Network Pharmacology and Molecular Docking Elucidate the Underlying Pharmacological Mechanisms of the Herb Houttuynia cordata in Treating Pneumonia Caused by SARS-CoV-2
Abstract
:1. Introduction
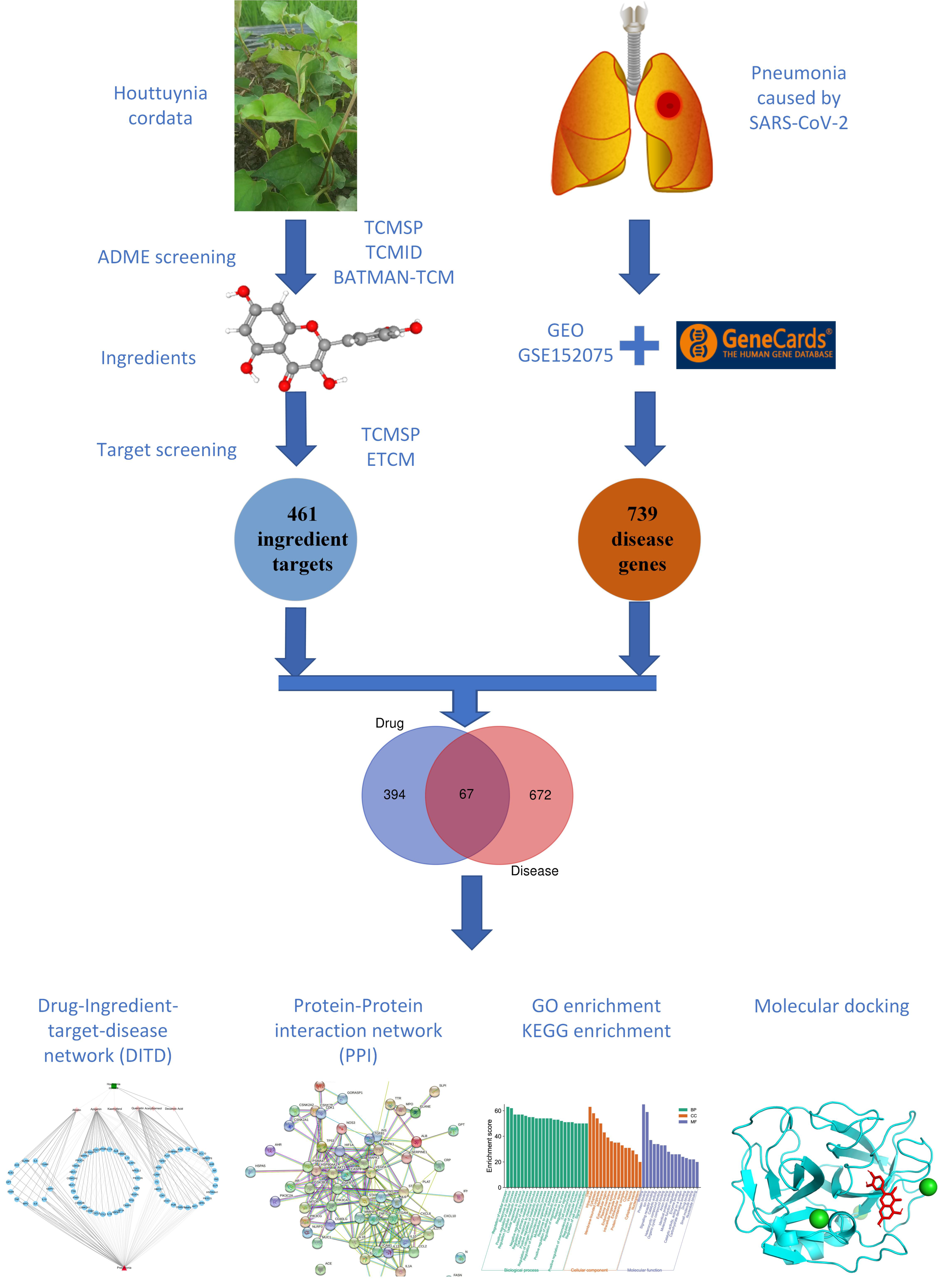
2. Materials and Methods
2.1. Bioactive Compound Screening and Pharmacokinetic Prediction
2.2. Potential Targets of HC Active Components
2.3. Identification of Pneumonia-Related Targets Database
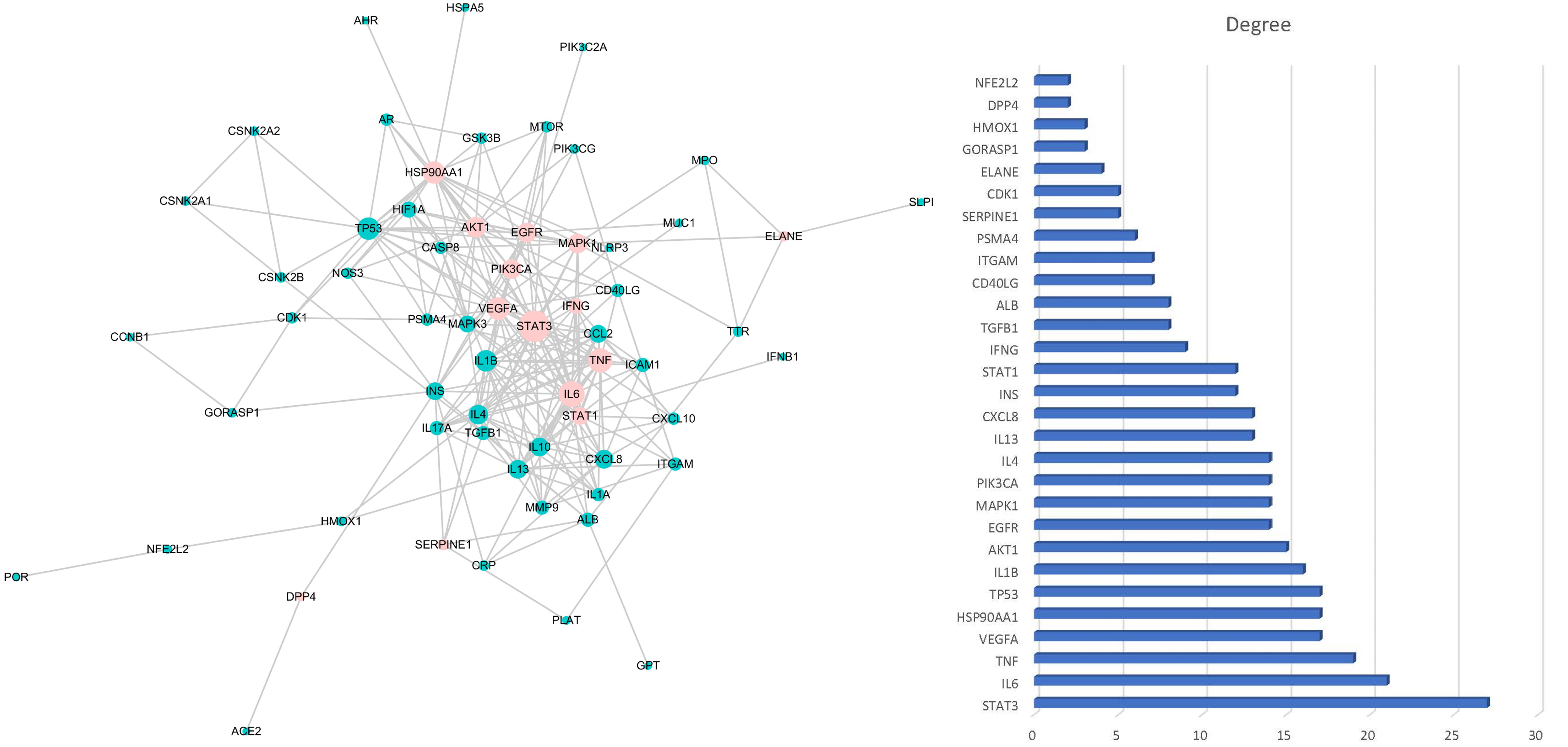
2.4. Construction of PPI Network and Herb-Metabolites-Targets-Disease (HMTD) Network
2.5. GO and KEGG Analysis
2.6. Molecular Docking
2.7. Molecular Dynamics Simulation
3. Results
3.1. Target Prediction and Analysis of HC
3.2. Disease Targets Analysis
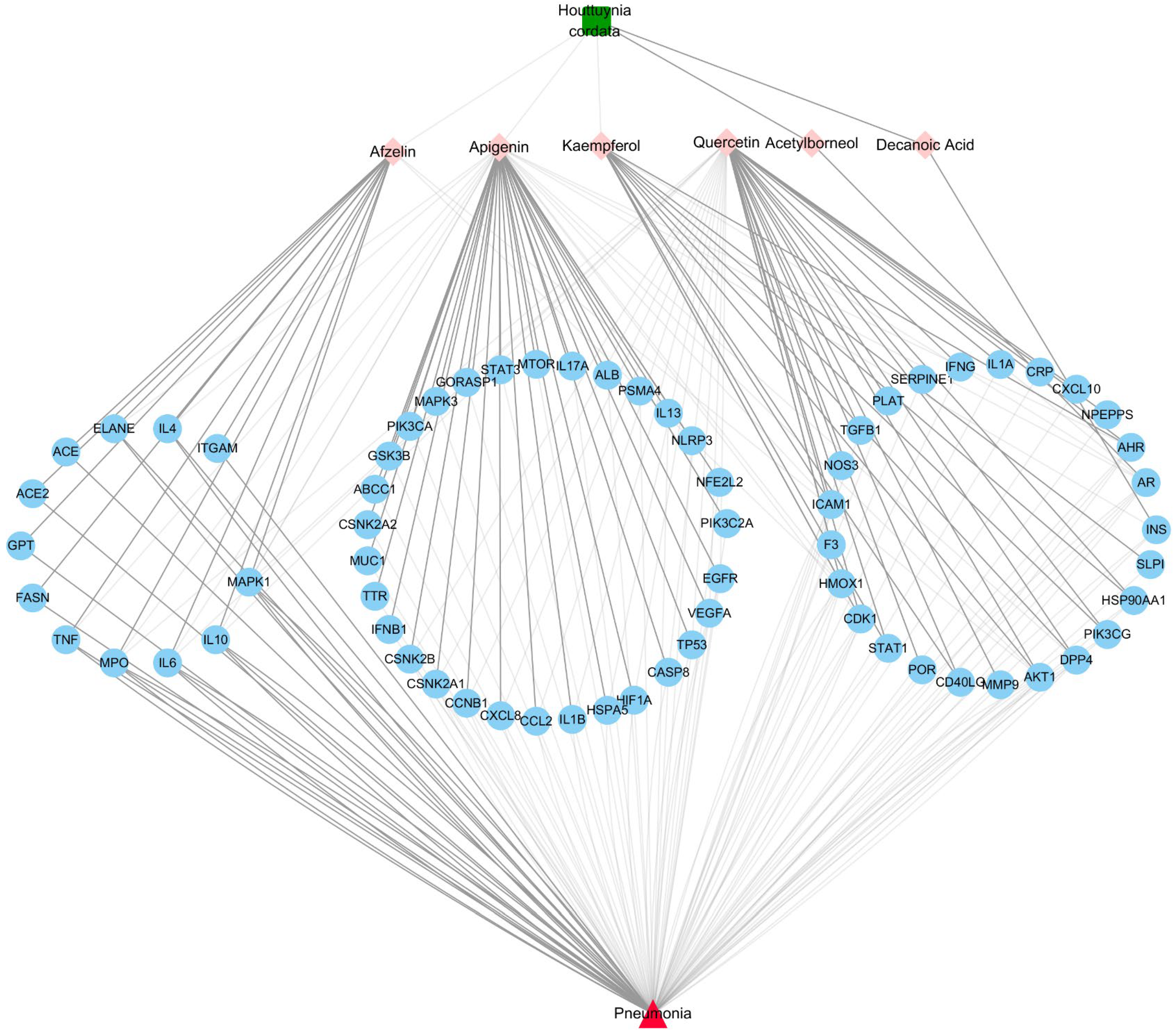
3.3. Herb-Ingredients-Targets-Disease Network of HC Analysis
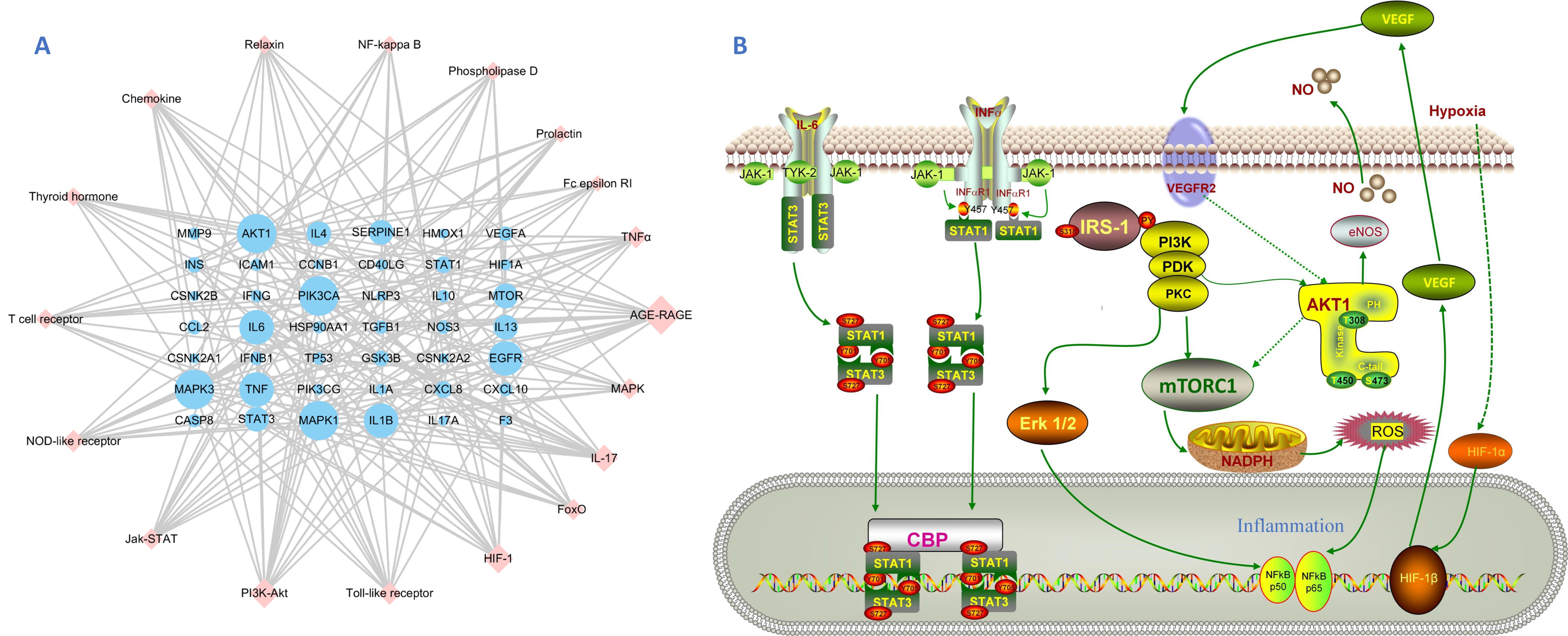
3.4. GO and KEGG Enrichment Analysis
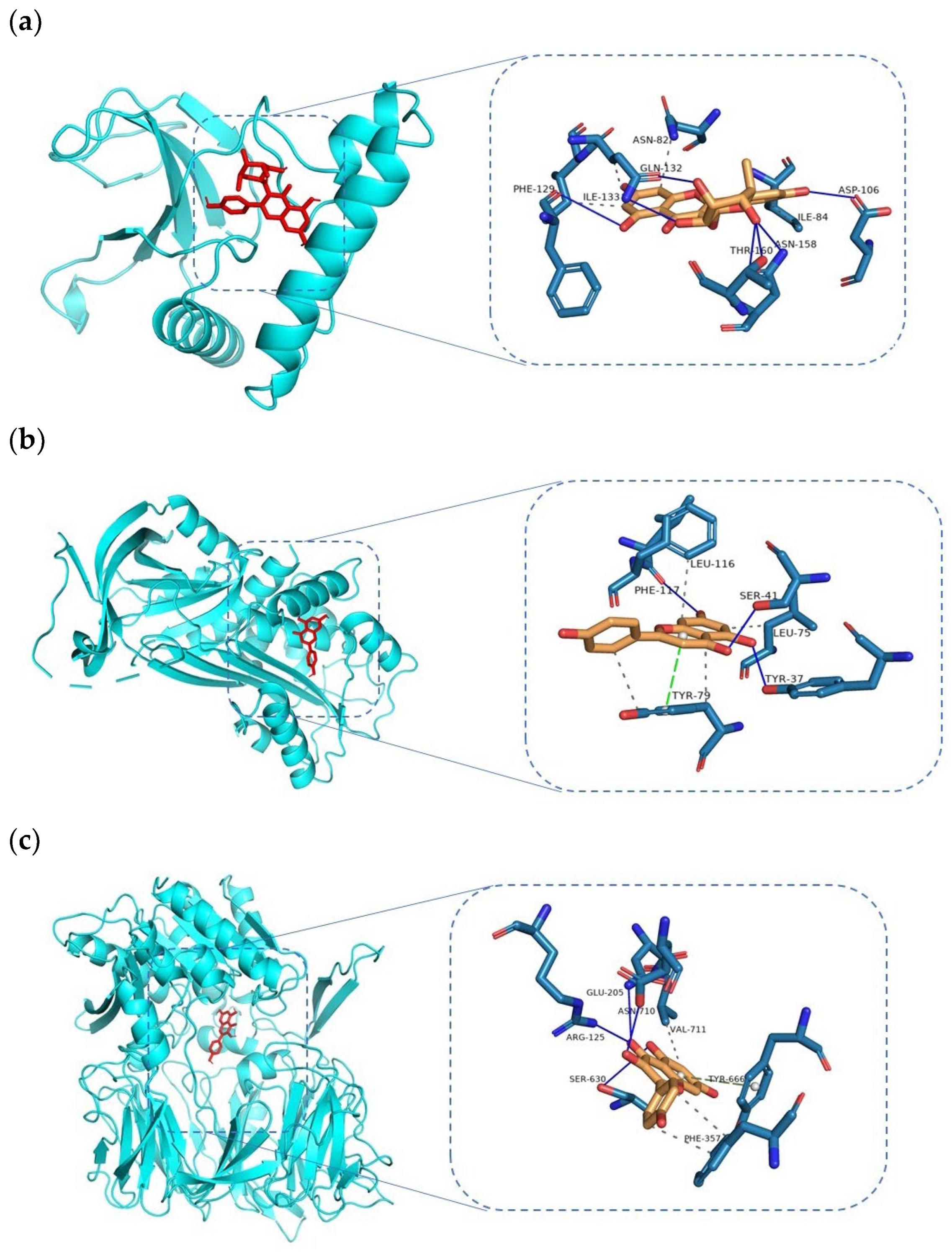
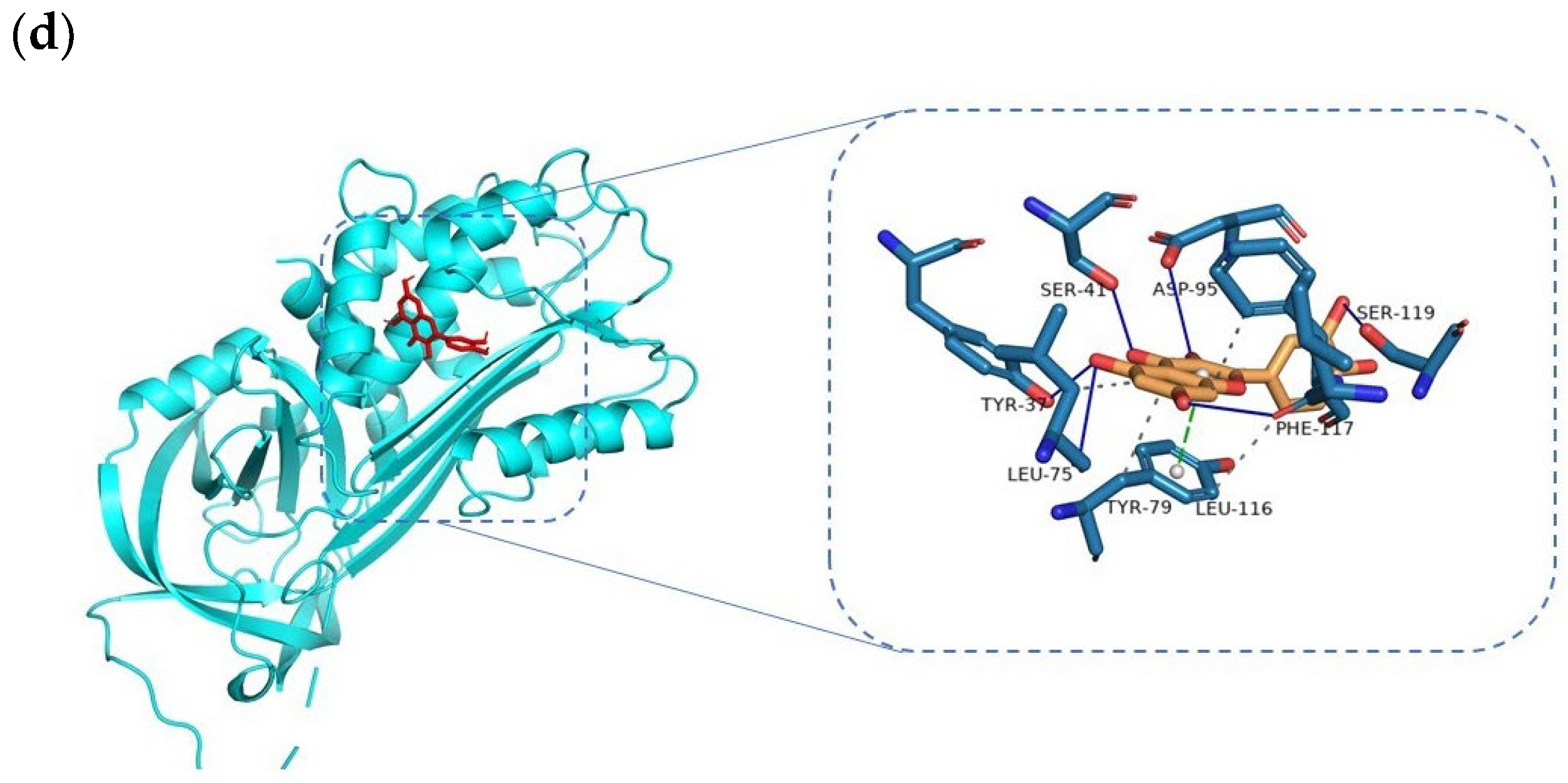
3.5. Docking and Molecular Dynamics Simulation Analysis of Ingredients-Targets
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.T.; Yang, C.L.; Yin, M.C. Protective effects from Houttuynia cordata aqueous extract against acetaminophen-induced liver injury. Biomedicine 2014, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, Y.; Ling, L.; Zhang, Y.; Li, H.; Chen, D. Beneficial effects of Houttuynia cordata polysaccharides on “two-hit” acute lung injury and endotoxic fever in rats associated with anti-complementary activities. Acta Pharm. Sin. B 2018, 8, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.-M.; Lee, K.-M.; Koon, C.-M.; Cheung, C.S.-F.; Lau, C.-P.; Ho, H.-M.; Lee, M.Y.-H.; Au, S.W.-N.; Cheng, C.H.-K.; Lau, C.B.-S.; et al. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008, 118, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-Y.; Zhang, Y.-Y.; Ou, Y.-Y.; Lu, X.-X.; Pan, L.-Y.; Li, H.; Lu, Y.; Chen, D.-F. Houttuynia cordata Thunb. polysaccharides ameliorates lipopolysaccharide-induced acute lung injury in mice. J. Ethnopharmacol. 2015, 173, 81–90. [Google Scholar] [CrossRef]
- Fuzimoto, A.D.; Isidoro, C. The antiviral and coronavirus-host protein pathways inhibiting properties of herbs and natural compounds—Additional weapons in the fight against the COVID-19 pandemic? J. Tradit. Complement. Med. 2020, 10, 405–419. [Google Scholar] [CrossRef]
