined cardiac and lung ultrasound to predict weaning failure in elderly, high-risk cardiac patients: A pilot study. Intensive Care Med. 2020, 46, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Koratala, A.; Ronco, C.; Kazory, A. The Promising Role of Lung Ultrasound in Assessment of Volume Status for Patients Receiving Maintenance Renal Replacement Therapy. Blood Purif. 2020, 49, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Moshavegh, R.; Hansen, K.L.; Moller-Sorensen, H.; Nielsen, M.B.; Jensen, J.A. Automatic Detection of B-Lines in In Vivo Lung Ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 309–317. [Google Scholar] [CrossRef]
- Sezgin, C.; Gunalp, M.; Genc, S.; Acar, N.; Ustuner, E.; Oguz, A.B.; Tanriverdi, A.K.; Demirkan, A.; Polat, O. Diagnostic Value of Bedside Lung Ultrasonography in Pneumonia. Ultrasound Med. Biol. 2020, 46, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Barssoum, K.; Victor, V.; Salem, A.; Kumar, A.; Mubasher, M.; Hassib, M.; Magdi, M.; Renjithlal, S.; Abdelazeem, M.; Shariff, M.; et al. Echocardiography, lung ultrasound, and cardiac magnetic resonance findings in COVID-19: A systematic review. Echocardiography 2021, 38, 1365–1404. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.J.; Bhatt, H.B.; Parikh, S.N.; Jhaveri, B.N.; Puranik, J.H. Bedside Lung Ultrasound in Emergency Protocol as a Diagnostic Tool in Patients of Acute Respiratory Distress Presenting to Emergency Department. J. Emerg. Trauma Shock 2018, 11, 125–129. [Google Scholar] [CrossRef]
- Asano, M.; Watanabe, H.; Sato, K.; Okuda, Y.; Sakamoto, S.; Hasegawa, Y.; Sudo, K.; Takeda, M.; Sano, M.; Kibira, S.; et al. Validity of Ultrasound Lung Comets for Assessment of the Severity of Interstitial Pneumonia. J. Ultrasound Med. 2018, 37, 1523–1531. [Google Scholar] [CrossRef]
- Gargani, L.; Bruni, C.; Romei, C.; Frumento, P.; Moreo, A.; Agoston, G.; Guiducci, S.; Bellando-Randone, S.; Lepri, G.; Belloli, L.; et al. Prognostic Value of Lung Ultrasound B-Lines in Systemic Sclerosis. Chest 2020, 158, 1515–1525. [Google Scholar] [CrossRef]
- Platz, E.; Merz, A.A.; Jhund, P.S.; Vazir, A.; Campbell, R.; McMurray, J.J. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: A systematic review. Eur. J. Heart Fail. 2017, 19, 1154–1163. [Google Scholar] [CrossRef]
- Volpicelli, G.; Gargani, L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J. 2020, 12, 22. [Google Scholar] [CrossRef]
- Yasukawa, K.; Minami, T. Point-of-Care Lung Ultrasound Findings in Patients with COVID-19 Pneumonia. Am. J. Trop. Med. Hyg. 2020, 102, 1198–1202. [Google Scholar] [CrossRef]
- Dargent, A.; Chatelain, E.; Si-Mohamed, S.; Simon, M.; Baudry, T.; Kreitmann, L.; Quenot, J.-P.; Cour, M.; Argaud, L. Lung ultrasound score as a tool to monitor disease progression and detect ventilator-associated pneumonia during COVID-19-associated ARDS. Heart Lung 2021, 50, 700–705. [Google Scholar] [CrossRef]
- Lomoro, P.; Verde, F.; Zerboni, F.; Simonetti, I.; Borghi, C.; Fachinetti, C.; Natalizi, A.; Martegani, A. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: Single-center study and comprehensive radiologic literature review. Eur. J. Radiol. Open 2020, 7, 100231. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Mathis, G.; Blaivas, M.; Volpicelli, G.; Seibel, A.; Wastl, D.; Atkinson, N.S.S.; Cui, X.-W.; Fan, M.; Yi, D. Lung B-line artefacts and their use. J. Thorac. Dis. 2016, 8, 1356–1365. [Google Scholar] [CrossRef]
- Allinovi, M.; Parise, A.; Giacalone, M.; Amerio, A.; Delsante, M.; Odone, A.; Franci, A.; Gigliotti, F.; Amadasi, S.; Delmonte, D.; et al. Lung Ultrasound May Support Diagnosis and Monitoring of COVID-19 Pneumonia. Ultrasound Med. Biol. 2020, 46, 2908–2917. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Jelic, T.; Woo, M.Y.; Heslop, C.; Olszynski, P. Just the Facts: Recommendations on point-of-care ultrasound use and machine infection control during the coronavirus disease 2019 pandemic. CJEM 2020, 22, 445–449. [Google Scholar] [CrossRef]
