Co-Delivery of the Human NY-ESO-1 Tumor-Associated Antigen and Alpha-GalactosylCeramide by Filamentous Bacteriophages Strongly Enhances the Expansion of Tumor-Specific CD8+ T Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Mice
2.3. Phage Nanoparticle Construction and Production
2.4. Alpha-GalactosylCeramide Decoration of Phage Particles
2.5. Viral Transduction of Jurkat Cells
2.6. Human and Mouse Dendritic Cell Generation
2.7. CD83 Expression on Human DCs and NFAT Assay
2.8. In Vitro iNKT Response
2.9. In Vivo Immunization and Dextramer Staining
3. Results
3.1. Production of NY-ESO-1 Bacteriophage Bound to α-GalCer
3.2. Antigen-Specific T Cell Response to Bacteriophages Carrying Tumor-Associated Antigens
3.3. iNKT Response to Phage Bound to α-GalCer
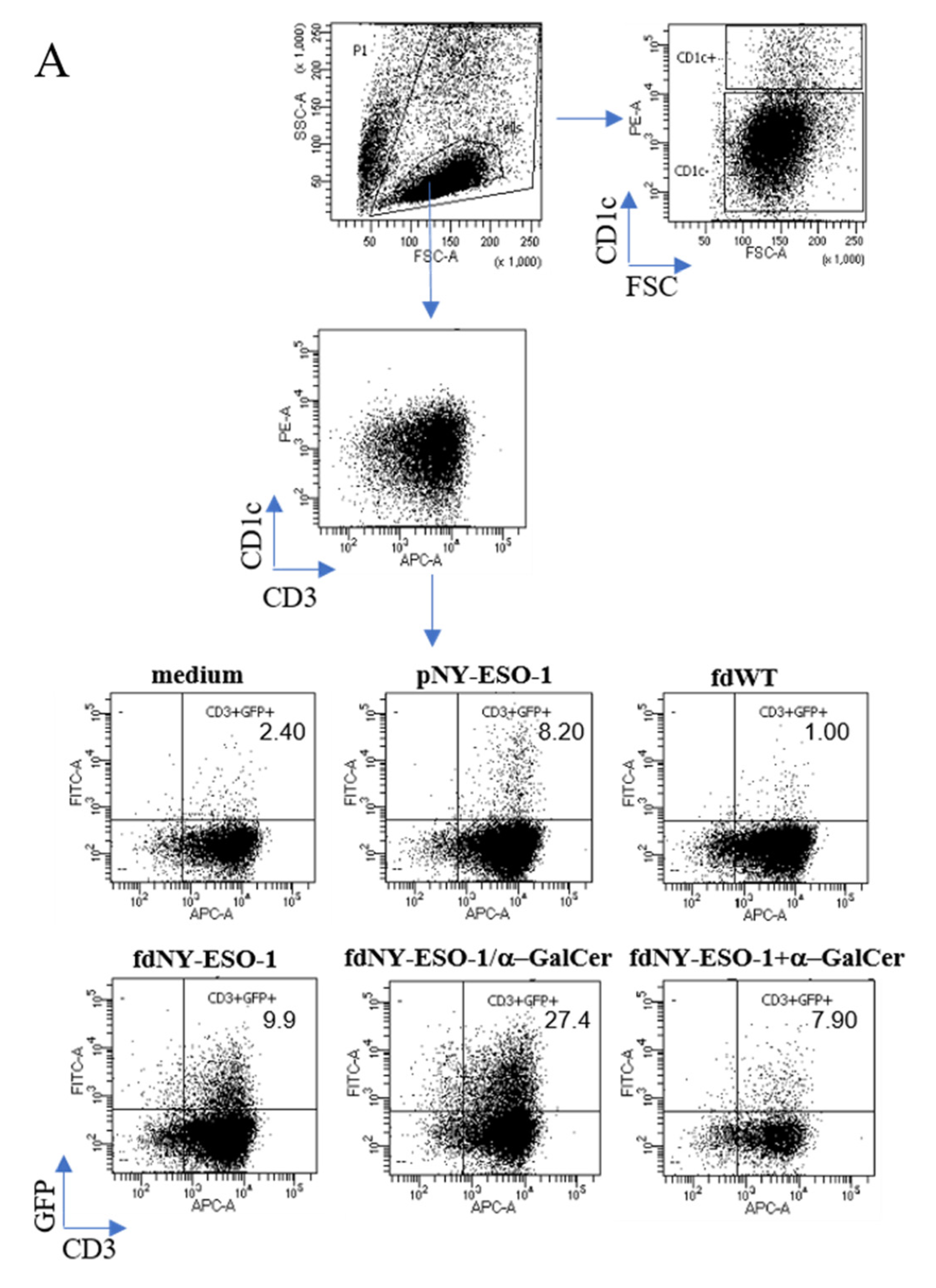
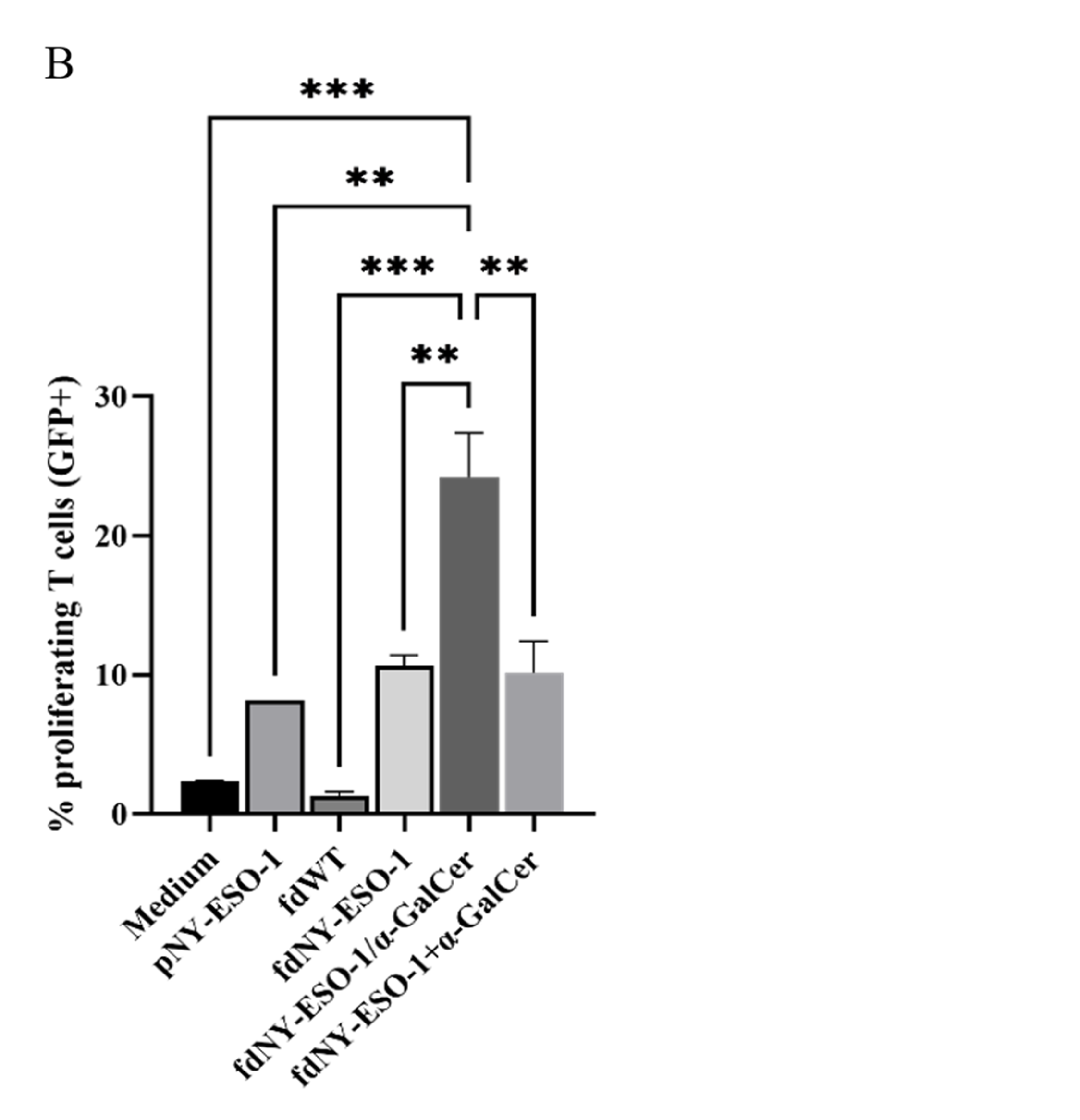
3.4. Co-Delivery of Peptides and α-GalCer Enhances CD8 T Cell Responses In Vivo
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buonaguro, L.; Petrizzo, A.; Tornesello, M.L.; Buonaguro, F.M. Translating Tumor Antigens into Cancer Vaccines. Clin. Vaccine Immunol. 2011, 18, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Le, I.; Dhandayuthapani, S.; Chacon, J.; Eiring, A.M.; Gadad, S.S. Harnessing the Immune System with Cancer Vaccines: From Prevention to Therapeutics. Vaccines 2022, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhao, X. Nanomaterial-Based Delivery Vehicles for Therapeutic Cancer Vaccine Development. Cancer Biol. Med. 2021, 18, 352–371. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Nishimura, Y. The Present Status and Future Prospects of Peptide-Based Cancer Vaccines. Int. Immunol. 2016, 28, 319–328. [Google Scholar] [CrossRef]
- Stephens, A.J.; Burgess-Brown, N.A.; Jiang, S. Beyond Just Peptide Antigens: The Complex World of Peptide-Based Cancer Vaccines. Front. Immunol. 2021, 12, 696791. [Google Scholar] [CrossRef]
- Raza, A.; Merhi, M.; Inchakalody, V.P.; Krishnankutty, R.; Relecom, A.; Uddin, S.; Dermime, S. Unleashing the Immune Response to NY-ESO-1 Cancer Testis Antigen as a Potential Target for Cancer Immunotherapy. J. Transl. Med. 2020, 18, 140. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kageyama, S.; Miyahara, Y.; Okayama, T.; Kokura, S.; Wang, L.; Sato, E.; Yagita, H.; Itoh, Y.; Shiku, H. Safety and Antibody Immune Response of CHP-NY-ESO-1 Vaccine Combined with Poly-ICLC in Advanced or Recurrent Esophageal Cancer Patients. Cancer Immunol. Immunother. 2021, 70, 3081–3091. [Google Scholar] [CrossRef]
