Causes of HIV Treatment Interruption during the Last 20 Years: A Multi-Cohort Real-Life Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
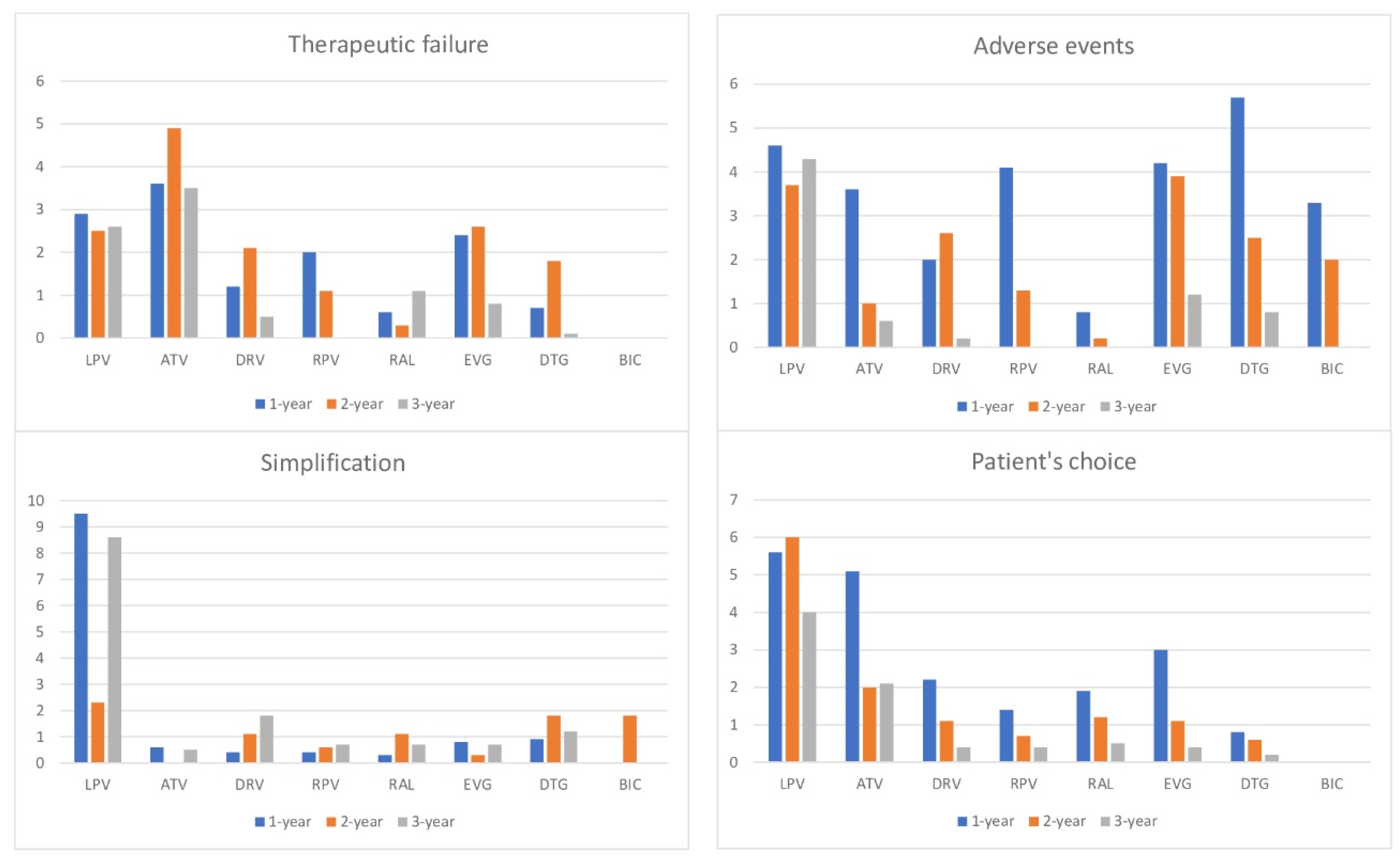
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Participating Centers
References
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N. Engl. J. Med. 2016, 375, 830–839. Available online: https://www.nejm.org/doi/full/10.1056/nejmoa1600693 (accessed on 10 November 2022). [CrossRef]
- Madeddu, G.; De Vito, A.; Cozzi-Lepri, A.; Cingolani, A.; Maggiolo, F.; Perno, C.F.; Gagliardini, R.; Marchetti, G.; Saracino, A.; Monforte, A.D.A.; et al. Time Spent with HIV-RNA ≤ 200 Copies/mL in a Cohort of People with HIV during the U=U Era. AIDS Epidemiol. Soc. 2021, 35, 1103–1112. Available online: https://journals.lww.com/aidsonline/Fulltext/2021/06010/Time_spent_with_HIV_RNA___200_copies_ml_in_a.11.aspx (accessed on 10 November 2022). [CrossRef] [PubMed]
- Group TEE. European guidelines for the clinical management and treatment of HIV-infected adults in Europe. AIDS 2003, 17, 3–26. [Google Scholar] [CrossRef]
- Lundgren, J.D.; Babiker, A.G.; Gordin, F.; Emery, S.; Grund, B.; Sharma, S.; Avihingsanon, A.; Cooper, D.A.; Fätkenheuer, G.; Llibre, J.M.; et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N. Engl. J. Med. 2015, 9, 795–807. [Google Scholar]
- EACS Guidelines 8.0; European AIDS Clinical Society: Brussels, Belgium, 2015.