- Du, S.; Li, H.; Cui, Y.; Yang, L.; Wu, J.; Huang, H.; Chen, Y.; Huang, W.; Zhang, R.; Yang, J.; et al. Houttuynia cordata inhibits lipopolysaccharide-induced rapid pulmonary fibrosis by up-regulating IFN-γ and inhibiting the TGF-β1/Smad pathway. Int. Immunopharmacol. 2012, 13, 331–340. [Google Scholar] [CrossRef]
- Fu, J.G.; Dai, L.; Lin, Z.; Lu, H.M. Houttuynia cordata thunb: A review of phytochemistry and pharmacology and quality control. Chin. Med. 2013, 04, 101–123. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.D.; Gao, H.; Zhu, Q.C.; Wang, Y.Q.; Li, T.; Mu, Z.Q.; Wu, H.L.; Peng, T.; Yao, X.S. Houttuynoids A–E, anti-herpes simplex virus active flavonoids with novel skeletons from Houttuynia cordata. Org. Lett. 2012, 14, 1772–1775. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Yang, Z.; Wang, J.; Xu, Y.; Tan, R.; Li, E. Houttuynia cordata blocks HSV infection through inhibition of NF-kB activation. Antivir. Res. 2011, 92, 341–345. [Google Scholar] [CrossRef]
- Shi, C.C.; Zhu, H.Y.; Li, H.; Zeng, D.L.; Shi, X.L.; Zhang, Y.Y.; Lu, Y.; Ling, L.J.; Wang, C.Y.; Chen, D.F. Regulating the balance of Th17/Treg cells in gut-lung axis contributed to the therapeutic effect of Houttuynia cordata polysaccharides on H1N1-induced acute lung injury. Int. J. Biol. Macromol. 2020, 158, 52–66. [Google Scholar] [CrossRef]
- Antonio, A.d.S.; Wiedemann, L.S.M.; Veiga-Junior, V.F. Natural products’ role against COVID-19. RSC Adv. 2020, 10, 23379–23393. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Sun, L.; Zou, S.; Chen, J.; Mao, H.; Zhang, Y.; Liao, N.; Zhang, R. Antiviral effects of Houttuynia cordata polysaccharide extract on murine norovirus-1 (mnv-1)-a human Norovirus surrogate. Molecules 2019, 24, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairns, L.; Abed El Khaleq, Y.; Storrar, W.; Scheuermann-Freestone, M. COVID-19 myopericarditis with cardiac tamponade in the absence of respiratory symptoms: A case report. J. Med. Case Rep. 2021, 15, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine storm in COVID-19: The current evidence and treatment strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, J.; Kim, J.W.; Lee, S.G.; Kim, H.P. Flavonoids from the aerial parts of Houttuynia cordata attenuate lung inflammation in mice. Arch. Pharmacal Res. 2015, 38, 1304–1311. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Liang, X.; Yang, L.; Su, M.; Lai, K.P. Network Pharmacology and bioinformatics analyses identify intersection genes of niacin and COVID-19 as potential therapeutic targets. Brief. Bioinform. 2020, 22, 1279–1290. [Google Scholar] [CrossRef]