- Pavlick, A.; Blazquez, A.B.; Meseck, M.; Lattanzi, M.; Ott, P.A.; Marron, T.U.; Holman, R.M.; Mandeli, J.; Salazar, A.M.; McClain, C.B.; et al. Combined Vaccination with NY-ESO-1 Protein, Poly-ICLC, and Montanide Improves Humoral and Cellular Immune Responses in Patients with High-Risk Melanoma. Cancer Immunol. Res. 2020, 8, 70–80. [Google Scholar] [CrossRef]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA Vaccine Drives Immunity in Checkpoint-Inhibitor-Treated Melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef]
- Holmberg-Thydén, S.; Dufva, I.H.; Gang, A.O.; Breinholt, M.F.; Schejbel, L.; Andersen, M.K.; Kadivar, M.; Svane, I.M.; Grønbæk, K.; Hadrup, S.R.; et al. Epigenetic Therapy in Combination with a Multi-Epitope Cancer Vaccine Targeting Shared Tumor Antigens for High-Risk Myelodysplastic Syndrome—A Phase I Clinical Trial. Cancer Immunol. Immunother. 2022, 71, 433–444. [Google Scholar] [CrossRef]
- Gableh, F.; Saeidi, M.; Hemati, S.; Hamdi, K.; Soleimanjahi, H.; Gorji, A.; Ghaemi, A. Combination of the Toll like Receptor Agonist and α-Galactosylceramide as an Efficient Adjuvant for Cancer Vaccine. J. Biomed. Sci. 2016, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Dellabona, P.; Padovan, E.; Casorati, G.; Brockhaus, M.; Lanzavecchia, A. An Invariant Vα24-JαQ/Vβ11 t Cell Receptor Is Expressed in All Individuals by Clonally Expanded CD4−8−t Cells. J. Exp. Med. 1994, 180, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Rossjohn, J.; Pellicci, D.G.; Patel, O.; Gapin, L.; Godfrey, D.I. Recognition of CD1d-Restricted Antigens by Natural Killer T Cells. Nat. Rev. Immunol. 2012, 12, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Springfield, R.; Chen, S.; Li, X.; Feng, X.; Moshirian, R.; Yang, R.; Yuan, W. α-GalCer and INKT Cell-Based Cancer Immunotherapy: Realizing the Therapeutic Potentials. Front. Immunol. 2019, 10, 1126. [Google Scholar] [CrossRef]
- Bedard, M.; Salio, M.; Cerundolo, V. Harnessing the Power of Invariant Natural Killer T Cells in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1829. [Google Scholar] [CrossRef]
- Kinjo, Y.; Takatsuka, S.; Kitano, N.; Kawakubo, S.; Abe, M.; Ueno, K.; Miyazaki, Y. Functions of CD1d-Restricted Invariant Natural Killer T Cells in Antimicrobial Immunity and Potential Applications for Infection Control. Front. Immunol. 2018, 9, 1266. [Google Scholar] [CrossRef]