- Arribas, J.R. The Rise and Fall of Triple Nucleoside Reverse Transcriptase Inhibitor (NRTI) Regimens. J. Antimicrob. Chemother. 2004, 54, 587–592. Available online: https://academic.oup.com/jac/article/54/3/587/741965 (accessed on 10 November 2022). [CrossRef] [PubMed]
- Luber, A.D. Genetic Barriers to Resistance and Impact on Clinical Response. Medscape Gen. Med. 2005, 7, 69. [Google Scholar] [CrossRef]
- Rossetti, B.; Baldin, G.; Sterrantino, G.; Rusconi, S.; De Vito, A.; Giacometti, A.; Gagliardini, R.; Colafigli, M.; Capetti, A.; D’Ettorre, G.; et al. Efficacy and safety of dolutegravir-based regimens in advanced HIV-infected naïve patients: Results from a multicenter cohort study. Antivir. Res. 2019, 169, 104552. [Google Scholar] [CrossRef]
- Madeddu, G.; De Socio, G.V.; Ricci, E.; Quirino, T.; Orofino, G.; Carenzi, L.; Franzetti, M.; Parruti, G.; Martinelli, C.; Vichi, F.; et al. Muscle symptoms and creatine phosphokinase elevations in patients receiving raltegravir in clinical practice: Results from the SCOLTA project long-term surveillance. Int. J. Antimicrob. Agents 2015, 45, 289–294. [Google Scholar] [CrossRef]
- Borghetti, A.; Baldin, G.; Capetti, A.; Sterrantino, G.; Rusconi, S.; Latini, A.; Giacometti, A.; Madeddu, G.; Picarelli, C.; De Marco, R.; et al. Efficacy and tolerability of dolutegravir and two nucleos(t)ide reverse transcriptase inhibitors in HIV-1-positive, virologically suppressed patients. AIDS 2017, 31, 457–459. [Google Scholar] [CrossRef]
- Madeddu, G.; Menzaghi, B.; Ricci, E.; Carenzi, L.; Martinelli, C.; Di Biagio, A.; Parruti, G.; Orofino, G.; Mura, M.S.; Bonfanti, P. Raltegravir central nervous system tolerability in clinical practice: Results from a multicenter observational study. AIDS 2012, 26, 2412–2415. Available online: https://pubmed.ncbi.nlm.nih.gov/23032413/ (accessed on 10 November 2022). [CrossRef] [PubMed]
- Squillace, N.; Ricci, E.; Quirino, T.; Gori, A.; Bandera, A.; Carenzi, L.; De Socio, G.V.; Orofino, G.; Martinelli, C.; Madeddu, G.; et al. Safety and tolerability of Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Disoproxil fumarate in a real life setting: Data from surveillance cohort long-term toxicity antiretrovirals/antivirals (SCOLTA) project. PLoS ONE 2017, 12, e0179254. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0179254 (accessed on 10 November 2022). [CrossRef] [PubMed]
- Ciccullo, A.; D’Angelillo, A.; Iannone, V.; Farinacci, D.; Lombardi, F.; Visconti, E.; Tamburrini, E.; Di Giambenedetto, S. Real-Life Safety of Doravirine in Treatment-Experienced, Virologically Suppressed PLWHIV. J. Acquir. Immune Defic. Syndr. 2021, 88, E5–E6. Available online: https://pubmed.ncbi.nlm.nih.gov/34397747/ (accessed on 10 November 2022). [CrossRef] [PubMed]
- Kanters, S.; Vitoria, M.; Doherty, M.; Socias, M.E.; Ford, N.; Forrest, J.; Popoff, E.; Bansback, N.; Nsanzimana, S.; Thorlund, K.; et al. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: A systematic review and network meta-analysis. Lancet HIV 2016, 3, e510–e520. [Google Scholar] [CrossRef] [PubMed]
- EACS GUIDELINES Version 11.1-October 2022; European AIDS Clinical Society: Brussels, Belgium, 2022.
- Ciccullo, A.; Baldin, G.; Borghi, V.; Sterrantino, G.; Madeddu, G.; Latini, A.; D’Ettorre, G.; Lanari, A.; Mazzitelli, M.; Colafigli, M.; et al. Overall Tolerability of Integrase Inhibitors in Clinical Practice: Results from a Multicenter Italian Cohort. AIDS Res. Hum. Retrovir. 2021, 37, 4–10. [Google Scholar] [CrossRef]
- Fabbiani, M.; Rossetti, B.; Ciccullo, A.; Oreni, L.; Lagi, F.; Celani, L.; Colafigli, M.; De Vito, A.; Mazzitelli, M.; Dusina, A.; et al. Efficacy and durability of two- vs. three-drug integrase inhibitor-based regimens in virologically suppressed HIV-infected patients: Data from real-life ODOACRE cohort. HIV Med. 2021, 22, 843–853. [Google Scholar] [CrossRef]
- De Vito, A.; Caruana, G.; Clark, F.; Nunnari, G.; Pellicanò, G.; Angioni, G.; Freedman, A.; Babudieri, S.; Madeddu, G. Efficacy, safety and tolerability of dolutegravir-based combination antiretroviral therapy in clinical practice in HIV-infected patients: Results from a multicenter study—Infectious Diseases & Tropical Medicine. Infect. Deases Trop. Med. 2019, 5, e565. Available online: https://www.infectiousjournal.com/article/565 (accessed on 10 November 2022).
- Baldin, G.; Ciccullo, A.; Capetti, A.; Rusconi, S.; Sterrantino, G.; Cossu, M.V.; Giacomelli, A.; Lagi, F.; Latini, A.; Bagella, P.; et al. Efficacy and safety of switching to dolutegravir plus emtricitabine/tenofovir disoproxil fumarate (TDF) or elvitegravir/cobicistat/emtricitabine/TDF in virologically suppressed HIV-infected patients in clinical practice: Results from a multicentre, observational study. HIV Med. 2018, 20, 164–168. Available online: https://pubmed.ncbi.nlm.nih.gov/30457197/ (accessed on 10 November 2022).
