Impact of SARS-CoV-2 Infection on Unvaccinated Pregnant Women: Non-Reassuring Fetal Heart Rate Tracing Because of Placentitis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Eligibility and Exclusion Criteria
- −
- age ≥ 18 years old;
- −
- maternal SARS-CoV-2 infection confirmed by RT-PCR on nasopharyngeal swab according to routine care;
- −
- delivery because of NRHFR tracing, after 24 weeks of pregnancy;
- −
- in utero fetal death;
- −
- placenta abruptio or ongoing severe preeclampsia [13];
- −
- Benckiser hemorrhage;
- −
- uterine rupture;
- −
- painful uterine contractions or labor in progress.
2.3. Data
2.4. Statistical Analyses
2.5. Ethics Statement
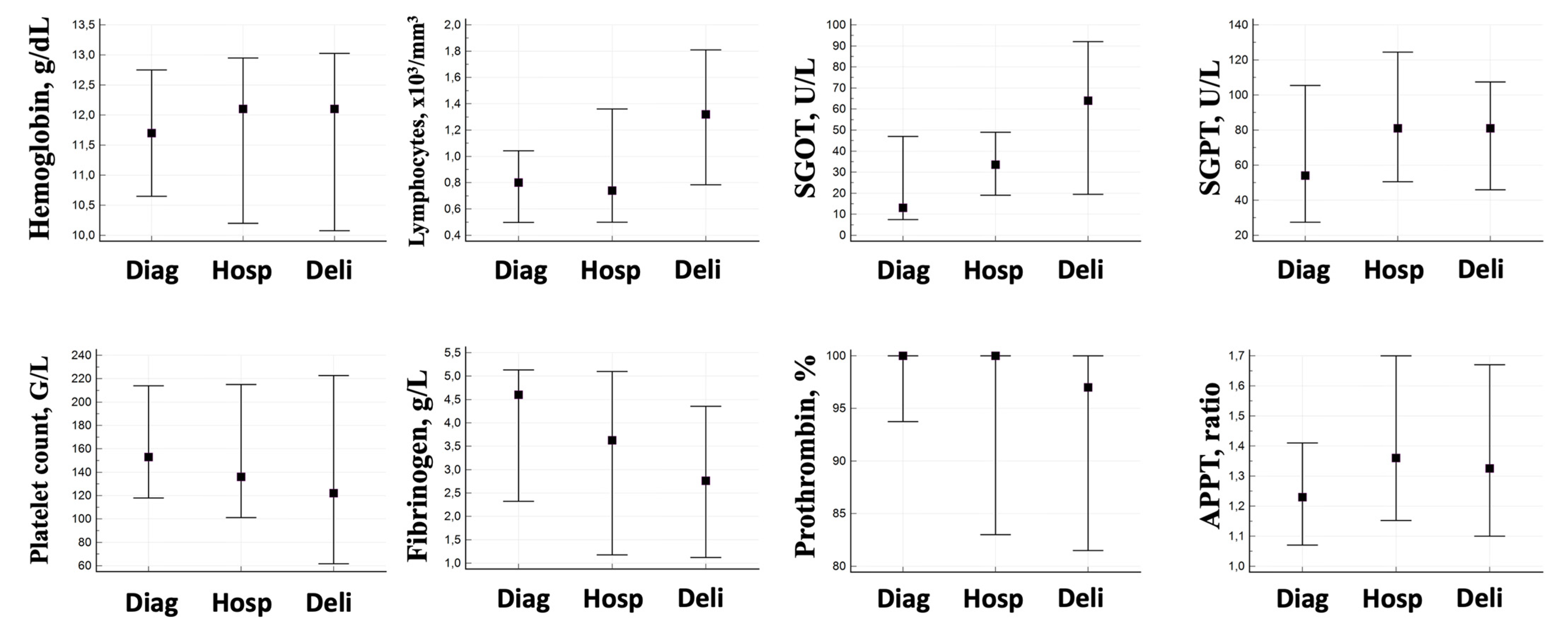
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| CTG | cardiotocographic |
| NRFHR | non-reassuring fetal heart rate |
| CEGORIF | Cercle d’Étude des Gynécologues-Obstétriciens de la Région Ile-de-France |
| APHP | Assistance Publique des Hôpitaux de Paris |
| BMI | body mass index |
| APTT | activated partial thromboplastin time |
| SGOT | serum glutamic-oxaloacetic transaminase |
| SGPT | serum glutamic-pyruvic transaminase |
| WHO | World Health Organization |
| NIH | National Institutes of Health |
| IQR | interquartile range |
| ICU | intensive care unit |
| RT-PCR | reverse transcription polymerase chain reaction |
| HELLP syndrom | hemolysis, elevated liver enzymes and low platelets syndrom |
| ACE2 | angiotensin converting enzyme 2 |
| TMPRSS2 | transmembrane protease serine 2 |
References
- Badr, D.A.; Picone, O.; Bevilacqua, E.; Carlin, A.; Meli, F.; Sibiude, J.; Mattern, J.; Fils, J.-F.; Mandelbrot, L.; Lanzone, A.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 and Pregnancy Outcomes According to Gestational Age at Time of Infection. Emerg. Infect. Dis. 2021, 27, 2535–2543. [Google Scholar] [CrossRef]
- Marchand, G.; Patil, A.S.; Masoud, A.T.; Ware, K.; King, A.; Ruther, S.; Brazil, G.; Calteux, N.; Ulibarri, H.; Parise, J.; et al. Systematic review and meta-analysis of COVID-19 maternal and neonatal clinical features and pregnancy outcomes up to June 3, 2021. AJOG Glob. Rep. 2022, 2, 100049. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Vale, M.S.D.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality among Pregnant Women with and without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Badr, D.A.; Mattern, J.; Carlin, A.; Cordier, A.-G.; Maillart, E.; El Hachem, L.; El Kenz, H.; Andronikof, M.; De Bels, D.; Damoisel, C.; et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am. J. Obstet. Gynecol. 2020, 223, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Favre, G.; Mazzetti, S.; Gengler, C.; Bertelli, C.; Schneider, J.; Laubscher, B.; Capoccia, R.; Pakniyat, F.; Ben Jazia, I.; Eggel-Hort, B.; et al. Decreased Fetal Movements: A Sign of Placental SARS-CoV-2 Infection with Perinatal Brain Injury. Viruses 2021, 13, 2517. