Clinical and Genetic Characteristics of the Heidenhain Variant of Creutzfeldt–Jakob Disease
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Clinical Data Collection
2.3. Laboratory Tests
2.4. Statistical Analysis
3. Results
3.1. The Clinical Features of HvCJD Cases Included in Our Cohort
3.2. The Clinical Features of Genetic HvCJD Cases
3.3. The Differences between Sporadic and Genetic HvCJD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kropp, S.; Schulz-Schaeffer, W.J.; Finkenstaedt, M.; Riedemann, C.; Windl, O.; Steinhoff, B.J.; Zerr, I.; Kretzschmar, H.A.; Poser, S. The Heidenhain Variant of Creutzfeldt-Jakob Disease. Arch. Neurol. 1999, 56, 55–61. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L.; Zhang, S.; Zhang, M.; Zhu, M.; Dong, Q.; Wang, Q.; Han, X. Clinical and prognostic features of Heidenhain variant of Creutzfeldt−Jakob disease: A retrospective case series study. Eur. J. Neurol. 2022, 29, 2412–2419. [Google Scholar] [CrossRef]
- Chen, S.; He, S.; Xu, Y.-Y.; Teng, H.-Y.; Zhang, J.-W. Clinical Presentation of Sporadic Creutzfeldt-Jakob Disease in Han-Chinese. Alzheimer Dis. Assoc. Disord. 2019, 34, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.A.; Murray, K.L.; Heath, C.A.; Will, R.G.; Knight, R.S.G. Isolated visual symptoms at onset in sporadic Creutzfeldt-Jakob disease: The clinical phenotype of the “Heidenhain variant”. Br. J. Ophthalmol. 2005, 89, 1341–1342. [Google Scholar] [CrossRef] [PubMed]
- Baiardi, S.; Capellari, S.; Ladogana, A.; Strumia, S.; Santangelo, M.; Pocchiari, M.; Parchi, P. Revisiting the Heidenhain Variant of Creutzfeldt-Jakob Disease: Evidence for Prion Type Variability Influencing Clinical Course and Laboratory Findings. J. Alzheimer’s Dis. 2016, 50, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Leigh, D.; Bagg, C.E. A rare presenile dementia associated with cortical blindness (Heidenhain’s syndrome). J. Neurol. Neurosurg. Psychiatry 1954, 17, 129–133. [Google Scholar] [CrossRef]
- Parker, S.E.; Gujrati, M.; Pula, J.H.; Zallek, S.N.; Kattah, J.C. The Heidenhain Variant of Creutzfeldt-Jakob Disease—A Case Series. J. Neuro-Ophthalmol. 2014, 34, 4–9. [Google Scholar] [CrossRef]
- Finsterer, J.; Bancher, C.; Mamoli, B. Giant visually-evoked potentials without myoclonus in the Heidenhain type of Creutzfeld-Jakob disease. J. Neurol. Sci. 1999, 167, 73–75. [Google Scholar] [CrossRef]
- Pachalska, M.; Kurzbauer, H.; MacQueen, B.D.; Formińska-Kapuścik, M.; Herman-Sucharska, I. Neuropsychological features of rapidly progressive dementia in a patient with an atypical presentation of Creutzfeldt-Jakob Disease. Experiment 2001, 7, 1307–1315. [Google Scholar]