- Zhou, Y.D.; Hou, Y.; Shen, J.Y.; Huang, Y.; Martin, W.; Cheng, F.X. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14–31. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, J.; Dasgupta, S.; Nagle, V.; Bhadra, B. COVID-19: Potential of microalgae derived natural astaxanthin as adjunctive supplement in alleviating cytokine storm. SSRN Electron. J. 2020, 1–17. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Bai, C.; He, F.; Xie, Y.; Zhou, H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19). Pharmacol. Res. 2020, 158, 104939. [Google Scholar] [CrossRef]
- Gao, Q.; Tian, D.; Han, Z.; Lin, J.; Chang, Z.; Zhang, D.; Ma, D. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Buyang Huanwu decoction in the treatment of ischemic stroke. Evid. Based Complement. Altern. Med. 2021, 2021, 8815447–8815476. [Google Scholar] [CrossRef] [PubMed]
- Jian, G.H.; Su, B.Z.; Zhou, W.J.; Xiong, H. Application of network pharmacology and molecular docking to elucidate the potential mechanism of Eucommia ulmoides-Radix Achyranthis Bidentatae against osteoarthritis. BioData Min. 2020, 13, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yang, K. Exploring the pharmacological mechanism of Yanghe Decoction on HER2-positive breast cancer by a network pharmacology approach. J. Ethnopharmacol. 2017, 199, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Zhang, Y.Q.; Liu, Z.M.; Chen, T.; Lv, C.Y.; Tang, S.H.; Zhang, X.B.; Zhang, W.; Li, Z.Y.; Zhou, R.R.; et al. ETCM: An encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019, 47, 976–982. [Google Scholar] [CrossRef]
- Vastrad, B.; Vastrad, C.; Tengli, A. Bioinformatics analyses of significant genes, related pathways, and candidate diagnostic biomarkers and molecular targets in SARS-CoV-2/COVID-19. Gene Rep. 2020, 21, 100956. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Xu, T.; Sun, X.G.; Zhang, S.; Liu, S.L.; Fan, S.N.; Lei, C.F.; Tang, F.F.; Zhai, C.M.; Li, C.X.; et al. Network pharmacology and bioinformatics approach reveals the therapeutic mechanism of action of Baicalein in Hepatocellular carcinoma. Evid. Based Complement. Altern. Med. 2019, 2019, 7518374. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Duan, X.; Wu, S.; Yanfang, Y.; Wu, H. Network Pharmacology Integrated Molecular Docking Reveals the Anti-COVID-19 Mechanism of Qing-Fei-Da-Yuan Granules. Nat. Prod. Commun. 2020, 15, 1–15. [Google Scholar] [CrossRef]
