Vector Competence for Zika Virus Changes Depending on the Aedes aegypti’s Region of Origin in Manaus: A Study of an Endemic Brazilian Amazonian City
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Collection
2.2. ZIKV Culture
2.3. ZIKV Infection of Aedes aegypti
2.4. Mosquito Dissection and Viral RNA Extraction
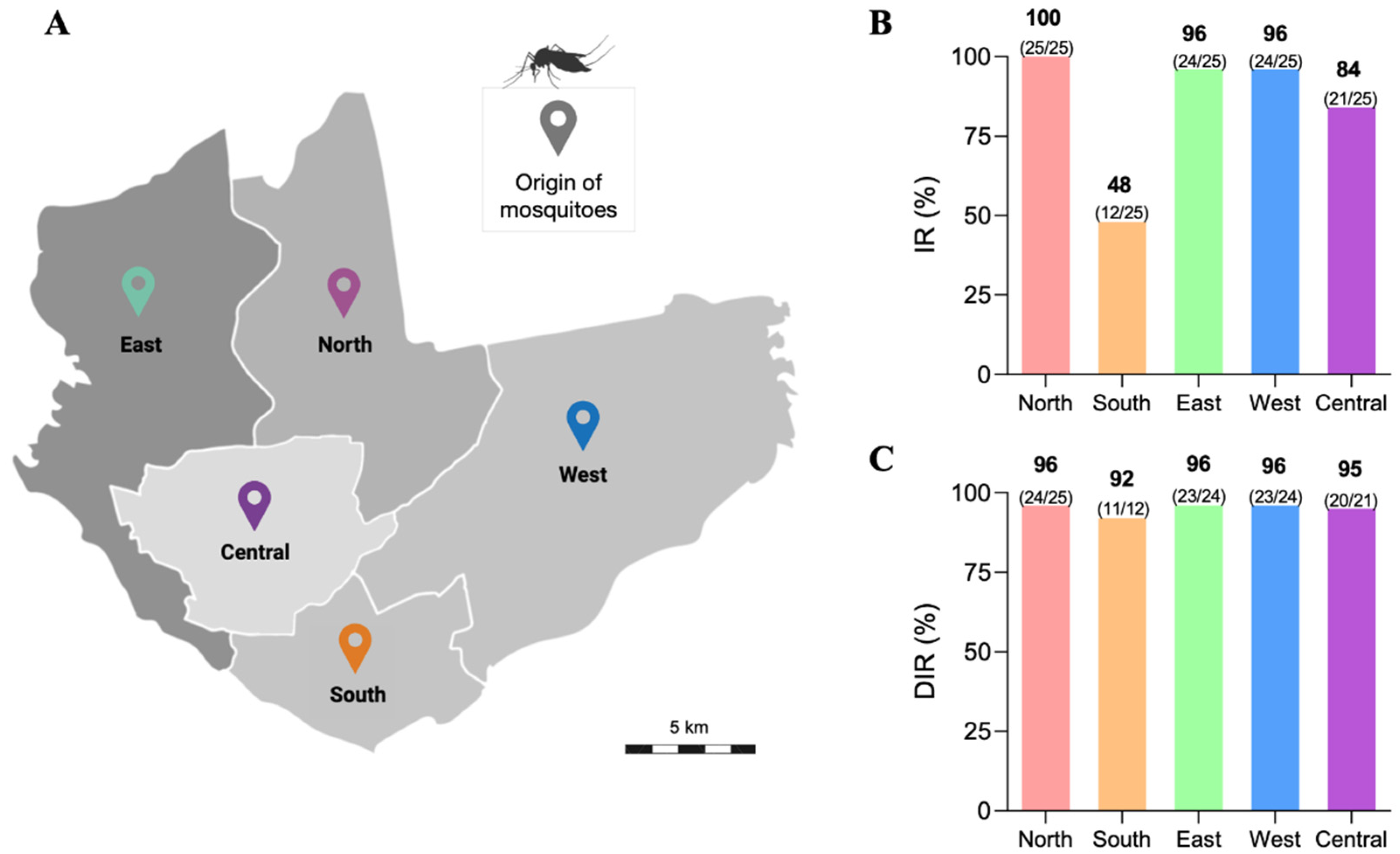
2.5. Infection Rate (IR), Disseminated Infection Rate (DIR), Transmission Rate (TR), and Transmission Efficiency (TE)
2.6. Ethical Approval
2.7. Statistical Analyses
3. Results
3.1. Infection Rate (IR) and Disseminated Infection Rate (DIR) of the Ae. aegypti Populations
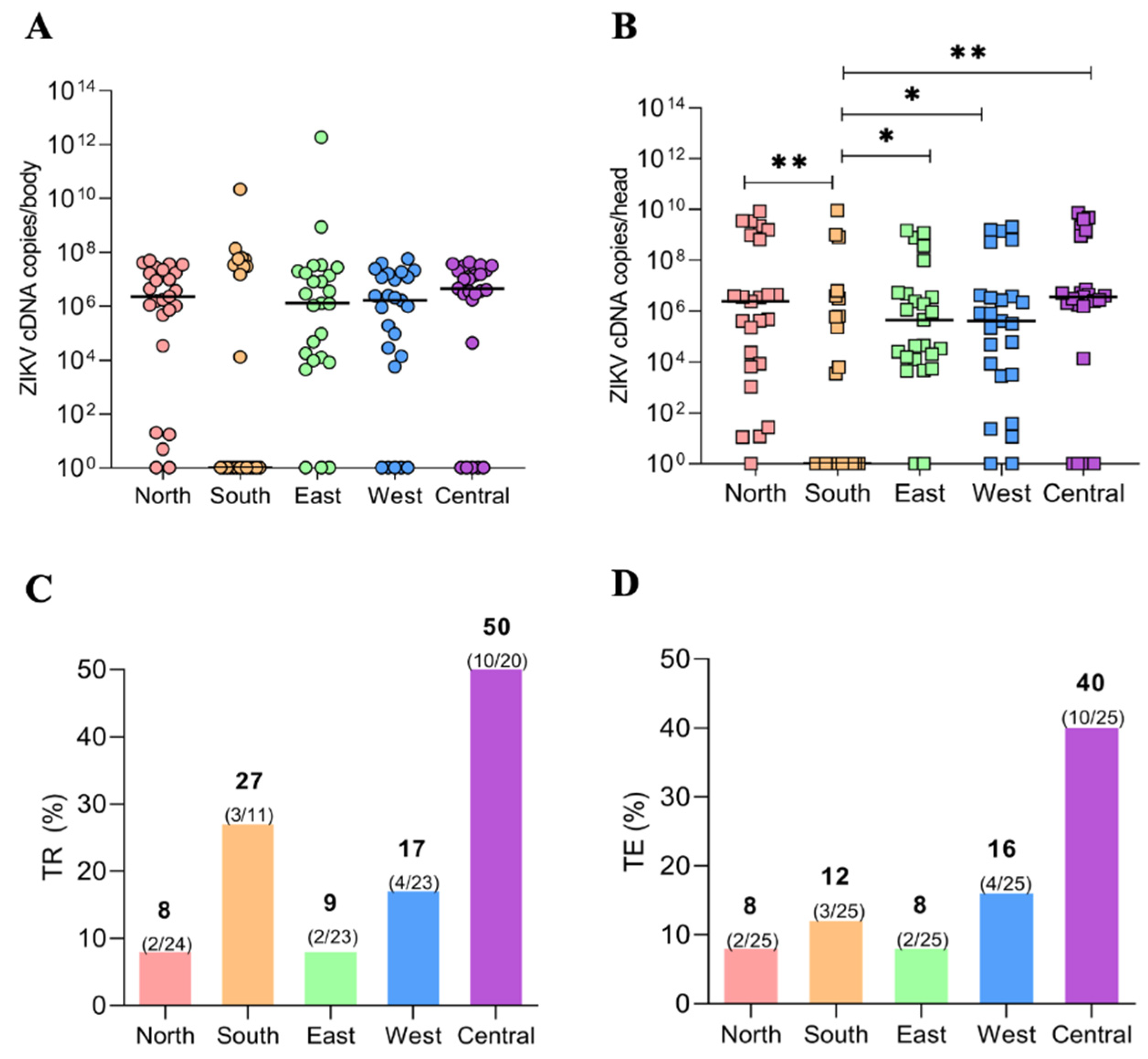
3.2. Viral Load (VL) of the ZIKV via qPCR of Ae. aegypti Populations
3.3. Transmission Rate (TR) and Transmission Efficiency (TE)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Chaves, B.A.; Godoy, R.S.M.; Campolina, T.B.; Júnior, A.B.V.; Paz, A.D.C.; Vaz, E.B.D.C.; Silva, B.M.; Nascimento, R.M.; Guerra, M.D.G.V.B.; Lacerda, M.V.G.; et al. Dengue infection susceptibility of five aedes aegypti populations from Manaus (Brazil) after challenge with virus serotypes 1–4. Viruses 2022, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Godoy, R.S.M.; Felix, L.D.S.; Orfanó, A.D.S.; Chaves, B.A.; Nogueira, P.M.; Costa, B.D.A.; Soares, A.S.; Oliveira, C.C.A.; Nacif-Pimenta, R.; Silva, B.M.; et al. Dengue and Zika virus infection patterns vary among Aedes aegypti field populations from Belo Horizonte, a Brazilian endemic city. PLoS Negl. Trop. Dis. 2021, 15, e0009839. [Google Scholar] [CrossRef] [PubMed]
- Chaves, B.A.; Orfano, A.S.; Nogueira, P.M.; Rodrigues, N.B.; Campolina, T.B.; Nacif-Pimenta, R.; Pires, A.C.A.M.; Júnior, A.B.V.; Paz, A.D.C.; Vaz, E.B.D.C.; et al. Coinfection with Zika Virus (ZIKV) and Dengue Virus Results in Preferential ZIKV Transmission by Vector Bite to Vertebrate Host. J. Infect. Dis. 2018, 218, 563–571. [Google Scholar] [CrossRef]
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika virus isolation and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 12. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232. [Google Scholar] [CrossRef]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef]