- Cornelius, J.R.; Boes, C.J.; Ghearing, G.; Leavitt, J.A.; Kumar, N. Visual symptoms in the Heidenhain variant of Creutzfeldt-Jakob Disease. J. Neuroimaging 2009, 19, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Appleby, B.S.; Appleby, K.K.; Crain, B.J.; Onyike, C.U.; Wallin, M.T.; Rabins, P.V. Characteristics of Established and Proposed Sporadic Creutzfeldt-Jakob Disease Variants. Arch. Neurol. 2009, 66, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Abbadessa, G.; Lavorgna, L.; Miele, G.; Cirillo, M.; Bonavita, S. Heidenhain variant of Creutzefeldt–Jackob disease in a patient carrying the V210I mutation with asymmetric MRI abnormalities. Acta Neurol. Belg. 2020, 120, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cui, Z.; Guomin, X.; Wang, H.; Zhang, X.; Li, Z.; Sun, Q.; Qi, F. Rare genetic E196A mutation in a patient with Creutzfeldt–Jakob disease: A case report and literature. Prion 2020, 14, 143–148. [Google Scholar] [CrossRef]
- Imbriani, P.; Marfia, G.A.; Marciani, M.G.; Poleggi, A.; Pocchiari, M.; Puoti, G.; Caltagirone, C.; Pisani, A. Heidenhain variant in two patients with inherited V210I Creutzfeldt-Jakob disease. Int. J. Neurosci. 2015, 126, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Tajima, Y.; Satoh, C.; Mito, Y.; Kitamoto, T. Creutzfeldt-Jakob Disease with a Codon 210 Mutation: First Pathological Observation in a Japanese Patient. Intern. Med. 2014, 53, 483–487. [Google Scholar] [CrossRef]
- Zerr, I.; Kallenberg, K.; Summers, D.M.; Romero, C.; Taratuto, A.; Heinemann, U.; Breithaupt, M.; Varges, D.; Meissner, B.; Ladogana, A.; et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009, 132, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- CDC’s Diagnostic Criteria for Creutzfeldt-Jakob Disease (CJD), 2018. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of High-Consequence Pathogens and Pathology (DHCPP). 2018. Available online: https://www.cdc.gov/prions/cjd/diagnostic-criteria.html (accessed on 15 August 2018).
- Ryu, D.W.; Hong, Y.J.; Park, J.W.; Lee, S.B.; Kim, S.H.; Kim, Y.; Seong, M.J.; Kim, B.S. Familial Creutzfeldt–Jakob Disease with a PRNP Mutation at Codon 180 Presented with Visual Hallucinations and Illusions. Dement. Neurocognitive Disord. 2019, 18, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cui, B.; Chu, M.; Cui, Y.; Jing, D.; Li, D.; Xie, K.; Kong, Y.; Xia, T.; Wang, C.; et al. The Frequency of Genetic Mutations Associated With Behavioral Variant Frontotemporal Dementia in Chinese Han Patients. Front. Aging Neurosci. 2021, 13, 699836. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Shi, Q.; Tian, C.; Chen, C.; Han, J.; Zhou, W.; Zhang, B.