- Horst, D.; Geerdink, R.J.; Gram, A.M.; Stoppelenburg, A.J.; Ressing, M.E. Hiding Lipid Presentation: Viral Interference with CD1d-Restricted Invariant Natural Killer T (INKT) Cell Activation. Viruses 2012, 4, 2379–2399. [Google Scholar] [CrossRef]
- Gálvez, N.M.S.; Bohmwald, K.; Pacheco, G.A.; Andrade, C.A.; Carreño, L.J.; Kalergis, A.M. Type I Natural Killer T Cells as Key Regulators of the Immune Response to Infectious Diseases. Clin. Microbiol. Rev. 2021, 34, e00232-20. [Google Scholar] [CrossRef]
- Anderson, R.J.; Compton, B.J.; Tang, C.W.; Authier-Hall, A.; Hayman, C.M.; Swinerd, G.W.; Kowalczyk, R.; Harris, P.; Brimble, M.A.; Larsen, D.S.; et al. NKT Cell-Dependent Glycolipid-Peptide Vaccines with Potent Anti-Tumour Activity. Chem. Sci. 2015, 6, 5120–5127. [Google Scholar] [CrossRef]
- Burn, O.K.; Farrand, K.; Pritchard, T.; Draper, S.; Tang, C.W.; Mooney, A.H.; Schmidt, A.J.; Yang, S.H.; Williams, G.M.; Brimble, M.A.; et al. Glycolipid-Peptide Conjugate Vaccines Elicit CD8+ T-Cell Responses and Prevent Breast Cancer Metastasis. Clin. Transl. Immunol. 2022, 11, e1401. [Google Scholar] [CrossRef]
- Hashiguchi, S.; Yamaguchi, Y.; Takeuchi, O.; Akira, S.; Sugimura, K. Immunological Basis of M13 Phage Vaccine: Regulation under MyD88 and TLR9 Signaling. Biochem. Biophys. Res. Commun. 2010, 402, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jiang, R.; Wang, Y.; Zhu, M.; Zhang, X.; Dong, S.; Shi, H.; Wang, L. Recombinant Phage Elicits Protective Immune Response against Systemic S. Globosa Infection in Mouse Model. Sci. Rep. 2017, 7, 42024. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, R.; D’Apice, L.; Trovato, M.; Cuccaro, F.; Costa, V.; De Leo, M.G.; Marzullo, V.M.; Biondo, C.; D’Auria, S.; De Matteis, M.A.; et al. Antigen Delivery by Filamentous Bacteriophage Fd Displaying an Anti-DEC-205 Single-chain Variable Fragment Confers Adjuvanticity by Triggering a TLR 9-mediated Immune Response. EMBO Mol. Med. 2015, 7, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Dapice, L.; Costa, V.; Sartorius, R.; Trovato, M.; Aprile, M.; De Berardinis, P. Stimulation of Innate and Adaptive Immunity by Using Filamentous Bacteriophage Fd Targeted to DEC-205. J. Immunol. Res. 2015, 2015, 585078. [Google Scholar] [CrossRef]
- Reardon, S. Microbiology: Phage Therapy Gets Revitalized. Nature 2014, 510, 15–17. [Google Scholar] [CrossRef]