- Zhang, M.X.; Yang, J.W.; Zhao, X.L.; Zhao, Y.; Zhu, S.Q. Network pharmacology and molecular docking study on the active ingredients of qidengmingmu capsule for the treatment of diabetic retinopathy. Sci. Rep. 2021, 11, 7382. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, Q.; Wang, X. “Network Target” theory and network pharmacology. In Network Pharmacology; Li, S., Ed.; Springer: Singapore, 2021; pp. 1–34. [Google Scholar] [CrossRef]
- Nacher, J.C.; Schwartz, J.-M. A global view of drug-therapy interactions. BMC Pharmacol. 2008, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.Y.; Lee, J.Y.; Chun, J.M. Exploring the mechanism of Gyejibokryeong-hwan against atherosclerosis using network pharmacology and molecular docking. Plants 2020, 9, 1750. [Google Scholar] [CrossRef]
- Tao, Q.; Du, J.; Li, X.; Zeng, J.; Tan, B.; Xu, J.; Lin, W.; Chen, X.-l. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Dev. Ind. Pharm. 2020, 46, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.H.; Wu, F.; Cao, W.Y.; Wu, Z.G.; Chao, Y.C.; Liang, C. Network pharmacology for the identification of phytochemicals in traditional Chinese medicine for COVID-19 that may regulate interleukin-6. Biosci. Rep. 2021, 41, BSR20202583. [Google Scholar] [CrossRef] [PubMed]
- Namoto, K.; Sirockin, F.; Ostermann, N.; Gessier, F.; Flohr, S.; Sedrani, R.; Gerhartz, B.; Trappe, J.; Hassiepen, U.; Duttaroy, A.; et al. Discovery of C-(1-aryl-cyclohexyl)-methylamines as selective, orally available inhibitors of dipeptidyl peptidase IV. Bioorg Med. Chem. Lett. 2014, 24, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Lechtenberg, B.C.; Kasperkiewicz, P.; Robinson, H.; Drag, M.; Riedl, S.J. The elastase-PK101 structure: Mechanism of an ultrasensitive activity-based probe revealed. ACS Chem. Biol. 2015, 10, 945–951. [Google Scholar] [CrossRef]
- Amaral, M.; Kokh, D.B.; Bomke, J.; Wegener, A.; Buchstaller, H.P.; Eggenweiler, H.M.; Matias, P.; Sirrenberg, C.; Wade, R.C.; Frech, M. Protein conformational flexibility modulates kinetics and thermodynamics of drug binding. Nat. Commun. 2017, 8, 2276. [Google Scholar] [CrossRef] [Green Version]
- Shaw, S.; Bourne, T.; Meier, C.; Carrington, B.; Gelinas, R.; Henry, A.; Popplewell, A.; Adams, R.; Baker, T.; Rapecki, S.; et al. Discovery and characterization of olokizumab: A humanized antibody targeting interleukin-6 and neutralizing gp130-signaling. MAbs 2014, 6, 774–782. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, M.; Cleasby, A.; Davies, T.G.; Hall, R.J.; Ludlow, R.F.; Murray, C.W.; Tisi, D.; Jhoti, H. Crystallographic screening using ultra-low-molecular-weight ligands to guide drug design. Drug Discov. Today 2019, 24, 1081–1086. [Google Scholar] [CrossRef]