- Taramasso, L.; De Vito, A.; Ricci, E.D.; Orofino, G.; Squillace, N.; Menzaghi, B.; Molteni, C.; Gulminetti, R.; De Socio, G.V.; Pellicanò, G.F.; et al. Durability of Dolutegravir-Based Regimens: A 5-Year Prospective Observational Study. AIDS Patient Care STDs 2021, 35, 342–353. Available online: https://www.liebertpub.com/doi/abs/10.1089/apc.2021.0089 (accessed on 10 November 2022). [CrossRef]
- Bonfanti, P.; De Vito, A.; Ricci, E.; Menzaghi, B.; Orofino, G.; Squillace, N.; Molteni, C.; De Socio, G.V.; Salomoni, E.; Celesia, B.M.; et al. Bone Safety of Dolutegravir-Containing Regimens in People Living with HIV: Results from a Real-World Cohort. Infect. Drug Resist. 2020, 13, 2291–2300. Available online: https://www.dovepress.com/bone-safety-of-dolutegravir-containing-regimens-in-people-living-with--peer-reviewed-article-IDR (accessed on 10 November 2022). [CrossRef]
- Mazzitelli, M.; Fusco, P.; Brogna, M.; Vallone, A.; D’Argenio, L.; Beradelli, G.; Foti, G.; Mangano, C.; Carpentieri, M.S.; Cosco, L.; et al. Weight of Clinical and Social Determinants of Metabolic Syndrome in People Living with HIV. Viruses 2022, 14, 1339. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, M.; Pereira, B.I.; Moyle, G.; Asboe, D.; Pozniak, A.; Boffito, M.; Milinkovic, A. Factors associated with overweight/obesity in a cohort of people living with HIV over 50 years of age. AIDS Care-Psychol. Socio-Med. Asp. AIDS/HIV 2022, 34, 542–544. [Google Scholar] [CrossRef]
- Taramasso, L.; Di Biagio, A.; Maggiolo, F.; Tavelli, A.; Lo Caputo, S.; Bonora, S.; Zaccarelli, M.; Caramello, P.; Costantini, A.; Viscoli, C. First-line antiretroviral therapy with efavirenz plus tenofovir disiproxil fumarate/emtricitabine or rilpivirine plus tenofovir disiproxil fumarate/emtricitabine: A durability comparison. HIV Med. 2018, 19, 475–584. Available online: https://pubmed.ncbi.nlm.nih.gov/29846042/ (accessed on 10 November 2022). [CrossRef] [PubMed]
- Bagella, P.; De Socio, G.V.; Ricci, E.; Menzaghi, B.; Martinelli, C.; Squillace, N.; Maggi, P.; Orofino, G.; Calza, L.; Carenzi, L.; et al. Durability, safety, and efficacy of rilpivirine in clinical practice: Results from the SCOLTA Project. Infect. Drug Resist. 2018, 11, 615–623. Available online: https://pubmed.ncbi.nlm.nih.gov/29731650/ (accessed on 10 November 2022). [CrossRef]
- Taramasso, L.; Tatarelli, P.; Ricci, E.; Madeddu, G.; Menzaghi, B.; Squillace, N.; De Socio, G.V.; Martinelli, C.; Gulminetti, R.; Maggi, P. Improvement of lipid profile after switching from efavirenz or ritonavir-boosted protease inhibitors to rilpivirine or once-daily integrase inhibitors: Results from a large observational cohort study (SCOLTA). BMC Infect. Dis. 2018, 18, 1–8. Available online: https://pubmed.ncbi.nlm.nih.gov/30064371/ (accessed on 10 November 2022). [CrossRef] [PubMed]
- Mazzitelli, M.; Degli Antoni, M.; Castelli, F.; Ripamonti, D.; Zuglian, G.; Lapadula, G.; Fabbiani, M.; Ferraresi, A.; Putaggio, C.; Cattelan, A.M.; et al. Real-life use of Doravirine in treatment-experienced people living with HIV: A multicenter Italian study. Medicine 2022, 101, E29855. Available online: https://pubmed.ncbi.nlm.nih.gov/35905209/ (accessed on 10 November 2022). [CrossRef]
- Bonfanti, P.; Martinelli, C.; Ricci, E.; Carradori, S.; Parruti, G.; Armignacco, O.; Magnani, C.; Quirino, T. An Italian approach to postmarketing monitoring: Preliminary results from the SCOLTA (Surveillance Cohort Long-term Toxicity Antiretrovirals) project on the safety of lopinavir/ritonavir. J. Acquir. Immune Defic. Syndr. 2005, 39, 317–320. [Google Scholar] [CrossRef] [PubMed]
- DAIDS. Division of Aids Table for Grading the Severity of Adult and Pediatric Adverse Events Division of Aids Table for Grading the Severity of Adult and Pediatric Adverse Events; National Institute of Allergy and Infectious Diseases: Bethesda, MD, USA, 2017; pp. 1–35. Available online: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf (accessed on 10 November 2022).