-Y.; Jiang, H.-Y.; Zhang, J.; Dong, X.-P. The Epidemiological, Clinical, and Laboratory Features of Sporadic Creutzfeldt-Jakob Disease Patients in China: Surveillance Data from 2006 to 2010. PLoS ONE 2011, 6, e24231. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, B.J.; Racker, S.; Herrendorf, G.; Poser, S.; Grosche, S.; Zerr, I.; Kretzschmar, H.; Weber, T. Accuracy and Reliability of Periodic Sharp Wave Complexes in Creutzfeldt-Jakob Disease. Arch. Neurol. 1996, 53, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Shi, Q.; Zhou, W.; Zhang, B.-Y.; Wang, Y.; Chen, C.; Ma, Y.; Gao, C.; Dong, X.-P. T188K-Familial Creutzfeldt-Jacob Disease, Predominant Among Chinese, has a Reactive Pattern in CSF RT-QuIC Different from D178N-Fatal Familial Insomnia and E200K-Familial CJD. Neurosci. Bull. 2019, 35, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhao, X.; Zhou, X.; Ye, X.; Yu, X.; Jiang, W.; Deng, Y.; Zhou, S.; Ma, L.; Shan, P.; et al. Epidemiological and Clinical Characteristics of Sporadic Creutzfeldt–Jakob Disease: A Retrospective Study in Eastern China. Front. Neurol. 2021, 12, 700485. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, A.; Pascuzzo, R.; Blevins, J.; Grisoli, M.; Lodi, R.; Moscatelli, M.; Castelli, G.; Cohen, M.L.; Schonberger, L.B.; Foutz, A.; et al. Evaluation of a New Criterion for Detecting Prion Disease With Diffusion Magnetic Resonance Imaging. JAMA Neurol. 2020, 77, 1141. [Google Scholar] [CrossRef] [PubMed]
- Shir, D.; Lazar, E.B.; Graff-Radford, J.; Aksamit, A.J.; Cutsforth-Gregory, J.K.; Jones, D.T.; Botha, H.; Ramanan, V.K.; Prusinski, C.; Porter, A.; et al. Analysis of Clinical Features, Diagnostic Tests, and Biomarkers in Patients With Suspected Creutzfeldt-Jakob Disease, 2014–2021. JAMA Netw. Open 2022, 5, e2225098. [Google Scholar] [CrossRef] [PubMed]
- Shiga, Y.; Miyazawa, K.; Sato, S.; Fukushima, R.; Shibuya, S.; Sato, Y.; Konno, H.; Doh-Ura, K.; Mugikura, S.; Tamura, H.; et al. Diffusion-weighted MRI abnormalities as an early diagnostic marker for Creutzfeldt-Jakob disease. Neurology 2004, 63, 443–449. [Google Scholar] [CrossRef]
- Zanusso, G.; Camporese, G.; Ferrari, S.; Santelli, L.; Bongianni, M.; Fiorini, M.; Monaco, S.; Manara, R.; Cagnin, A. Long-term preclinical magnetic resonance imaging alterations in sporadic Creutzfeldt-Jakob disease. Ann. Neurol. 2016, 80, 629–632. [Google Scholar] [CrossRef]
- Satoh, K.; Shirabe, S.; Tsujino, A.; Eguchi, H.; Motomura, M.; Honda, H.; Tomita, I.; Satoh, A.; Tsujihata, M.; Matsuo, H.; et al. Total Tau Protein in Cerebrospinal Fluid and Diffusion-Weighted MRI as an Early Diagnostic Marker for Creutzfeldt-Jakob Disease. Dement. Geriatr. Cogn. Disord. 2007, 24, 207–212. [Google Scholar] [CrossRef]