- Sartorius, R.; D’Apice, L.; Barba, P.; Cipria, D.; Grauso, L.; Cutignano, A.; De Berardinis, P. Vectorized Delivery of Alpha-Galactosylceramide and Tumor Antigen on Filamentous Bacteriophage Fd Induces Protective Immunity by Enhancing Tumor-Specific T Cell Response. Front. Immunol. 2018, 9, 1496. [Google Scholar] [CrossRef]
- González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.N.; Rito-Palomares, M.; Benavides, J. Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef]
- Stern, Z.; Stylianou, D.C.; Kostrikis, L.G. The Development of Inovirus-Associated Vector Vaccines Using Phage-Display Technologies. Expert Rev. Vaccines 2019, 18, 913–920. [Google Scholar] [CrossRef]
- Zou, J.; Dickerson, M.T.; Owen, N.K.; Landon, L.A.; Deutscher, S.L. Biodistribution of Filamentous Phage Peptide Libraries in Mice. Mol. Biol. Rep. 2004, 31, 121–129. [Google Scholar] [CrossRef]
- Berardinis, P.; Sartorius, R.; Caivano, A.; Mascolo, D.; Domingo, G.; Pozzo, G.; Gaubin, M.; Perham, R.; Piatier-Tonneau, D.; Guardiola, J. Use of Fusion Proteins and Procaryotic Display Systems for Delivery of HIV-1 Antigens: Development of Novel Vaccines for HIV-1 Infection. Curr. HIV Res. 2005, 1, 441–446. [Google Scholar] [CrossRef]
- Murgas, P.; Bustamante, N.; Araya, N.; Cruz-Gómez, S.; Durán, E.; Gaete, D.; Oyarce, C.; López, E.; Herrada, A.A.; Ferreira, N.; et al. A Filamentous Bacteriophage Targeted to Carcinoembryonic Antigen Induces Tumor Regression in Mouse Models of Colorectal Cancer. Cancer Immunol. Immunother. 2018, 67, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Safaei Ghaderi, S.; Riazi-Rad, F.; Safaie Qamsari, E.; Bagheri, S.; Rahimi-Jamnani, F.; Sharifzadeh, Z. Development of a Human Phage Display-Derived Anti-Pd-1 Scfv Antibody: An Attractive Tool for Immune Checkpoint Therapy. SSRN Electron. J. 2022, 22, 22. [Google Scholar] [CrossRef]
- Jutz, S.; Leitner, J.; Schmetterer, K.; Doel-Perez, I.; Majdic, O.; Grabmeier-Pfistershammer, K.; Paster, W.; Huppa, J.B.; Steinberger, P. Assessment of Costimulation and Coinhibition in a Triple Parameter T Cell Reporter Line: Simultaneous Measurement of NF-ΚB, NFAT and AP-1. J. Immunol. Methods 2016, 430, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Schümann, J.; Facciotti, F.; Panza, L.; Michieletti, M.; Compostella, F.; Collmann, A.; Mori, L.; De Libero, G. Differential Alteration of Lipid Antigen Presentation to NKT Cells Due to Imbalances in Lipid Metabolism. Eur. J. Immunol. 2007, 37, 1431–1441. [Google Scholar] [CrossRef]