- Monforte, A.D.A.; Lepri, A.C.; Rezza, G.; Pezzotti, P.; Antinori, A.; Phillips, A.N.; Angarano, G.; Colangeli, V.; De Luca, A.; Ippolito, G.; et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. AIDS 2000, 14, 499–507. Available online: https://pubmed.ncbi.nlm.nih.gov/10780712/ (accessed on 10 November 2022). [CrossRef] [PubMed]
- Vo, T.T.N.; Ledergerber, B.; Keiser, O.; Hirschel, B.; Furrer, H.; Battegay, M.; Cavassini, M.; Bernasconi, E.; Vernazza, P.; Weber, R.; et al. Durability and outcome of initial antiretroviral treatments received during 2000--2005 by patients in the Swiss HIV Cohort Study. J. Infect. Dis. 2008, 197, 1685–1694. Available online: https://pubmed.ncbi.nlm.nih.gov/18513155/ (accessed on 10 November 2022). [CrossRef]
- Robison, L.S.; Westfall, A.O.; Mugavero, M.J.; Kempf, M.C.; Cole, S.R.; Allison, J.J.; Willig, J.H.; Raper, J.L.; Wilcox, C.M.; Saag, M.S. Short-term discontinuation of HAART regimens more common in vulnerable patient populations. AIDS Res. Hum. Retrovir. 2008, 24, 1347–1355. Available online: https://pubmed.ncbi.nlm.nih.gov/19032064/ (accessed on 10 November 2022). [CrossRef]
- Cicconi, P.; Cozzi-Lepri, A.; Castagna, A.; Trecarichi, E.M.; Antinori, A.; Gatti, F.; Cassola, G.; Sighinolfi, L.; Castelli, P.; d’Arminio Monforte, A.; et al. Insights into reasons for discontinuation according to year of starting first regimen of highly active antiretroviral therapy in a cohort of antiretroviral-naïve patients. HIV Med. 2010, 11, 104–113. Available online: https://pubmed.ncbi.nlm.nih.gov/19732176/ (accessed on 10 November 2022). [CrossRef] [PubMed]
- Abgrall, S.; Ingle, S.; May, M.; Costagliola, D.; Mercie, P.; Cavassini, M.; Reekie, J.; Samji, H.; Gill, M.J.; Crane, H.M.; et al. Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and North America 2002–2009. AIDS 2013, 27, 803–813. Available online: https://pubmed.ncbi.nlm.nih.gov/23719350/ (accessed on 10 November 2022). [PubMed]
- Di Biagio, A.; Cozzi-Lepri, A.; Prinapori, R.; Angarano, G.; Gori, A.; Quirino, T.; De Luca, A.; Costantini, A.; Mussini, C.; Rizzardini, G.; et al. Treatment discontinuation in HIV-1-infected individuals starting their first-line HAART after 2008: Data from the ICONA Foundation Study Cohort. J. Int. AIDS Soc. 2014, 17, 19825. Available online: https://pubmed.ncbi.nlm.nih.gov/25397569/ (accessed on 10 November 2022). [CrossRef]
- Jiamsakul, A.; Azwa, I.; Zhang, F.; Yunihastuti, E.; Ditangco, R.; Kumarasamy, N.; Ng, O.T.; Chan, Y.-J.; Ly, P.S.; Choi, J.Y.; et al. Treatment modification after second-line failure among people living with HIV in the Asia-Pacific. Antivir. Ther. 2019, 25, 377–387. [Google Scholar] [CrossRef]
- Onoya, D.; Brennan, A.T.; Berhanu, R.; van der Berg, L.; Buthelezi, T.; Fox, M.P. Changes in second-line regimen durability and continuity of care in relation to national ART guideline changes in South Africa. J. Int. AIDS Soc. 2016, 19, 20675. [Google Scholar] [CrossRef]
- Cardoso, S.W.; Luz, P.M.; Velasque, L.; Torres, T.S.; Tavares, I.C.; Ribeiro, S.R.; Moreira, R.; Veloso, V.G.; Moore, R.D.; Grinsztejn, B. Outcomes of second-line combination antiretroviral therapy for HIV-infected patients: A cohort study from Rio de Janeiro, Brazil. BMC Infect. Dis. 2014, 14, 699. Available online: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-014-0699-5 (accessed on 10 November 2022). [CrossRef]
- Ross, J.; Jiamsakul, A.; Kumarasamy, N.; Azwa, I.; Merati, T.; Do, C.; Lee, M.; Ly, P.; Yunihastuti, E.; Nguyen, K.; et al. Virological failure and HIV drug resistance among adults living with HIV on second-line antiretroviral therapy in the Asia-Pacific. HIV Med. 2020, 22, 201–211. Available online: https://pubmed.ncbi.nlm.nih.gov/33151020/ (accessed on 10 November 2022). [CrossRef]