- Pecho-Silva, S.; Navarro-Solsol, A.C.; Taype-Rondan, A.; Torres-Valencia, J.; Arteaga-Livias, K.; Herriman, D.A.; Acosta-Pinzas, K.; Valenzuela-Rodriguez, G.; Barboza, J.J.; Panduro-Correa, V. Pulmonary Ultrasound in the Diagnosis and Monitoring of Coronavirus Disease (COVID-19): A Systematic Review. Ultrasound Med. Biol. 2021, 47, 1997–2005. [Google Scholar] [CrossRef]
- Peng, Q.-Y.; Wang, X.-T.; Zhang, L.-N. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020, 46, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Tung-Chen, Y.; Martí de Gracia, M.; Díez-Tascón, A.; Alonso-González, R.; Agudo-Fernández, S.; Parra-Gordo, M.L.; Ossaba-Vélez, S.; Rodríguez-Fuertes, P.; Llamas-Fuentes, R. Correlation between Chest Computed Tomography and Lung Ultrasonography in Patients with Coronavirus Disease 2019 (COVID-19). Ultrasound Med. Biol. 2020, 46, 2918–2926. [Google Scholar] [CrossRef]
- Pare, J.R.; Camelo, I.; Mayo, K.C.; Leo, M.M.; Dugas, J.N.; Nelson, K.P.; Baker, W.E.; Shareef, F.; Mitchell, P.M.; Schechter-Perkins, E.M. Point-of-care Lung Ultrasound Is More Sensitive than Chest Radiograph for Evaluation of COVID-19. West. J. Emerg. Med. 2020, 21, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Via, G.; Melniker, L.; Goffi, A.; Tavazzi, G.; Neri, L.; Villen, T.; Hoppmann, R.; Mojoli, F.; Noble, V.; et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): International expert consensus. Crit. Care 2020, 24, 702. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, S.; Chen, B.; Chen, J.; Xian, J.; Lin, Y.; Shan, H.; Su, Z.Z. Nicht-invasive Beurteilung von pulmonalen Läsionen bei Patienten mit Coronavirus-Erkrankung (COVID-19) durch Ultraschall direkt am Krankenbett. Ultraschall Med. 2020, 41, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Cao, C.; Gao, Y.; Zhang, W.; Xie, Y.; Duan, Y.; Kong, S.; You, M.; Ma, R.; Jiang, L.; et al. Prognostic value of bedside lung ultrasound score in patients with COVID-19. Crit. Care 2020, 24, 700. [Google Scholar] [CrossRef] [PubMed]
- Laursen, C.B.; Clive, A.; Hallifax, R.; Pietersen, P.I.; Asciak, R.; Davidsen, J.R.; Bhatnagar, R.; Bedawi, E.O.; Jacobsen, N.; Coleman, C.; et al. European Respiratory Society statement on thoracic ultrasound. Eur. Respir. J. 2021, 57, 2001519. [Google Scholar] [CrossRef] [PubMed]
- Alharthy, A.; Faqihi, F.; Abuhamdah, M.; Noor, A.; Naseem, N.; Balhamar, A.; Al Saud, A.A.A.S.B.A.; Brindley, P.G.; Memish, Z.A.; Karakitsos, D.; et al. Prospective Longitudinal Evaluation of Point-of-Care Lung Ultrasound in Critically Ill Patients With Severe COVID-19 Pneumonia. J. Ultrasound Med. 2020, 40, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Ravetti, C.G.; Vassallo, P.F.; de Barros, G.M.; Rocha, G.C.; Chamon, S.; Borges, I.N.; Marinho, C.C.; Cabral, M.A.d.S.; Duani, H.; de Andrade, M.V.M.; et al. Lung Ultrasound Can Predict the Clinical Course and Severity of COVID-19 Disease. Ultrasound Med. Biol. 2021, 47, 2090–2096. [Google Scholar] [CrossRef]
- Møller-Sørensen, H.; Gjedsted, J.; Lind Jørgensen, V.; Lindskov Hansen, K. COVID-19 Assessment with Bedside Lung Ultrasound in a Population of Intensive Care Patients Treated with Mechanical Ventilation and ECMO. Diagnostics 2020, 10, 447. [Google Scholar] [CrossRef]
- Lazzeri, C.; Bonizzoli, M.; Batacchi, S.; Socci, F.; Matucci-Cerinic, M.; Peris, A. Combined lung and cardiac ultrasound in COVID-related acute respiratory distress syndrome. Intern. Emerg. Med. 2021, 16, 1779–1785. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19: A Simple, Quantitative, Reproducible Method. J. Ultrasound Med. 2020, 39, 1413–1419. [Google Scholar] [CrossRef]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Down, B.; Jha, S. Point-of-care lung ultrasound in intensive care during the COVID-19 pandemic. Clin. Radiol. 2020, 75, 710.e1–710e4. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, J.S.; Basseal, J.M. World Federation for Ultrasound in Medicine and Biology Position Statement: How to Perform a Safe Ultrasound Examination and Clean Equipment in the Context of COVID-19. Ultrasound Med. Biol. 2020, 46, 1821–1826. [Google Scholar] [CrossRef]