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do, C.a.o.J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Vauloup-Fellous, C.; Escourrou, G.; Rosenblatt, J.; Jouannic, J.M.; Laurent-Bellue, A.; De Luca, D. Factors associated with SARS-CoV-2 transplacental transmission. Am. J. Obstet. Gynecol. 2022, 227, 541–543.e11. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Baldewijns, M.; Benachi, A.; Bugatti, M.; Collins, R.R.J.; De Luca, D.; Facchetti, F.; Linn, R.L.; Marcelis, L.; Morotti, D.; et al. Chronic Histiocytic Intervillositis with Trophoblast Necrosis Is a Risk Factor Associated with Placental Infection from Coronavirus Disease 2019 (COVID-19) and Intrauterine Maternal-Fetal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission in Live-Born and Stillborn Infants. Arch. Pathol. Lab. Med. 2021, 145, 517–528. [Google Scholar]
- DeSisto, C.L.; Wallace, B.; Simeone, R.M.; Polen, K.; Ko, J.Y.; Meaney-Delman, D.; Ellington, S.R. Risk for Stillbirth among Women with and without COVID-19 at Delivery Hospitalization—United States, March 2020–September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1640–1645. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Avvad-Portari, E.; Babál, P.; Baldewijns, M.; Blomberg, M.; Bouachba, A.; Camacho, J.; Collardeau-Frachon, S.; Colson, A.; Dehaene, I.; et al. Placental Tissue Destruction and Insufficiency from COVID-19 Causes Stillbirth and Neonatal Death from Hypoxic-Ischemic Injury. Arch. Pathol. Lab. Med. 2022, 146, 660–676. [Google Scholar] [CrossRef]
- Mongula, J.E.; Frenken, M.W.E.; van Lijnschoten, G.; Arents, N.L.A.; de Wit-Zuurendonk, L.D.; Kok, A.P.A.S.-D.; van Runnard Heimel, P.J.; Porath, M.M.; Goossens, S.M.T.A. COVID-19 during pregnancy: Non-reassuring fetal heart rate, placental pathology and coagulopathy. Ultrasound Obstet. Gynecol. 2020, 56, 773–776. [Google Scholar] [CrossRef]
- Gracia-Perez-Bonfils, A.; Martinez-Perez, O.; Llurba, E.; Chandraharan, E. Fetal heart rate changes on the cardiotocograph trace secondary to maternal COVID-19 infection. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 286–293. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Brown, M.A.; Zeeman, G.G.; Dekker, G.; Sibai, B.M. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens Int. J. Women’s Cardiovasc. Health 2013, 3, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Mamelle, N.; Munoz, F.; Grandjean, H. Fetal growth from the AUDIPOG study. I. Establishment of reference curves. J. Gynecol. Obstet. Biol. Reprod. (Paris) 1996, 25, 61–70. [Google Scholar] [PubMed]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed]
- NIH COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 26 January 2023).