- Sillen, M.; Miyata, T.; Vaughan, D.E.; Strelkov, S.V.; Declerck, P.J. Structural Insight into the Two-Step Mechanism of PAI-1 Inhibition by Small Molecule TM5484. Int. J. Mol. Sci. 2021, 22, 1482. [Google Scholar] [CrossRef]
- Shingnaisui, K.; Dey, T.; Manna, P.; Kalita, J. Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: A review. J. Ethnopharmacol. 2018, 220, 35–43. [Google Scholar] [CrossRef]
- Yang, S.H.; Zhang, J.L.; Yan, Y.Q.; Yang, M.; Li, C.; Li, J.M.; Zhong, L.Y.; Gong, Q.F.; Yu, H. Network pharmacology-based strategy to investigate the pharmacologic mechanisms of Atractylodes macrocephala Koidz. For the treatment of Chronic Gastritis. Front. Pharmacol. 2020, 10, 1629. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wu, L.; Ren, X.; Wu, X.; Chen, Y.; Ran, G.; Huang, A.; Huang, L.; Zhong, D. Traditional Chinese medicine for corona virus disease 2019: A protocol for systematic review. Medicine 2020, 99, e21774. [Google Scholar] [CrossRef]
- Bai, X.; Tang, Y.; Li, Q.; Chen, Y.; Liu, D.; Liu, G.; Fan, X.; Ma, R.; Wang, S.; Li, L.; et al. Network pharmacology integrated molecular docking reveals the bioactive components and potential targets of Morinda officinalis–Lycium barbarum coupled-herbs against oligoasthenozoospermia. Sci. Rep. 2021, 11, 2220. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.M.; Nho, K.J.; Kim, H.S.; Lee, A.Y.; Moon, B.C.; Kim, H.K. An ethyl acetate fraction derived from Houttuynia cordata extract inhibits the production of inflammatory markers by suppressing NF-κB and MAPK activation in lipopolysaccharide-stimulated RAW 264.7 macrophages. BMC Complement. Altern. Med. 2014, 14, 234–247. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Chen, J.; Zhang, W.; Li, H.; Wang, W.; Chen, J. Network pharmacology based investigation of the effects of herbal ingredients on the immune dysfunction in heart disease. Pharmacol. Res. 2019, 141, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ai, X.; Duan, Y.; Xue, M.; He, W.; Wang, C.; Xu, T.; Xu, M.; Liu, B.; Li, C.; et al. Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-κB and MAPK signaling pathways. Biomed. Pharmacother. 2017, 89, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Zhang, J.C.; Qiao, Y.; Liu, B.; Zhang, S. Uncovering synergistic mechanism of chinese herbal medicine in the treatment of atrial fibrillation with obstructive sleep apnea hypopnea syndrome by network pharmacology. Evid. Based Complement. Altern. Med. 2019, 2019, 8691608. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, J.; Jing, W.; Wang, Q.; Liu, Y.; Cheng, X.; Ye, F.; Tian, J.; Wei, F.; Ma, S. Systemic elucidation on the potential bioactive compounds and hypoglycemic mechanism of Polygonum multiflorum based on network pharmacology. Chin. Med. 2020, 15, 121–135. [Google Scholar] [CrossRef]