- Zanluca, C.; De Melo, V.C.A.; Mosimann, A.L.P.; Dos Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz. 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Pan American Health Organization. Neurological Syndrome, Congenital Malformations, and Zika Virus Infection; Implications for Public Health in the Americas; Epidemiol Alert, 2015; pp. 1–11. Available online: https://iris.paho.org/bitstream/handle/10665.2/50697/EpiUpdate1December2015_eng.pdf?sequence=1&isAllowed=y (accessed on 15 June 2022).
- Brasil, S.E. Monitoramento dos casos de Arboviroses urbanas transmitidas pelo Aedes (dengue, chikungunya e Zika). Bol. Epidemiol. 2020, 51, 1–17. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2020/boletim-epidemiologico-vol-51-no-11.pdf (accessed on 20 August 2022).
- Chowdhury, A.; Modahl, C.M.; Missé, D.; Kini, R.M.; Pompon, J. High resolution proteomics of Aedes aegypti salivary glands infected with either dengue, Zika or chikungunya viruses identify new virus specific and broad antiviral factors. Sci. Rep. 2021, 11, 23696. [Google Scholar] [CrossRef]
- Lambrechts, L.; Paaijmans, K.P.; Fansiri, T.; Carrington, L.B.; Kramer, L.D.; Thomas, M.B.; Scott, T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 7460–7465. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Ebel, G.D. Dynamics of Flavivirus Infection in Mosquitoes; BT-A in VR; Academic Press: Cambridge, MA, USA, 2003; pp. 187–232. [Google Scholar]
- Franz, A.W.E.; Kantor, A.M.; Passarelli, A.L.; Clem, R.J. Tissue barriers to arbovirus infection in mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, A.; Jansen, S.; Lühken, R.; Leggewie, M.; Schmidt-Chanasit, J.; Tannich, E. Forced salivation as a method to analyze vector competence of mosquitoes. J. Vis. Exp. 2018, 138, e57980. [Google Scholar]
- Calvez, E.; O’Connor, O.; Pol, M.; Rousset, D.; Faye, O.; Richard, V.; Tarantola, A.; Dupont-Rouzeyrol, M. Differential transmission of Asian and African Zika virus lineages by Aedes aegypti from New Caledonia. Emerg. Microbes Infect. 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.I.; Richardson, J.H.; Sánchez-vargas, I.; Olson, K.E.; Beaty, B.J. Dengue virus type 2: Replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Ayres, C.F.J.; Melo-Santos, M.A.V.; Solé -Cava, A.M.; Furtado, A.F. Genetic Differentiation of Aedes aegypti (Diptera: Culicidae), the Major Dengue Vector in Brazil. J. Med. Entomol. 2003, 40, 430–435. [Google Scholar] [CrossRef]