- Yasuda, M.; Sugiyama, A.; Hokkoku, H.; Suichi, T.; Ito, K.; Satoh, K.; Kitamoto, T.; Kuwabara, S. Propagation of Diffusion-Weighted MRI Abnormalities in the Preclinical Stage of Sporadic Creutzfeldt-Jakob Disease. Neurology 2022, 99, 699–702. [Google Scholar] [CrossRef]
- Hermann, P.; Appleby, B.; Brandel, J.-P.; Caughey, B.; Collins, S.; Geschwind, M.D.; Green, A.; Haïk, S.; Kovacs, G.G.; Ladogana, A.; et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 2021, 20, 235–246. [Google Scholar] [CrossRef]
- Minikel, E.V.; Vallabh, S.M.; Lek, M.; Estrada, K.; Samocha, K.E.; Sathirapongsasuti, J.F.; McLean, C.Y.; Tung, J.Y.; Yu, L.P.C.; Gambetti, P.; et al. Quantifying prion disease penetrance using large population control cohorts. Sci. Transl. Med. 2016, 8, 322ra9. [Google Scholar] [CrossRef]
- Kim, M.O.; Takada, L.T.; Wong, K.; Forner, S.A.; Geschwind, M.D. Genetic PrP Prion Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033134. [Google Scholar] [CrossRef] [PubMed]
- Capellari, S.; Strammiello, R.; Saverioni, D.; Kretzschmar, H.; Parchi, P. Genetic Creutzfeldt–Jakob disease and fatal familial insomnia: Insights into phenotypic variability and disease pathogenesis. Acta Neuropathol. 2010, 121, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Ladogana, A.; Puopolo, M.; Poleggi, A.; Almonti, S.; Mellina, V.; Equestre, M.; Pocchiari, M. High incidence of genetic human transmissible spongiform encephalopathies in Italy. Neurology 2005, 64, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Puopolo, M.; Ladogana, A.; Pocchiari, M.; Budka, H.; Van Duijn, C.; Collins, S.J.; Boyd, A.; Giulivi, A.; Coulthart, M.; et al. Genetic prion disease: The EUROCJD experience. Hum. Genet. 2005, 118, 166–174. [Google Scholar] [CrossRef]
- Krasnianski, A.; Heinemann, U.; Ponto, C.; Kortt, J.; Kallenberg, K.; Varges, D.; Schulz-Schaeffer, W.J.; Kretzschmar, H.A.; Zerr, I. Clinical findings and diagnosis in genetic prion diseases in Germany. Eur. J. Epidemiol. 2015, 31, 187–196. [Google Scholar] [CrossRef]
- Gandoglia, I.; Strada, L.; Poleggi, A.; Castaldi, A.; Del Sette, M.; Di Maria, E. Penetrance of the V203I variant of the PRNP gene: Report of a patient with stroke-like onset of Creutzfeld-Jacob Disease and review of published cases. Prion 2022, 16, 19–22. [Google Scholar] [CrossRef]
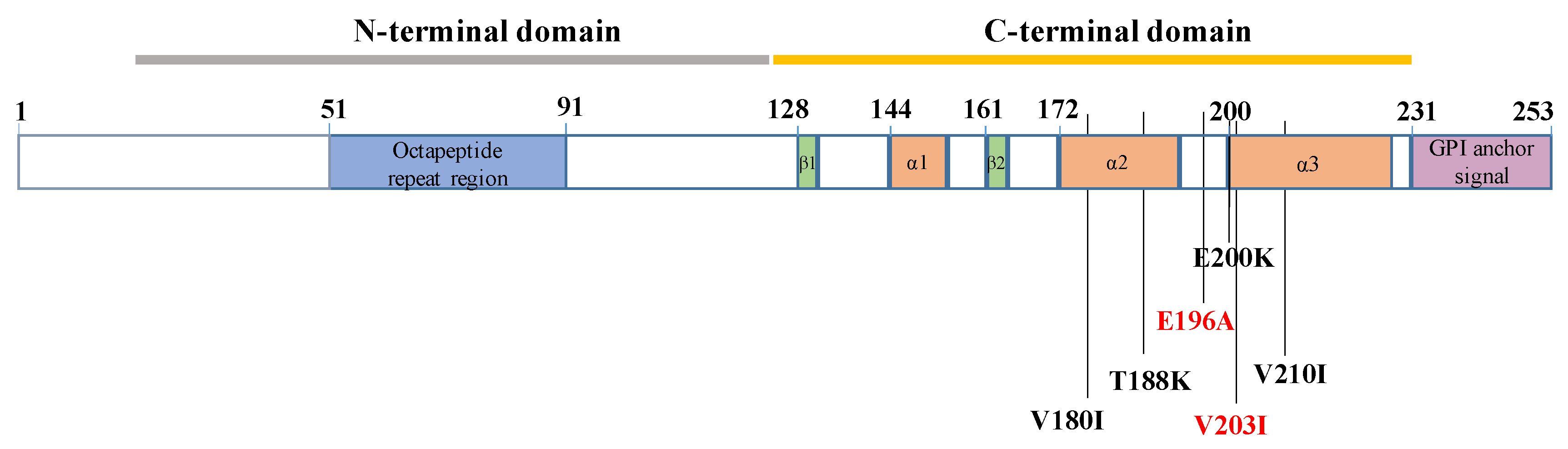
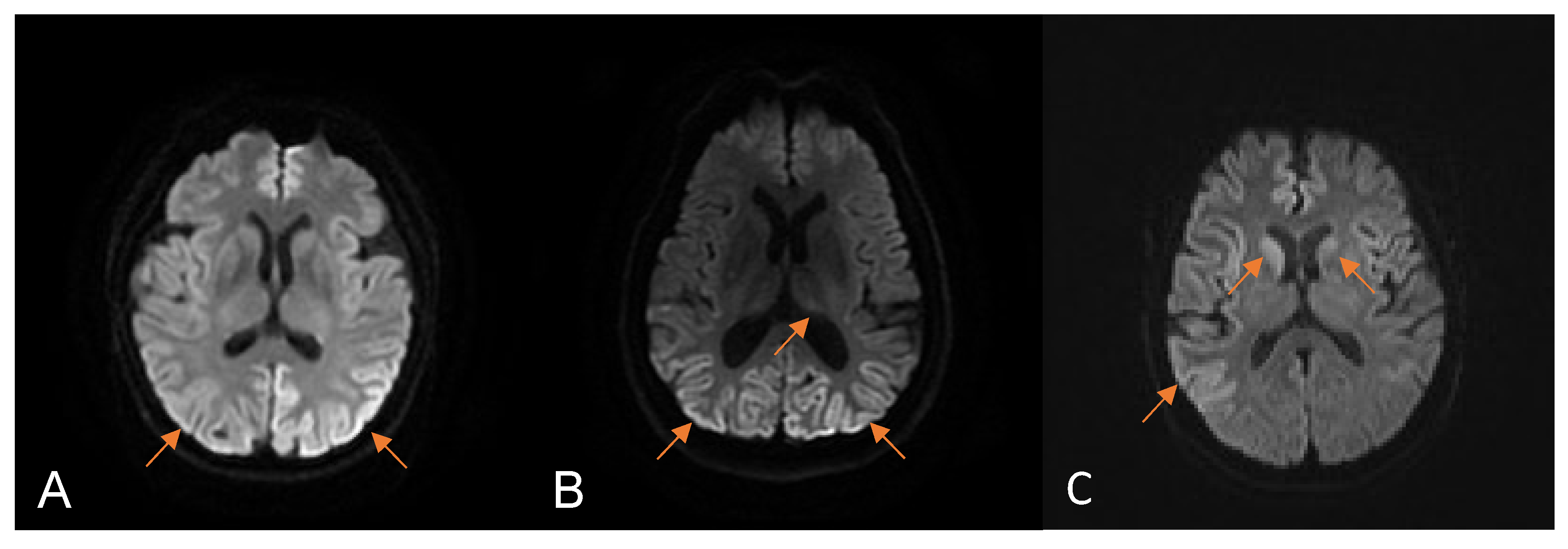
| Variables | Total HvCJD (N = 18) |
|---|---|
| Female, n (%) | 8 (44.4%) |
| Age at onset, mean ± SD, years | 61.8 ± 7.7 |