- Nicole, L.; Cooper, D.A.; Russell, D.; Smith, D.; Woolley, I.; Sullivan, M.O.; Wright, S.; Law, M. Treatment durability and virological response in treatment-experienced HIV-positive patients on an integrase inhibitor-based regimen: An Australian cohort study. Sex Health 2016, 13, 335–344. [Google Scholar]
- de Benedetto, I.; Trunfio, M.; Guastamacchia, G.; Bonora, S.; Calcagno, A. A review of the potential mechanisms of neuronal toxicity associated with antiretroviral drugs. J. Neurovirol. 2020, 26, 642–651. [Google Scholar] [CrossRef]
- Calcagno, A.; Moltó, J.; Borghetti, A.; Gervasoni, C.; Milesi, M.; Valle, M.; Avataneo, V.; Alcantarini, C.; Pla-Junca, F.; Trunfio, M.; et al. Older Age is Associated with Higher Dolutegravir Exposure in Plasma and Cerebrospinal Fluid of People Living with HIV. Clin. Pharmacokinet. 2020, 60, 103–109. [Google Scholar] [CrossRef]
- Calcagno, A.; Trunfio, M.; D’Avolio, A.; Di Perri, G.; Bonora, S. The impact of age on antiretroviral drug pharmacokinetics in the treatment of adults living with HIV. Expert Opin. Drug Metab. Toxicol. 2021, 17, 665–676. [Google Scholar] [CrossRef] [PubMed]
| LPV | ATV | DRV | RPV | RAL | EVG | DTG | BIC | p-Value * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 690 15.7% | N = 527 12.0% | N = 646 14.7% | N = 344 7.8% | N = 469 10.6% | N = 332 7.5% | N = 1125 25.5% | N = 242 6.2% | ||||||||||
| Period | 2002–2006 | 2003–2008 | 2006–2019 | 2013–2017 | 2007–2014 | 2014–2019 | 2014–Ongoing | 2019–Ongoing | |||||||||
| Male gender | 502 | 72.8 | 357 | 67.7 | 475 | 73.5 | 250 | 72.7 | 314 | 67.0 | 255 | 76.8 | 842 | 74.8 | 217 | 79.8 | 0.0003 |
| Age, years (mean ± SD) | 40.3 ± 7.8 | 42.8 ± 8.1 | 45.8 ± 9.3 | 43.6 ± 10.2 | 45.8 ± 9.2 | 43.8 ± 10.8 | 48.1 ± 12.0 | 49.1 ± 11.8 | <0.0001 | ||||||||
| Caucasian ethnicity | 633 | 91.7 | 493 | 93.5 | 607 | 94.0 | 316 | 91.9 | 438 | 93.4 | 301 | 90.7 | 1024 | 91.0 | 243 | 89.3 | 0.12 |
| Risk factor for HIV acquisition | |||||||||||||||||
| Heterosexual | 236 | 34.2 | 189 | 35.9 | 195 | 30.2 | 153 | 44.5 | 163 | 34.8 | 126 | 38.0 | 417 | 37.1 | 105 | 38.6 | |
| MSM | 139 | 20.1 | 81 | 15.4 | 132 | 20.4 | 118 | 34.3 | 97 | 20.7 | 121 | 36.4 | 376 | 33.4 | 83 | 30.5 | |
| PWID | 268 | 38.8 | 223 | 42.3 | 228 | 35.3 | 54 | 15.7 | 168 | 35.8 | 53 | 16.0 | 199 | 17.7 | 47 | 17.3 | |
| Other/unknown | 47 | 6.8 | 34 | 6.4 | 91 | 14.1 | 19 | 5.5 | 41 | 8.7 | 32 | 9.6 | 133 | 11.8 | 37 | 13.6 | <0.0001 |
| HbsAg positive | 50 | 7.2 | 27 | 5.1 | 40 | 6.2 | 20 | 5.8 | 28 | 6.0 | 31 | 9.3 | 31 | 2.8 | 26 | 9.6 | <0.0001 |
| HCV positive | 267 | 38.7 | 221 | 41.9 | 238 | 36.8 | 57 | 16.6 | 172 | 36.7 | 65 | 19.6 | 240 | 21.3 | 58 | 21.3 | <0.0001 |
| CDC stage | |||||||||||||||||
| A | 231 | 33.5 | 169 | 32.1 | 170 | 26.3 | 222 | 64.5 | 142 | 30.3 | 170 | 51.2 | 571 | 50.8 | 158 | 58.1 | |
| B | 187 | 27.1 | 170 | 32.3 | 242 | 37.5 | 76 | 22.1 | 143 | 30.5 | 88 | 26.5 | 305 | 27.1 | 61 | 22.4 | |
| C | 272 | 39.4 | 188 | 35.7 | 234 | 36.2 | 46 | 13.4 | 184 | 39.2 | 74 | 22.3 | 249 | 22.1 | 53 | 19.5 | <0.0001 |
| Naïve | 135 | 19.6 | 18 | 3.4 | 47 | 7.3 | 103 | 29.9 | 27 | 5.8 | 100 | 30.1 | 283 | 25.2 | 36 | 13.2 | <0.0001 |
| Detectable HIV-RNA (experienced, n = 3656) | 488 | 87.9 | 347 | 68.2 | 335 | 55.9 | 36 | 14.9 | 253 | 57.2 | 61 | 26.3 | 142 | 16.7 | 20 | 8.5 | <0.0001 |
| CD4 cells/mm3 | |||||||||||||||||
| <250 | 385 | 55.8 | 190 | 36.1 | 249 | 38.5 | 31 | 9.0 | 169 | 36.0 | 83 | 25.0 | 201 | 17.9 | 23 | 8.5 | |