- Salomon, L.J.; Bernard, J.P.; Ville, Y. Estimation of fetal weight: Reference range at 20–36 weeks’ gestation and comparison with actual birth-weight reference range. Ultrasound Obstet. Gynecol. 2007, 29, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Vlachodimitropoulou Koumoutsea, E.; Vivanti, A.J.; Shehata, N.; Benachi, A.; Le Gouez, A.; Desconclois, C.; Whittle, W.; Snelgrove, J.; Malinowski, A.K. COVID-19 and acute coagulopathy in pregnancy. J. Thromb. Haemost. 2020, 18, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Futterman, I.; Toaff, M.; Navi, L.; Clare, C.A. COVID-19 and HELLP: Overlapping Clinical Pictures in Two Gravid Patients. Am. J. Perinatol. Rep. 2020, 10, e179–e182. [Google Scholar] [CrossRef] [PubMed]
- Sichitiu, J.; Bourgon, N.; Guilleminot, T.; Bessieres, B.; Leruez-Ville, M.; Ville, Y. Third trimester placentitis: An underreported complication of SARS-CoV-2 infection. Am. J. Obstet. Gynecol. MFM 2022, 4, 100703. [Google Scholar] [CrossRef]
- Alcover, N.; Regiroli, G.; Benachi, A.; Vauloup-fellous, C.; Vivanti, A.J.; De Luca, D. Systematic review and synthesis of stillbirths and late miscarriages following SARS-CoV-2 infections. Am. J. Obstet. Gynecol. 2023, S0002937823000261. [Google Scholar] [CrossRef]
- Hui, L.; Marzan, M.B.; Rolnik, D.L.; Potenza, S.; Pritchard, N.; Said, J.M.; Palmer, K.R.; Whitehead, C.L.; Sheehan, P.M.; Ford, J.; et al. Reductions in stillbirths and preterm birth in COVID-19 vaccinated women: A multi-center cohort study of vaccination uptake and perinatal outcomes. Am. J. Obstet. Gynecol. 2022, S0002937822008821. [Google Scholar] [CrossRef]
- Kim, H. Impact of vaccination and the omicron variant on COVID-19 severity in pregnant women. Am. J. Infect. Control 2023, 51, 351–353. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Morotti, D.; Beigi, B.; Moshfegh, F.; Zafaranloo, N.; Patanè, L. Confirming Vertical Fetal Infection with Coronavirus Disease 2019: Neonatal and Pathology Criteria for Early Onset and Transplacental Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 from Infected Pregnant Mothers. Arch. Pathol. Lab. Med. 2020, 144, 1451–1456. [Google Scholar] [CrossRef]
- Sinaci, S.; Ocal, D.F.; Tokalioglu, E.O.; Ozturk, F.H.; Senel, S.A.; Keskin, L.H.; Tekin, O.M.; Sahin, D. Cardiotocographic features in COVID-19 infected pregnant women. J. Périnat. Med. 2021, 50, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, E.; Jang, M.; Burd, I. COVID-19 in pregnancy: Placental and neonatal involvement. Am. J. Reprod. Immunol. 2020, 84, e13306. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Watkins, J.C.; Torous, V.F.; Roberts, D.J. Defining Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Placentitis. Arch. Pathol. Lab. Med. 2021, 145, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Puri, M.; Agarwal, K.; Singh, S.; Yadav, R.; Tiwary, N.; Tayal, P.; Vats, B. COVID-19 as an independent risk factor for subclinical placental dysfunction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 7–11. [Google Scholar] [CrossRef]