- Aurisicchio, L.; Salvatori, E.; Lione, L.; Bandini, S.; Pallocca, M.; Maggio, R.; Fanciulli, M.; De Nicola, F.; Goeman, F.; Ciliberto, G.; et al. Poly-Specific Neoantigen-Targeted Cancer Vaccines Delay Patient Derived Tumor Growth. J. Exp. Clin. Cancer Res. 2019, 38, 78. [Google Scholar] [CrossRef]
- Del Pozzo, G.; Mascolo, D.; Sartorius, R.; Citro, A.; Barba, P.; D’Apice, L.; De Berardinis, P. Triggering DTH and CTL Activity by Fd Filamentous Bacteriophages: Role of CD4+ T Cells in Memory Responses. J. Biomed. Biotechnol. 2010, 2010, 894971. [Google Scholar] [CrossRef]
- Sartorius, R.; Bettua, C.; D’Apice, L.; Caivano, A.; Trovato, M.; Russo, D.; Zanoni, I.; Granucci, F.; Mascolo, D.; Barba, P.; et al. Vaccination with Filamentous Bacteriophages Targeting DEC-205 Induces DC Maturation and Potent Anti-Tumor T-Cell Responses in the Absence of Adjuvants. Eur. J. Immunol. 2011, 41, 2573–2584. [Google Scholar] [CrossRef]
- D’Apice, L.; Cuccaro, F.; Varriale, S.; Cipria, D.; Sartorius, R.; Circosta, P.; Cignetti, A.; Salerno, M.; Coscia, M.R.; Oreste, U.; et al. An Ig Transmembrane Domain Motif Improves the Function of TCRs Transduced in Human T Cells: Implications for Immunotherapy. J. Immunother. 2019, 42, 97–109. [Google Scholar] [CrossRef]
- Shenderov, E.; Kandasamy, M.; Gileadi, U.; Chen, J.; Shepherd, D.; Gibbs, J.; Prota, G.; Silk, J.D.; Yewdell, J.W.; Cerundolo, V. Generation and Characterization of HLA-A2 Transgenic Mice Expressing the Human TCR 1G4 Specific for the HLA-A2 Restricted NY-ESO-1 157-165 Tumor-Specific Peptide. J. Immunother. Cancer 2021, 9, e002544. [Google Scholar] [CrossRef]
- Chen, J.-L.; Dunbar, P.R.; Gileadi, U.; Jäger, E.; Gnjatic, S.; Nagata, Y.; Stockert, E.; Panicali, D.L.; Chen, Y.-T.; Knuth, A.; et al. Identification of NY-ESO-1 Peptide Analogues Capable of Improved Stimulation of Tumor-Reactive CTL. J. Immunol. 2000, 165, 948–955. [Google Scholar] [CrossRef]
- Brossay, L.; Chioda, M.; Burdin, N.; Koezuka, Y.; Casorati, G.; Dellabona, P.; Kronenberg, M. CD1d- Mediated Recognition of an α-Galactosylceramide by Natural Killer T Cells Is Highly Conserved through Mammalian Evolution. J. Exp. Med. 1998, 188, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Brigl, M.; Brenner, M.B. CD1: Antigen Presentation and T Cell Function. Annu. Rev. Immunol. 2004, 22, 817–890. [Google Scholar] [CrossRef] [PubMed]