| 250–500 | 220 | 31.9 | 219 | 41.6 | 196 | 30.3 | 122 | 35.5 | 172 | 36.7 | 92 | 27.7 | 278 | 24.7 | 67 | 24.6 | |
| >500 | 84 | 12.2 | 118 | 22.4 | 196 | 30.3 | 191 | 55.5 | 126 | 26.9 | 157 | 47.3 | 623 | 55.4 | 182 | 66.9 | <0.0001 |
| Year of first ART | |||||||||||||||||
| <1996 | 190 | 27.5 | 168 | 31.9 | 183 | 28.3 | 19 | 5.5 | 136 | 29.0 | 26 | 7.8 | 87 | 7.7 | 19 | 7.0 | |
| 1996–2002 | 411 | 59.6 | 265 | 50.3 | 232 | 35.9 | 61 | 17.7 | 207 | 44.1 | 58 | 17.5 | 233 | 20.7 | 41 | 15.1 | |
| 2003–2012 | 89 | 12.9 | 94 | 17.8 | 182 | 28.2 | 132 | 38.4 | 126 | 26.9 | 98 | 29.5 | 341 | 30.3 | 64 | 23.5 | |
| >2012 | . | . | . | . | 49 | 7.6 | 132 | 38.4 | . | . | 150 | 45.2 | 464 | 41.2 | 148 | 54.4 | <0.0001 |
| LPV | ATV | DRV | RPV | RAL | EVG | DTG | BIC | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 690 15.7% | N = 527 12.0% | N = 646 14.7% | N = 344 7.8% | N = 469 10.6% | N = 332 7.5% | N = 1125 25.5% | N = 242 6.2% | N = 4405 | ||||||||||
| Overall | 177 | 25.6 | 122 | 23.2 | 79 | 12.2 | 42 | 12.2 | 46 | 9.8 | 50 | 15.1 | 125 | 11.1 | 23 | 9.5 | 664 | 15.1 |
| Adverse events | 32 | 4.6 | 19 | 23.2 | 13 | 2.0 | 14 | 4.1 | 4 | 0.8 | 14 | 4.2 | 64 | 5.7 | 9 | 3.3 | 169 | 3.8 |
| Clinical event * | 6 | 0.9 | 1 | 0.2 | 0 | 0.0 | 1 | 0.3 | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 10 | 0.2 |
| Therapeutic failure | 20 | 2.9 | 19 | 3.6 | 8 | 1.2 | 7 | 2.0 | 3 | 0.6 | 8 | 2.4 | 8 | 0.7 | 0 | 0.0 | 73 | 1.7 |
| Pregnancy | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 | 0 | 0.0 | 2 | 0.1 |
| Drug-Drug interactions | 0 | 0.0 | 0 | 0.0 | 4 | 0.6 | 4 | 1.2 | 0 | 0.0 | 4 | 1.2 | 1 | 0.1 | 1 | 0.4 | 14 | 0.3 |
| Death | 4 | 0.6 | 4 | 0.8 | 5 | 0.8 | 1 | 0.3 | 3 | 0.6 | 2 | 0.6 | 6 | 0.5 | 1 | 0.4 | 26 | 0.6 |
| Unknown | 2 | 0.3 | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 2 | 0.4 | 0 | 0.0 | 1 | 0.1 | 0 | 0.0 | 7 | 0.2 |
| Optimization | 4 | 0.6 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.2 | 0 | 0.0 | 8 | 0.2 |
| Lost to follow-up | 49 | 7.1 | 43 | 8.2 | 24 | 3.7 | 7 | 2.0 | 16 | 3.4 | 9 | 2.7 | 11 | 1.0 | 5 | 1.8 | 164 | 3.7 |
| Simplification | 16 | 2.3 | 0 | 0.0 | 7 | 1.1 | 2 | 0.6 | 5 | 1.1 | 1 | 0.3 | 20 | 1.8 | 5 | 1.8 | 56 | 1.3 |
| Patients’ decision | 39 | 5.7 | 27 | 5.1 | 14 | 2.2 | 5 | 1.4 | 9 | 1.9 | 10 | 3.0 | 9 | 0.8 | 0 | 0.0 | 113 | 2.6 |
| Others ** | 5 | 0.7 | 5 | 1.0 | 3 | 0.5 | 1 | 0.3 | 2 | 0.4 | 2 | 0.6 | 2 | 0.2 | 2 | 0.7 | 22 | 0.5 |
| Age-Sex-Adjusted Model | Complete Model * | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | RR | 95% CI | p-Value | RR | 95% CI | p-Value | ||
| Low | High | Low | High | |||||
| Female sex (ref. Male) | 1.22 | 1.03 | 1.43 | 0.02 | 1.20 | 1.02 | 1.43 | 0.03 |
| Age > 50 years (ref. Age ≤ 50 years) | 0.86 | 0.72 | 1.02 | 0.08 | 1.20 | 0.99 | 1.45 | 0.06 |
| Other ethnicity (ref. Caucasian) | 1.17 | 0.89 | 1.54 | 0.26 | ||||
| Risk factor for HIV acquisition (ref. Heterosexual) | ||||||||
| MSM | 0.88 | 0.68 | 1.13 | 0.32 | 0.95 | 0.74 | 1.22 | 0.70 |
| PWID | 1.40 | 1.16 | 1.68 | 0.0004 | 1.24 | 0.97 | 1.58 | 0.08 |
| Other/unknown | 0.99 | 0.73 | 1.33 | 0.94 | 1.14 | 0.84 | 1.53 | 0.40 |
| HBsAg positive (ref. negative) | 0.99 | 0.70 | 1.39 | 0.96 | ||||
| HCV positive (ref. negative) | 1.36 | 1.16 | 1.60 | 0.0001 | 1.06 | 0.85 | 1.32 | 0.62 |
| CDC stage (ref. A) | ||||||||
| B | 1.03 | 0.84 | 1.07 | 0.74 | 0.93 | 0.76 | 1.14 | 0.50 |
| C | 1.50 | 1.25 | 1.80 | <0.0001 | 1.21 | 1.00 | 1.47 | 0.046 |
| Baseline detectable HIVRNA (ref. undetectable) | 1.83 | 1.55 | 2.15 | <0.0001 | 1.36 | 1.11 | 1.66 | 0.003 |