- Bouachba, A.; Allias, F.; Nadaud, B.; Massardier, J.; Mekki, Y.; Duchamp, M.B.; Fourniere, B.D.; Huissoud, C.; Trecourt, A.; Collardeau-Frachon, S. Placental lesions and SARS-Cov-2 infection: Diffuse placenta damage associated to poor fetal outcome. Placenta 2021, 112, 97–104. [Google Scholar] [CrossRef]
- Mosnino, E.; Bernardes, L.S.; Mattern, J.; Hipólito Micheletti, B.; Aparecida de Castro Maldonado, A.; Vauloup-Fellous, C.; Doucet-Populaire, F.; De Luca, D.; Benachi, A.; Vivanti, A.J. Impact of SARS-CoV-2 Alpha and Gamma Variants among Symptomatic Pregnant Women: A Two-Center Retrospective Cohort Study between France and Brazil. J. Clin. Med. 2022, 11, 2663. [Google Scholar] [CrossRef]
| Clinical Characteristics (n = 17) | |
|---|---|
| Maternal age | 36.0 (30.0–38.0) |
| Maternal BMI, kg/m2 | 25.0 (23.0–28.0) |
| Maternal BMI > 30 kg/m2 | 4/17 (23.5) |
| Geographic origin | |
| Europe | 9/17 (52.9) |
| America | 2/17 (11.8) |
| North Africa | 4/17 (23.5) |
| South Africa | 2/17 (11.8) |
| Previous pregnancies | |
| Nulliparous | 4/17 (23.5) |
| Multiparous | 13/17 (76.5) |
| Maternal comorbidities | |
| Smoking | 2/17 (11.8) |
| Pregestational chronic hypertension | 2/17 (11.8) |
| Pregestational diabetes mellitus | 0 |
| Pregestational liver disease 1 | 2/17 (11.8) |
| Asthma | 3/17 (17.6) |
| Other 2 | 3/17 (17.6) |
| Complete anti-SARS-CoV-2 vaccination | 0 |
| Pregnancy characteristics | |
| Gestational diabetes | 2/17 (11.8) |
| Moderate pre-eclampsia | 1/17 (5.9) |
| Gravidic cholestasis | 1/17 (5.9) |
| Characteristics of SARS-CoV-2 Infection (n = 17) | |
|---|---|
| Gestational age at diagnosis, weeks | 326/7 (305/7–352/7) |
| Symptoms | |
| Cough | 8/17 (47) |
| Fever | 12/17 (70.5) |
| Dyspnea | 3/17 (17.6) |
| Headache | 1/17 (5.8) |
| Nausea/vomiting | 1/17 (5.8) |
| Myalgia | 3/17 (17.6) |
| Anosmia/ageusia | 2/17 (11.8) |
| WHO classification | |
| No symptoms | 0 |
| Mild | 12/17 (70.5) |
| Moderate | 3/17 (17.6) |
| Severe | 2/17 (11.8) |
| Implicated viral strains | |
| Performed and reported (n = 7) Wild type (Wuhan-China) B.1.1.7 “Alpha” (UK strain) B.1.617.2 “Delta” (India) | 1/7 (14.2) 5/7 (71.4) 1/7 (14.2) |
| Unknown | 10/17 (58.8) |
| Obstetrical symptoms | |
| Uterine contractions | 0 |
| Decreased fetal movements | 2/17 (11.8) |
| Premature rupture of membranes | 0 |
| Metrorrhagia | 0 |
| Hospitalization | |
| Interval between diagnosis and hospitalization (day, median IQR) | 4 (2–6) |
| Gestational age at hospitalization | 335/7 (313/7–356/7) |