- Kjer-Nielsen, L.; Borg, N.A.; Pellicci, D.G.; Beddoe, T.; Kostenko, L.; Clements, C.S.; Williamson, N.A.; Smyth, M.J.; Besra, G.S.; Reid, H.H.; et al. A Structural Basis for Selection and Cross-Species Reactivity of the Semi-Invariant NKT Cell Receptor in CD1d/Glycolipid Recognition. J. Exp. Med. 2006, 203, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Meijlink, M.A.; Chua, Y.C.; Chan, S.T.S.; Anderson, R.J.; Rosenberg, M.W.; Cozijnsen, A.; Mollard, V.; McFadden, G.I.; Draper, S.L.; Holz, L.E.; et al. 6″-Modifed α-GalCer-Peptide Conjugate Vaccine Candidates Protect against Liver-Stage Malaria†. RSC Chem. Biol. 2022, 3, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Tang, C.W.; Daniels, N.J.; Compton, B.J.; Hayman, C.M.; Johnston, K.A.; Knight, D.A.; Gasser, O.; Poyntz, H.C.; Ferguson, P.M.; et al. A Self-Adjuvanting Vaccine Induces Cytotoxic Tlymphocytes That Suppress Allergy. Nat. Chem. Biol. 2014, 10, 943–949. [Google Scholar] [CrossRef]
- Grasso, C.; Field, C.S.; Tang, C.W.; Ferguson, P.M.; Compton, B.J.; Anderson, R.J.; Painter, G.F.; Weinkove, R.; Hermans, I.F.; Berridge, M.V. Vaccines Adjuvanted with an NKT Cell Agonist Induce Effective T-Cell Responses in Models of CNS Lymphoma. Immunotherapy 2020, 12, 395–406. [Google Scholar] [CrossRef]
- Speir, M.; Authier-Hall, A.; Brooks, C.R.; Farrand, K.J.; Compton, B.J.; Anderson, R.J.; Heiser, A.; Osmond, T.L.; Tang, C.W.; Berzofsky, J.A.; et al. Glycolipid-Peptide Conjugate Vaccines Enhance CD8+ T Cell Responses against Human Viral Proteins. Sci. Rep. 2017, 7, 14273. [Google Scholar] [CrossRef]
- Hung, L.-C.; Lin, C.-C.; Hung, S.-K.; Wu, B.-C.; Jan, M.-D.; Liou, S.-H.; Fu, S.-L. A Synthetic Analog of α-Galactosylceramide Induces Macrophage Activation via the TLR4-Signaling Pathways. Biochem. Pharmacol. 2007, 73, 1957–1970. [Google Scholar] [CrossRef]
- Firat, H.; Cochet, M.; Rohrlich, P.S.; Garcia-Pons, F.; Darche, S.; Danos, O.; Lemonnier, F.A.; Langlade-Demoyen, P. Comparative Analysis of the CD8+ T Cell Repertoires of H-2 Class I Wild-Type/HLA-A2.1 and H-2 Class I Knockout/HLA-A2.1 Transgenic Mice. Int. Immunol. 2002, 14, 925–934. [Google Scholar] [CrossRef]
- Dölen, Y.; Gileadi, U.; Chen, J.L.; Valente, M.; Creemers, J.H.A.; Van Dinther, E.A.W.; van Riessen, N.K.; Jäger, E.; Hruby, M.; Cerundolo, V.; et al. PLGA Nanoparticles Co-Encapsulating NY-ESO-1 Peptides and IMM60 Induce Robust CD8 and CD4 T Cell and B Cell Responses. Front. Immunol. 2021, 12, 641703. [Google Scholar] [CrossRef]