| Family history, n (%) | 0 (0%) |
| Duration of isolated visual symptoms, median (IQR), days | 30.0 (14.8–40.0) |
| Survival time, median (IQR), months | 7.0 (4.0–16.0) |
| Initial visual symptom | |
| Blurred vision, n (%) | 15 (83.3%) |
| Diplopia, n (%) | 2 (11.1%) |
| Metamorphopsia, n (%) | 1 (5.6%) |
| Visual symptoms during the entire course | |
| Blurred vision, n (%) | 16 (88.9%) |
| Diplopia, n (%) | 3 (16.7%) |
| Visual hallucination, n (%) | 5 (27.8%) |
| Cortical blindness, n (%) | 1 (5.6%) |
| Visuospatial dysfunction, n (%) | 1 (5.6%) |
| Hemianopsia, n (%) | 2 (11.1%) |
| Metamorphopsia, n (%) | 3 (16.7%) |
| Dyschromatopsia, n (%) | 1 (5.6%) |
| The first non-visual symptom | |
| Cognitive decline, n (%) | 8 (44.4%) |
| Unsteady gait, n (%) | 6 (33.3%) |
| Psychiatric symptoms, n (%) | 1 (5.6%) |
| Sensory disturbance, n (%) | 1 (5.6%) |
| Speech disorder, n (%) | 1 (5.6%) |
| Extrapyramidal signs, n (%) | 1 (5.6%) |
| Auxiliary examinations | |
| Restricted diffusion in the first MRI, n (%) | 13 (72.2%) |
| MRI fulfilled the WHO criteria for CJD, n (%) | 10 (55.6%) |
| Restricted diffusion on occipital lobe, n (%) | 10 (55.6%) |
| Restricted diffusion in the serial MRI, n (%) | 17 (94.4%) |
| MRI fulfilled the WHO criteria for CJD, n (%) | 16 (88.9%) |
| Restricted diffusion on occipital lobe, n (%) | 14 (77.8%) |
| PSWCs on initial EEG, n (%) | 7 (38.9%) |
| PSWCs on the serial EEG, n (%) | 11 (61.1%) |
| Positive CSF 14-3-3 protein, n/total (%) | 9/14 (64.3%) |
| CSF RT-QuIC, n/total (%) | 1/1 (100%) |
| Hypometabolism on PET, n/total (%) | 3/3 (100%) |
| PRNP mutation, n (%) | 3 (16.7%) |
| MM homozygote at codon 129, n (%) | 18 (100%) |
| Variables | Case 1 | Case 2 | Case 3 | Case 4 [15] | Case 5 [14] | Case 6 [14] | Case 7 [18] | Case 8 [12] | Case 9 [13] |
|---|---|---|---|---|---|---|---|---|---|
| Sex | Female | Male | Female | Female | Male | Female | Male | Female | Male |
| Age at onset, years | 66 | 57 | 63 | 54 | 59 | 66 | 78 | 69 | 42 |
| Family history | − | − | − | − | + | + | − | ND | − |
| Duration of isolated visual symptoms, days | 14 | 120 | 40 | 21 | 21 | 14 | 30 | 28 | ND |
| Survival time, months | 4 (alive) | 16 | 18 | 19 | 3 | 9 | ND | 2 | ND |
| Initial visual symptom | |||||||||
| Blurred vision | + | + | − | − | − | − | − | − | − |
| Diplopia | − | − | + | + | − | − | − | − | − |
| Visuospatial dysfunction | − | − | − | − | − | − | − | − | + |
| Hemianopsia | − | − | − | − | + | − | − | − | − |
| Metamorphopsia | − | − | − | + | − | + | − | + | − |
| Dyschromatopsia | − | − | − | − | − | − | − | − | + |