- Cunha, M.S.; Alves, L.; Rocco, M.; Maeda, Y.; Silva, G.; Nogueira, J.S.; de Souza, R.P.; Suzuki, A.; Addas-Carvalho, M.; Barjas-Castro, M.D.L.; et al. First Complete Genome Sequence of Zika Virus (Flaviviridae, Flavivirus) from an Autochthonous Transmission in Brazil. Genoma Annoucements 2016, 4, e00032-16. [Google Scholar] [CrossRef]
- White, L.A. Susceptibility of Aedes albopictus C6/36 Cells to Viral Infection. J. Clin. Microbiol. 1987, 25, 1221–1224. [Google Scholar] [CrossRef]
- Labarre, D.D.; Lowy, R.J. Improvements in methods for calculating virus titer estimates from TCID 50 and plaque assays. J. Virol. Methods 2001, 96, 107–126. [Google Scholar] [CrossRef]
- Secundino, N.F.C.; Chaves, B.A.; Orfano, A.S.; Silveira, K.R.D.; Rodrigues, N.B.; Campolina, T.B.; Nacif-Pimenta, R.; Villegas, L.E.M.; Silva, B.M.; Lacerda, M.V.G.; et al. Zika virus transmission to mouse ear by mosquito bite: A laboratory model that replicates the natural transmission process. Parasites Vectors 2017, 10, 4–10. [Google Scholar] [CrossRef]
- Bennett, K.E.; Olson, K.E.; Muñoz, M.D.L.; Fernández Salas, I.; Farfán Ale, J.A.; Higgs, S.; Black, W.C.; Beaty, B.J. Variation in vector competence for Dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am. J. Trop. Med. Hyg. 2002, 67, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chouin-carneiro, T.; Vega-rua, A.; Vazeille, M.; Yebakima, A. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, B.; Vazeille, M.; Tedjou, A.; Yougang, A.P.; Wilson-Bahun, T.A.; Mousson, L.; Wondji, C.S.; Failloux, A.B. Different populations of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Central Africa are susceptible to Zika virus infection. PLoS Negl. Trop. Dis. 2020, 14, e0008163. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Pavan, M.G.; Fernandes, R.S.; Busquets, N.; David, M.R.; Lourenço-Oliveira, R.; García-Pérez, A.L.; Maciel-de-Freitas, R. Limited risk of Zika virus transmission by five Aedes albopictus populations from Spain. Parasites Vectors 2019, 12, 150. [Google Scholar] [CrossRef]