- Yang, M.; Shang, Y.x.; Tian, Z.Y.; Xiong, M.; Lu, C.L.; Jiang, Y.; Zhang, Y.; Yingying, Z.; Jin, X.Y.; Jin, Q.B.; et al. Characteristics of registered studies for coronavirus disease 2019 (COVID-19): A systematic review. Integr. Med. Res. 2020, 9, 100426. [Google Scholar] [CrossRef]
- Zhou, S.S.; Ai, Z.Z.; Li, W.N.; You, P.T.; Wu, C.Y.; Li, L.; Hu, Y.Y.; Ba, Y.M. Deciphering the pharmacological mechanisms of taohe-chengqi decoction extract against renal fibrosis through integrating network pharmacology and experimental validation in vitro and in vivo. Front. Pharmacol. 2020, 11, 425. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Tian, S.; Zhao, J.; Zhang, W. Exploring the mechanism of TCM formulae in the treatment of different types of coronary heart disease by network pharmacology and machining learning. Pharmacol. Res. 2020, 159, 105034. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, L.; Yuan, Y.; Liu, L.; Feng, F.; Lv, P.; Ma, S.; Chen, K.; Yao, Q. Network pharmacology-based dissection of the anti-diabetic mechanism of Lobelia chinensis. Front. Pharmacol. 2020, 11, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalimuthu, S.; Kim, S.-K. Fucoidan, a sulfated polysaccharides from brown algae as therapeutic target for cancer. In Handbook of Anticancer Drugs from Marine Origin; Kim, S.-K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 145–164. [Google Scholar] [CrossRef]
- Cong, L.H.; Li, T.; Wang, H.; Wu, Y.N.; Wang, S.P.; Zhao, Y.Y.; Zhang, G.Q.; Duan, J. IL-17A-producing T cells exacerbate fine particulate matter-induced lung inflammation and fibrosis by inhibiting PI3K/Akt/mTOR-mediated autophagy. J. Cell. Mol. Med. 2020, 24, 8532–8544. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.D.; Xun, Y.; Lu, J.L.; Lu, Y.C.; Yang, Y.Y.; Zhou, P.; Hu, J.; Li, C.; Wang, S.G. Network pharmacology and molecular docking analyses on Lianhua Qingwen capsule indicate Akt1 is a potential target to treat and prevent COVID-19. Cell Prolif. 2020, 53, e12949. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Qiang, W.; Gui, Y.; Tan, X.; Pei, T.; Lin, K.; Cai, S.; Sun, L.; Ning, G.; Wang, J.; et al. A large-scale transcriptional study reveals inhibition of COVID-19 related cytokine storm by traditional Chinese medicines. Sci. Bull. 2021, 66, 884–888. [Google Scholar] [CrossRef]