| Baseline CD4+ (ref. < 250), cells/mm3 | ||||||||
| CD4+ 250–500 | 0.85 | 0.69 | 1.04 | 0.11 | 0.74 | 0.60 | 0.90 | 0.002 |
| CD4+ ≥ 500 | 1.58 | 1.31 | 1.92 | <0.0001 | 0.79 | 0.63 | 0.98 | 0.04 |
| Cohort (ref. DTG) | ||||||||
| LPV | 2.72 | 2.08 | 3.55 | <0.0001 | 1.92 | 1.41 | 2.60 | <0.0001 |
| ATV | 2.57 | 1.96 | 3.37 | <0.0001 | 2.03 | 1.51 | 2.74 | <0.0001 |
| DRV | 1.30 | 0.96 | 1.77 | 0.09 | 1.06 | 0.77 | 1.46 | 0.73 |
| RPV | 1.70 | 1.18 | 2.44 | 0.004 | 1.85 | 1.29 | 2.66 | 0.0008 |
| RAL | 0.97 | 0.67 | 1.50 | 0.88 | 0.79 | 0.54 | 1.16 | 0.23 |
| EVG | 1.91 | 1.35 | 2.71 | 0.0003 | 1.87 | 1.32 | 2.65 | 0.0004 |
| BIC | 0.81 | 0.50 | 1.32 | 0.40 | 0.88 | 0.54 | 1.44 | 0.61 |
| Age-Sex-Adjusted Model | Complete Model * | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | RR | 95% CI | p-Value | RR | 95% CI | p-Value | ||
| Low | High | Low | High | |||||
| Female sex (ref. Male) | 1.01 | 0.66 | 1.53 | 0.96 | 1.03 | 0.68 | 1.55 | 0.89 |
| Age > 50 years (ref. Age ≤ 50 years) | 1.04 | 0.69 | 1.58 | 0.84 | 1.04 | 0.69 | 1.58 | 0.84 |
| Other ethnicity (ref. Caucasian) | 1.00 | 0.60 | 1.68 | 0.99 | ||||
| Risk factor for HIV acquisition (ref. Heterosexual) | ||||||||
| MSM | 0.73 | 0.48 | 1.12 | 0.15 | ||||
| PWID | 1.11 | 0.62 | 1.96 | 0.73 | ||||
| Other/unknown | 1.25 | 0.74 | 2.10 | 0.40 | ||||
| HBsAg positive (ref. negative) | 0.64 | 0.25 | 1.66 | 0.36 | ||||
| HCV positive (ref. negative) | 1.53 | 0.97 | 2.41 | 0.06 | ||||
| CDC stage (ref. A) | ||||||||
| B | 0.86 | 0.53 | 1.40 | 0.52 | 0.75 | 0.47 | 1.20 | 0.23 |
| C | 1.58 | 1.09 | 2.29 | 0.02 | 1.11 | 0.75 | 1.63 | 0.60 |
| Baseline CD4+ (ref. < 250), cells/mm3 | ||||||||
| CD4+ 250–500 | 0.69 | 0.47 | 1.02 | 0.06 | ||||
| CD4+ >= 500 | 0.67 | 0.42 | 1.06 | 0.09 | ||||
| Cohort (ref. DTG) | ||||||||
| LPV | 1.87 | 1.28 | 2.72 | 0.001 | 1.80 | 1.22 | 2.67 | 0.003 |
| ATV | 0.70 | 0.18 | 2.79 | 0.61 | 0.73 | 0.19 | 2.78 | 0.64 |
| DRV | 0.94 | 0.45 | 1.95 | 0.86 | 0.92 | 0.44 | 1.94 | 0.83 |
| RPV | 0.24 | 0.09 | 0.67 | 0.006 | 0.24 | 0.09 | 0.66 | 0.006 |
| RAL | 1.40 | 0.65 | 2.97 | 0.39 | 1.34 | 0.63 | 2.85 | 0.45 |
| EVG | 0.57 | 0.29 | 1.12 | 0.10 | 0.56 | 0.29 | 1.11 | 0.10 |
| BIC | 0.87 | 0.37 | 2.05 | 0.75 | 0.84 | 0.35 | 1.99 | 0.69 |
| Age-Sex-Adjusted Model | Complete Model * | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Low | High | Low | High | |||||
| Female sex (ref. Male) | 1.10 | 0.98 | 1.23 | 0.12 | 1.15 | 1.01 | 1.31 | 0.04 |
| Age > 50 years (ref. Age ≤ 50 years) | 0.72 | 0.63 | 0.81 | <0.0001 | 1.12 | 0.97 | 1.28 | 0.13 |
| Other ethnicity (ref. Caucasian) | 1.28 | 1.05 | 1.57 | 0.01 | 1.44 | 1.17 | 1.77 | 0.0005 |
| Risk factor for HIV acquisition (ref. Heterosexual) | ||||||||
| MSM | 1.00 | 0.86 | 1.19 | 0.91 | 1.12 | 0.95 | 1.32 | 0.19 |
| PWID | 1.36 | 1.19 | 1.55 | <0.0001 | 1.21 | 1.01 | 1.45 | 0.04 |
| Other/unknown | 0.97 | 0.78 | 1.19 | 0.75 | 1.19 | 0.96 | 1.46 | 0.11 |
| HBsAg positive (ref. negative) | 1.18 | 0.95 | 1.47 | 0.12 | ||||
| HCV positive (ref. negative) | 1.31 | 1.17 | 1.46 | <0.0001 | 1.06 | 0.90 | 1.24 | 0.48 |
| CDC stage (ref. A) | ||||||||
| B | 1.00 | 0.88 | 1.15 | 0.94 | 0.93 | 0.81 | 1.07 | 0.29 |
| C | 1.36 | 1.20 | 1.56 | <0.0001 | 1.13 | 0.98 | 1.29 | 0.08 |
| Baseline detectable HIVRNA (ref. undetectable) | 1.86 | 1.66 | 2.09 | <0.0001 | 1.25 | 1.09 | 1.44 | 0.002 |
| Baseline CD4+ (ref. < 250), cells/mm3 | ||||||||
| CD4+ 250–500 | 0.67 | 0.59 | 0.76 | <0.0001 | 0.79 | 0.69 | 0.90 | 0.0006 |
| CD4+ >= 500 | 0.53 | 0.46 | 0.60 | <0.0001 | 0.82 | 0.70 | 0.96 | 0.01 |
| Cohort (ref. DTG) | ||||||||