| Visual hallucination | − | − | − | − | − | − | + | − | − |
| Visual symptoms during the entire course | |||||||||
| Blurred vision | + | + | − | − | − | − | − | + | − |
| Diplopia | − | − | + | + | − | − | − | − | − |
| Visual hallucination | − | − | − | − | + | + | + | − | − |
| Cortical blindness | + | − | − | − | + | − | − | − | + |
| Visuospatial dysfunction | − | − | − | − | − | − | + | − | + |
| Hemianopsia | − | − | − | − | + | − | − | − | − |
| Metamorphopsia | − | − | − | + | − | + | − | + | + |
| Dyschromatopsia | − | − | − | − | − | − | − | − | + |
| Auxiliary examinations | |||||||||
| OCT | − | ND | ND | ND | ND | ND | ND | ND | ND |
| Visual field test | ND | ND | ND | ND | ND | + | ND | ND | ND |
| VEP | + | ND | ND | ND | ND | ND | ND | ND | ND |
| Restricted diffusion in the first MRI | + | + | − | + | − | + | + | − | + |
| MRI fulfilled the WHO criteria for CJD | − | + | − | + | − | + | + | − | + |
| Restricted diffusion on occipital lobe | + | + | − | + | − | − | + | − | + |
| Restricted diffusion on basal ganglia | − | − | − | + | − | + | − | − | − |
| Restricted diffusion on thalamus | − | + | − | − | − | − | − | − | − |
| Restricted diffusion in the subsequent MRI | + | + | + | + | ND | ND | ND | + | ND |
| MRI fulfilled the WHO criteria for CJD | + | + | + | + | + | ||||
| Restricted diffusion on occipital lobe | + | + | + | + | − | ||||
| Restricted diffusion on basal ganglia | − | − | + | + | + | ||||
| Restricted diffusion on thalamus | − | + | − | − | − | ||||
| PSWCs on initial EEG | − | − | + | − | + | ND | − | − | + |
| PSWCs on subsequent EEG | ND | ND | ND | + | ND | ND | ND | ND | ND |
| Positive CSF 14-3-3 protein | + | + | ND | ND | − | + | + | + | + |
| RT-QuIC | + | ND | ND | ND | + | + | − | ND | ND |
| Hypometabolism on PET | + | ND | ND | ND | ND | ND | ND | ND | ND |
| PRNP mutation | T188K | E200K | V203I | V210I | V210I | V210I | V180I | V210I | E196A |
| MM homozygote at codon 129 | MM | MM | MM | MM | MM | MM | MM | MM | MM |
| Variables | Genetic HvCJD (n = 9) | Sporadic HvCJD (n = 15) | p Value |
|---|---|---|---|
| Female, n (%) | 5 (55.6%) | 6 (40%) | 0.675 |
| Age, mean ± SD, years | 61.6 ± 10.2 | 61.8 ± 8.4 | 0.950 |
| Family history | 2 (22.2%) | 0 (0%) | 0.111 |
| Initial visual symptom | |||
| Blurred vision, n (%) | 2 (22.2%) | 13 (86.7%) | 0.003 |
| Diplopia, n (%) | 2 (22.2%) | 1 (6.7%) | 0.533 |
| Visuospatial dysfunction, n (%) | 1 (11.1%) | 0 (0%) | 0.375 |
| Hemianopsia, n (%) | 1 (11.1%) | 0 (0%) | 0.375 |
| Metamorphopsia, n (%) | 3 (33.3%) | 1 (6.7%) | 0.130 |
| Dyschromatopsia, n (%) | 1 (11.1%) | 0 (0%) | 0.375 |
| Visual hallucination, n (%) | 1 (11.1%) | 0 (0%) | 0.375 |