- Huang, X.F.; Cheng, W.B.; Jiang, Y.; Liu, Q.; Liu, X.H.; Xu, W.F.; Huang, H.T. A network pharmacology-based strategy for predicting anti-inflammatory targets of ephedra in treating asthma. Int. Immunopharmacol. 2020, 83, 106423. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.P.; Peng, Y.S.; Huang, B.Y.; Ding, X.; Wang, X.Y.; Niu, P.H.; Meng, J.; Zhu, Z.Z.; Zhang, Z.; Wang, J.Y.; et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Lukassen, S.; Chua, R.L.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.W.; et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020, 39, e105114. [Google Scholar] [CrossRef]
- Subbarayan, K.; Ulagappan, K.; Wickenhauser, C.; Bachmann, M.; Seliger, B. Immune interaction map of human SARS-CoV-2 target genes: Implications for therapeutic avenues. Front. Immunol. 2021, 12, 597399. [Google Scholar] [CrossRef]
- Gong, P.Y.; Guo, Y.J.; LI, X.P.; Wang, N.; Gu, J. Exploring active compounds of Jinhua Qinggan Granules for prevention of COVID-19 based on network pharmacology and molecular docking. Chin. Tradit. Herb. Drugs 2020, 51, 1685–1693. [Google Scholar] [CrossRef]
- Ramesh, P.; Veerappapillai, S.; Karuppasamy, R. Gene expression profiling of corona virus microarray datasets to identify crucial targets in COVID-19 patients. Gene Rep. 2021, 22, 100980. [Google Scholar] [CrossRef]
- Kelly, E.; Greene, C.M.; McElvaney, N.G. Targeting neutrophil elastase in cystic fibrosis. Expert Opin. Ther. Targets 2008, 12, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhu, W.; Diao, Y.; Xu, G.; Wang, L.; Qu, S.; Shi, Y. Elucidation of the mechanism of action of ginseng against acute lung injury/acute respiratory distress syndrome by a network pharmacology-based strategy. Front. Pharmacol. 2021, 11, 611794. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V.; Hayashi, Y.; Jung, S.-H. An overview of severe acute respiratory syndrome–coronavirus (SARS-CoV) 3CL protease inhibitors: Peptidomimetics and small molecule chemotherapy. J. Med. Chem. 2016, 59, 6595–6628. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Patamia, V.; Scala, A.; Sciortino, M.T.; Piperno, A.; Rescifina, A. Putative inhibitors of SARS-CoV-2 main protease from a library of marine natural products: A virtual screening and molecular modeling study. Mar. Drugs 2020, 18, 225. [Google Scholar] [CrossRef] [Green Version]


| PubChem CID | Name | OB (%) | DL | Structure |