- Paczesny, J.; Bielec, K. Application of Bacteriophages in Nanotechnology. Nanomaterials 2020, 10, 1944. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, R.; D’Apice, L.; Prisco, A.; Berardinis, P. De Arming Filamentous Bacteriophage, a Nature-Made Nanoparticle, for New Vaccine and Immunotherapeutic Strategies. Pharmaceutics 2019, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.S.; Krag, D.N.; Peletskaya, E.N.; Pero, S.C.; Sun, Y.J.; Carman, C.L.; McCahill, L.E.; Roland, T.A. Intravenous Infusion of Phage-Displayed Antibody Library in Human Cancer Patients: Enrichment and Cancer-Specificity of Tumor-Homing Phage-Antibodies. Cancer Immunol. Immunother. 2013, 62, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Speck, P.; Smithyman, A. Safety and Efficacy of Phage Therapy via the Intravenous Route. FEMS Microbiol. Lett. 2015, 363, fnv242. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manco, R.; D’Apice, L.; Trovato, M.; Lione, L.; Salvatori, E.; Pinto, E.; Compagnone, M.; Aurisicchio, L.; De Berardinis, P.; Sartorius, R. Co-Delivery of the Human NY-ESO-1 Tumor-Associated Antigen and Alpha-GalactosylCeramide by Filamentous Bacteriophages Strongly Enhances the Expansion of Tumor-Specific CD8+ T Cells. Viruses 2023, 15, 672. https://doi.org/10.3390/v15030672
Manco R, D’Apice L, Trovato M, Lione L, Salvatori E, Pinto E, Compagnone M, Aurisicchio L, De Berardinis P, Sartorius R. Co-Delivery of the Human NY-ESO-1 Tumor-Associated Antigen and Alpha-GalactosylCeramide by Filamentous Bacteriophages Strongly Enhances the Expansion of Tumor-Specific CD8+ T Cells. Viruses. 2023; 15(3):672. https://doi.org/10.3390/v15030672
Chicago/Turabian StyleManco, Roberta, Luciana D’Apice, Maria Trovato, Lucia Lione, Erika Salvatori, Eleonora Pinto, Mirco Compagnone, Luigi Aurisicchio, Piergiuseppe De Berardinis, and Rossella Sartorius. 2023. "Co-Delivery of the Human NY-ESO-1 Tumor-Associated Antigen and Alpha-GalactosylCeramide by Filamentous Bacteriophages Strongly Enhances the Expansion of Tumor-Specific CD8+ T Cells" Viruses 15, no. 3: 672. https://doi.org/10.3390/v15030672
APA StyleManco, R., D’Apice, L., Trovato, M., Lione, L., Salvatori, E., Pinto, E., Compagnone, M., Aurisicchio, L., De Berardinis, P., & Sartorius, R. (2023). Co-Delivery of the Human NY-ESO-1 Tumor-Associated Antigen and Alpha-GalactosylCeramide by Filamentous Bacteriophages Strongly Enhances the Expansion of Tumor-Specific CD8+ T Cells. Viruses, 15(3), 672. https://doi.org/10.3390/v15030672