- Lazzeri, C.; Bonizzoli, M.; Batacchi, S.; Cianchi, G.; Franci, A.; Fulceri, G.E.; Peris, A. Cardiac Involvment in COVID-19-Related Acute Respiratory Distress Syndrome. Am. J. Cardiol. 2020, 132, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Bonadia, N.; Carnicelli, A.; Piano, A.; Buonsenso, D.; Gilardi, E.; Kadhim, C.; Torelli, E.; Petrucci, M.; Di Maurizio, L.; Biasucci, D.G.; et al. Lung Ultrasound Findings Are Associated with Mortality and Need for Intensive Care Admission in COVID-19 Patients Evaluated in the Emergency Department. Ultrasound Med. Biol. 2020, 46, 2927–2937. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, K.; Zhao, Q.; Tian, R.; Xie, H.; Xie, L.; Deng, P.; Xie, G.; Bao, A.; Du, J. Lung Ultrasound Score in Evaluating the Severity of Coronavirus Disease 2019 (COVID-19) Pneumonia. Ultrasound Med. Biol. 2020, 46, 2938–2944. [Google Scholar] [CrossRef]
- Haji-Hassan, M.; Lenghel, L.M.; Bolboacă, S.D. Hand-Held Ultrasound of the Lung: A Systematic Review. Diagnostics 2021, 11, 1381. [Google Scholar] [CrossRef]
- Šustić, A.; Mirošević, M.; Szuldrzynski, K.; Marčun, R.; Haznadar, M.; Podbegar, M.; Protić, A. Inter-observer reliability for different point-of-care lung ultrasound findings in mechanically ventilated critically ill COVID-19 patients. J. Clin. Monit. Comput. 2022, 36, 279–281. [Google Scholar] [CrossRef]

| Parameter | n | Classical Correlation with LUS Score | Repeated Measures Correlation [rmcorr] with LUS Score | 95% CI Low [rmcorr] | 95% CI High [rmcorr] | p Value [rmcorr] |
|---|---|---|---|---|---|---|
| Hemodynamic status (Extravascular lung water) | 444 | 0.36 | 0.11 | 0.01 | 0.20 | 0.031 |
| P/F ratio | 425 | −0.40 | −0.26 | −0.34 | −0.15 | <0.001 |
| pH value | 470 | 0.19 | 0.12 | 0.03 | 0.21 | 0.001 |
| Pulmonary compliance | 449 | −0.21 | −0.10 | −0.20 | −0.01 | 0.034 |
| Catecholamine circulatory support | 478 | 0.14 | −0.10 | −0.19 | −0.004 | 0.040 |
| Base excess | 429 | 0.12 | 0.14 | 0.05 | 0.24 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, V.S.; Recker, F.; Kretschmer, E.; Putensen, C.; Ehrentraut, S.F.; Staerk, C.; Fleckenstein, T.; Mayr, A.; Seibel, A.; Schewe, J.-C.; et al. Lung Ultrasound in Predicting Outcomes in Patients with COVID-19 Treated with Extracorporeal Membrane Oxygenation. Viruses 2023, 15, 1796. https://doi.org/10.3390/v15091796
Schäfer VS, Recker F, Kretschmer E, Putensen C, Ehrentraut SF, Staerk C, Fleckenstein T, Mayr A, Seibel A, Schewe J-C, et al. Lung Ultrasound in Predicting Outcomes in Patients with COVID-19 Treated with Extracorporeal Membrane Oxygenation. Viruses. 2023; 15(9):1796. https://doi.org/10.3390/v15091796
Chicago/Turabian StyleSchäfer, Valentin Sebastian, Florian Recker, Edgar Kretschmer, Christian Putensen, Stefan Felix Ehrentraut, Christian Staerk, Tobias Fleckenstein, Andreas Mayr, Armin Seibel, Jens-Christian Schewe, and et al. 2023. "Lung Ultrasound in Predicting Outcomes in Patients with COVID-19 Treated with Extracorporeal Membrane Oxygenation" Viruses 15, no. 9: 1796. https://doi.org/10.3390/v15091796
APA StyleSchäfer, V. S., Recker, F., Kretschmer, E., Putensen, C., Ehrentraut, S. F., Staerk, C., Fleckenstein, T., Mayr, A., Seibel, A., Schewe, J.-C., & Petzinna, S. M. (2023). Lung Ultrasound in Predicting Outcomes in Patients with COVID-19 Treated with Extracorporeal Membrane Oxygenation. Viruses, 15(9), 1796. https://doi.org/10.3390/v15091796