| Visual symptoms during the CJD course | |||
| Blurred vision, n (%) | 3 (33.3%) | 14 (93.3%) | 0.004 |
| Diplopia, n (%) | 2 (22.2%) | 2 (13.3%) | 0.615 |
| Visual hallucination, n (%) | 3 (33.3%) | 5 (33.3%) | 1.000 |
| Cortical blindness, n (%) | 3 (33.3%) | 0 (0%) | 0.042 |
| Visuospatial dysfunction, n (%) | 2 (22.2%) | 1 (6.7%) | 0.533 |
| Hemianopsia, n (%) | 1 (11.1%) | 2 (13.3%) | 1.000 |
| Metamorphopsia, n (%) | 4 (44.4%) | 3 (20%) | 0.356 |
| Dyschromatopsia, n (%) | 1 (11.1%) | 1 (6.7%) | 1.000 |
| Non-visual neuropsychiatric symptoms | |||
| Cognitive decline, n (%) | 7 (77.8%) | 15 (100%) | 0.130 |
| Myoclonus, n (%) | 6 (66.7%) | 10 (66.7%) | 1.000 |
| Pyramidal signs, n (%) | 2 (22.2%) | 11 (73.3%) | 0.033 |
| Extrapyramidal signs, n (%) | 5 (55.6%) | 10 (66.7%) | 0.678 |
| Cerebellar signs, n (%) | 5 (55.6%) | 9 (60%) | 1.000 |
| Epilepsy, n (%) | 2 (22.2%) | 2 (13.3%) | 0.615 |
| Psychiatric symptoms, n (%) | 5 (55.6%) | 4 (26.7%) | 0.212 |
| Akinetic mutism, n (%) | 5 (55.6%) | 5 (33.3%) | 0.403 |
| Auxiliary examinations | |||
| Typical hyperintensity on DWI, n (%) | 8 (88.9%) | 14 (93.3%) | 1.000 |
| DWI hyperintensity on occipital lobe, n (%) | 6 (66.7%) | 11 (73.3%) | 1.000 |
| DWI hyperintensity on basal ganglia, n (%) | 5 (55.6%) | 6 (40%) | 0.675 |
| PSWC on EEG, n/total (%) | 4/8 (50%) | 10/15 (66.7%) | 0.657 |
| Positive CSF 14-3-3 protein, n/total (%) | 6/7 (85.7%) | 7/12 (58.3%) | 0.333 |
| MM homozygote at codon 129, n/total (%) | 8/8 (100%) | 15/15 (100%) | 1.000 |
| Duration of isolated visual symptoms, median(IQR), days | 24.5 (15.8–37.5) | 30.0 (15.0–30.0) | 0.925 |
| Survival time, median (IQR), months | 12.5 (2.8–18.3) | 7.0 (4.0–10.0) | 0.525 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.; Chen, Z.; Zhang, J.; Wang, X.; Wu, L. Clinical and Genetic Characteristics of the Heidenhain Variant of Creutzfeldt–Jakob Disease. Viruses 2023, 15, 1092. https://doi.org/10.3390/v15051092
Kong Y, Chen Z, Zhang J, Wang X, Wu L. Clinical and Genetic Characteristics of the Heidenhain Variant of Creutzfeldt–Jakob Disease. Viruses. 2023; 15(5):1092. https://doi.org/10.3390/v15051092
Chicago/Turabian StyleKong, Yu, Zhongyun Chen, Jing Zhang, Xue Wang, and Liyong Wu. 2023. "Clinical and Genetic Characteristics of the Heidenhain Variant of Creutzfeldt–Jakob Disease" Viruses 15, no. 5: 1092. https://doi.org/10.3390/v15051092
APA StyleKong, Y., Chen, Z., Zhang, J., Wang, X., & Wu, L. (2023). Clinical and Genetic Characteristics of the Heidenhain Variant of Creutzfeldt–Jakob Disease. Viruses, 15(5), 1092. https://doi.org/10.3390/v15051092