| LPV | 4.37 | 3.66 | 5.21 | <0.0001 | 3.48 | 2.84 | 4.27 | <0.0001 |
| ATV | 2.61 | 2.15 | 3.17 | <0.0001 | 2.30 | 1.86 | 2.84 | <0.0001 |
| DRV | 1.32 | 1.08 | 1.61 | 0.007 | 1.16 | 0.93 | 1.44 | 0.19 |
| RPV | 1.62 | 1.20 | 2.19 | 0.002 | 1.76 | 1.30 | 2.37 | 0.0002 |
| RAL | 1.16 | 0.93 | 1.46 | 0.18 | 1.05 | 0.83 | 1.32 | 0.69 |
| EVG | 2.07 | 1.62 | 2.64 | <0.0001 | 2.12 | 1.66 | 2.72 | <0.0001 |
| BIC | 0.86 | 0.57 | 1.29 | 0.46 | 0.90 | 0.60 | 1.36 | 0.62 |
| Age-Sex-Adjusted Model | Complete Model * | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Low | High | Low | High | |||||
| Female sex (ref. Male) | 1.11 | 0.83 | 1.48 | 0.49 | 1.05 | 0.76 | 1.46 | 0.76 |
| Age > 50 years (ref. Age ≤ 50 years) | 1.18 | 0.88 | 1.58 | 0.26 | 1.15 | 0.86 | 1.56 | 0.35 |
| Other ethnicity (ref. Caucasian) | 1.28 | 0.90 | 1.83 | 0.17 | ||||
| Risk factor for HIV acquisition (ref. Heterosexual) | ||||||||
| MSM | 0.73 | 0.54 | 0.98 | 0.04 | 0.89 | 0.65 | 1.22 | 0.48 |
| PWID | 1.38 | 0.95 | 2.01 | 0.09 | 0.83 | 0.52 | 1.33 | 0.43 |
| Other/unknown | 1.03 | 0.69 | 1.55 | 0.88 | 1.09 | 0.72 | 1.64 | 0.69 |
| HBsAg positive (ref. negative) | 0.90 | 0.54 | 1.52 | 0.70 | ||||
| HCV positive (ref. negative) | 1.87 | 1.37 | 2.56 | <0.0001 | 1.58 | 1.05 | 2.39 | 0.03 |
| CDC stage (ref. A) | ||||||||
| B | 1.08 | 0.80 | 1.46 | 0.60 | 0.85 | 0.62 | 1.17 | 0.31 |
| C | 1.81 | 1.38 | 2.39 | <0.0001 | 1.12 | 0.80 | 1.57 | 0.50 |
| Baseline CD4+ (ref. <250), cells/mm3 | ||||||||
| CD4+ 250–500 | 0.51 | 0.38 | 0.67 | <0.0001 | 0.80 | 0.58 | 1.13 | 0.21 |
| CD4+ >= 500 | 0.61 | 0.45 | 0.83 | 0.001 | 1.01 | 0.69 | 1.46 | 0.97 |
| Cohort (ref. DTG) | ||||||||
| LPV | 3.12 | 2.35 | 4.16 | <0.0001 | 2.74 | 1.97 | 3.81 | <0.0001 |
| ATV | 1.83 | 0.91 | 3.68 | 0.09 | 1.64 | 0.79 | 3.38 | 0.18 |
| DRV | 1.13 | 0.63 | 2.02 | 0.68 | 1.10 | 0.60 | 2.00 | 0.76 |
| RPV | 0.83 | 0.17 | 0.66 | 0.002 | 0.36 | 0.18 | 0.72 | 0.004 |
| RAL | 1.89 | 1.01 | 3.54 | 0.047 | 1.89 | 1.01 | 3.55 | 0.048 |
| EVG | 1.04 | 0.70 | 1.54 | 0.85 | 1.10 | 0.74 | 1.64 | 0.65 |
| BIC | 1.51 | 0.80 | 2.85 | 0.20 | 1.54 | 0.81 | 2.91 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vito, A.; Ricci, E.; Menzaghi, B.; Orofino, G.; Martinelli, C.V.; Squillace, N.; Taramasso, L.; De Socio, G.V.; Molteni, C.; Valsecchi, L.; et al. Causes of HIV Treatment Interruption during the Last 20 Years: A Multi-Cohort Real-Life Study. Viruses 2023, 15, 720. https://doi.org/10.3390/v15030720
De Vito A, Ricci E, Menzaghi B, Orofino G, Martinelli CV, Squillace N, Taramasso L, De Socio GV, Molteni C, Valsecchi L, et al. Causes of HIV Treatment Interruption during the Last 20 Years: A Multi-Cohort Real-Life Study. Viruses. 2023; 15(3):720. https://doi.org/10.3390/v15030720
Chicago/Turabian StyleDe Vito, Andrea, Elena Ricci, Barbara Menzaghi, Giancarlo Orofino, Canio Vito Martinelli, Nicola Squillace, Lucia Taramasso, Giuseppe Vittorio De Socio, Chiara Molteni, Laura Valsecchi, and et al. 2023. "Causes of HIV Treatment Interruption during the Last 20 Years: A Multi-Cohort Real-Life Study" Viruses 15, no. 3: 720. https://doi.org/10.3390/v15030720
APA StyleDe Vito, A., Ricci, E., Menzaghi, B., Orofino, G., Martinelli, C. V., Squillace, N., Taramasso, L., De Socio, G. V., Molteni, C., Valsecchi, L., Costa, C., Celesia, B. M., Parruti, G., Pellicanò, G. F., Sarchi, E., Cascio, A., Cenderello, G., Falasca, K., Di Biagio, A., ... Madeddu, G. (2023). Causes of HIV Treatment Interruption during the Last 20 Years: A Multi-Cohort Real-Life Study. Viruses, 15(3), 720. https://doi.org/10.3390/v15030720