| Conventional hospitalization | 15/17 (88.2) |
| Admission to ICU | 2/17 (11.8) |
| Need for oxygen support | 2/17 (11.8) |
| Orotracheal intubation | 2/17 (11.8) |
| Obstetrical and Neonatal Characteristics | |
|---|---|
| Delivery (n = 17) | |
| Cesarean delivery | 17 (100) |
| Gestational age at delivery, weeks | 336/7 (312/7–354/7) |
Prematurity
| 1/17 (6) 5/17 (29) 9/17 (53) |
| Interval between hospitalization and delivery, days | 5.0 (3.0–7.0) |
| Neonatal ouctomes | |
| Birthweight, g | 2090 (1480–2200) |
| Birthweight, Z-score | −0.1 (−1.3–0.1) |
Birthweight, Z-score (no, %)
| 5/17 (29) 2/17 (11.8) |
| <5 min Apgar score | 6/15 (40) |
| Respiratory distress | 11/17 (64.7) |
| NICU admission | 12/17 (70.6) |
| Neonatal death | 1/17 (5.9) |
| Umbilical cord pH (n = 15) | |
| Median, IQR | 7.2 (7.1–7.3) |
Acidosis (No., %)
| 6/15 (40) 1/15 (7) |
| Neonatal SARS-CoV-2 RT-PCR by nasopharyngeal swab (n = 7) | |
| Positive | 3/7 (42.8) |
| Negative | 4/7 (57.1) |
| Placental analysis (n = 15) | |
| Placental SARS-CoV-2 identification (n = 8/15) | |
| Positive | 8/15 (47) |
| Negative | 0 |
| Placentitis | |
| Fibrin deposition | 10/15 (67) |
| Chronic intervillositis | 10/15 (67) |
| Trophoblast necrosis | 9/15 (60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claudet, A.; De Luca, D.; Mosnino, E.; Mattern, J.; Picone, O.; Sibiude, J.; Wafo, E.; Tsatsaris, V.; Giral, E.; Grefenstette, I.; et al. Impact of SARS-CoV-2 Infection on Unvaccinated Pregnant Women: Non-Reassuring Fetal Heart Rate Tracing Because of Placentitis. Viruses 2023, 15, 1069. https://doi.org/10.3390/v15051069
Claudet A, De Luca D, Mosnino E, Mattern J, Picone O, Sibiude J, Wafo E, Tsatsaris V, Giral E, Grefenstette I, et al. Impact of SARS-CoV-2 Infection on Unvaccinated Pregnant Women: Non-Reassuring Fetal Heart Rate Tracing Because of Placentitis. Viruses. 2023; 15(5):1069. https://doi.org/10.3390/v15051069
Chicago/Turabian StyleClaudet, Alexandra, Daniele De Luca, Elie Mosnino, Jérémie Mattern, Olivier Picone, Jeanne Sibiude, Estelle Wafo, Vassilis Tsatsaris, Emilie Giral, Irène Grefenstette, and et al. 2023. "Impact of SARS-CoV-2 Infection on Unvaccinated Pregnant Women: Non-Reassuring Fetal Heart Rate Tracing Because of Placentitis" Viruses 15, no. 5: 1069. https://doi.org/10.3390/v15051069
APA StyleClaudet, A., De Luca, D., Mosnino, E., Mattern, J., Picone, O., Sibiude, J., Wafo, E., Tsatsaris, V., Giral, E., Grefenstette, I., Carrara, J., Badr, D. A., Saint-Frison, M.-H., Prevot, S., Benachi, A., & Vivanti, A. J. (2023). Impact of SARS-CoV-2 Infection on Unvaccinated Pregnant Women: Non-Reassuring Fetal Heart Rate Tracing Because of Placentitis. Viruses, 15(5), 1069. https://doi.org/10.3390/v15051069