- dos Santos, J.M.M.; Fraga, E.C.; Maia, J.F.; Tadei, W.P. Genetic diversity in dengue mosquito, Aedes aegypti (Diptera: Culicidae) from Amazon region: Comparative analysis with isozymes and RAPD loci. Open Trop. Med. J. 2011, 4, 11–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa Paz, A.; Chaves, B.A.; Godoy, R.S.M.; Coelho, D.F.; Vieira Júnior, A.B.; Alencar, R.M.; Alcântara, J.A.; Félix, L.d.S.; Oliveira, C.C.A.; Monteiro, W.M.; et al. Vector Competence for Zika Virus Changes Depending on the Aedes aegypti’s Region of Origin in Manaus: A Study of an Endemic Brazilian Amazonian City. Viruses 2023, 15, 770. https://doi.org/10.3390/v15030770
da Costa Paz A, Chaves BA, Godoy RSM, Coelho DF, Vieira Júnior AB, Alencar RM, Alcântara JA, Félix LdS, Oliveira CCA, Monteiro WM, et al. Vector Competence for Zika Virus Changes Depending on the Aedes aegypti’s Region of Origin in Manaus: A Study of an Endemic Brazilian Amazonian City. Viruses. 2023; 15(3):770. https://doi.org/10.3390/v15030770
Chicago/Turabian Styleda Costa Paz, Andréia, Bárbara Aparecida Chaves, Raquel Soares Maia Godoy, Deilane Ferreira Coelho, Ademir Bentes Vieira Júnior, Rodrigo Maciel Alencar, João Arthur Alcântara, Luiza dos Santos Félix, Cinthia Catharina Azevedo Oliveira, Wuelton Marcelo Monteiro, and et al. 2023. "Vector Competence for Zika Virus Changes Depending on the Aedes aegypti’s Region of Origin in Manaus: A Study of an Endemic Brazilian Amazonian City" Viruses 15, no. 3: 770. https://doi.org/10.3390/v15030770
APA Styleda Costa Paz, A., Chaves, B. A., Godoy, R. S. M., Coelho, D. F., Vieira Júnior, A. B., Alencar, R. M., Alcântara, J. A., Félix, L. d. S., Oliveira, C. C. A., Monteiro, W. M., Lacerda, M. V. G., Secundino, N. F. C., & Pimenta, P. F. P. (2023). Vector Competence for Zika Virus Changes Depending on the Aedes aegypti’s Region of Origin in Manaus: A Study of an Endemic Brazilian Amazonian City. Viruses, 15(3), 770. https://doi.org/10.3390/v15030770