|---|---|---|---|---|
| 5280343 | Quercetin | 46.4 | 0.27 |  |
| 5280459 | Quercitrin | 4.03 | 0.73 | 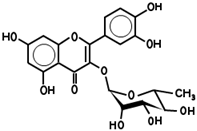 |
| 5280863 | Kaempferol | 41.8 | 0.24 |  |
| 2969 | Decanoic Acid | 26.74 | 0.03 | 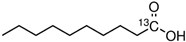 |
| 73083158 | Acetylborneol | 0.63 | N/A | |
| 5316673 | Afzelin | 3.83 | 0.69 | 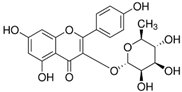 |
| 5280443 | Apigenin | 23.06 | 0.21 | 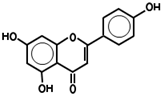 |
| No | UniProt ID | Gene Symbol | Protein Name | Degree |
|---|---|---|---|---|
| 1 | P10275 | AR | Androgen receptor | 8 |
| 2 | P31749 | AKT1 | RAC-alpha serine | 8 |
| 3 | P09601 | HMOX1 | Heme oxygenase 1 | 8 |
| 4 | P27487 | DPP4 | Dipeptidyl peptidase 4 | 6 |
| 5 | P06493 | CDK1 | Cyclin-dependent kinase 1 | 6 |
| 6 | P05362 | ICAM1 | Intercellular adhesion molecule 1 | 6 |
| 7 | P35869 | AHR | Aryl hydrocarbon receptor | 6 |
| 8 | P28482 | MAPK1 | Mitogen-activated protein kinase 1 | 6 |
| 9 | P22301 | IL10 | Interleukin-10 | 6 |
| 10 | P05231 | IL6 | Interleukin-6 | 6 |
| 11 | P05164 | MPO | Myeloperoxidase | 6 |
| 12 | P01308 | INS | Insulin | 4 |
| 13 | P07900 | HSP90AA1 | Heat shock protein HSP 90-alpha | 4 |
| 14 | P48736 | PIK3CG | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | 4 |
| 15 | P29965 | CD40LG | CD40 ligand | 4 |
| 16 | P42224 | STAT1 | Signal transducer and activator of transcription 1-alpha/beta | 4 |
| 17 | P01375 | TNF | Tumour necrosis factor | 4 |
| 18 | P08246 | ELANE | Neutrophil elastase | 4 |
| 19 | P05112 | IL4 | Interleukin-4 | 4 |
| 20 | P00533 | EGFR | Epidermal growth factor receptor | 4 |
| 21 | P15692 | VEGFA | Vascular endothelial growth factor A | 4 |
| ID | Description | Count | Gene Ratio | FDR |
|---|---|---|---|---|
| hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 18 | 18/67 | 2.78 × 10−23 |
| hsa04657 | IL-17 signaling pathway | 16 | 16/67 | 1.91 × 10−20 |
| hsa04066 | HIF-1 signaling pathway | 15 | 15/67 | 1.01 × 10−18 |
| hsa04620 | Toll-like receptor signaling pathway | 12 | 12/67 | 4.25 × 10−14 |
| hsa04668 | TNF signaling pathway | 12 | 12/67 | 6.79 × 10−14 |
| hsa04151 | PI3K-Akt signaling pathway | 16 | 16/67 | 5.19 × 10−13 |
| hsa04630 | Jak-STAT signaling pathway | 12 | 12/67 | 3.54 × 10−12 |
| hsa04621 | NOD-like receptor signaling pathway | 12 | 12/67 | 5.17 × 10−12 |
| hsa04068 | FoxO signaling pathway | 11 | 11/67 | 9.46 × 10−12 |
| hsa04660 | T cell receptor signaling pathway | 10 | 10/67 | 1.98 × 10−11 |
| hsa04917 | Prolactin signaling pathway | 8 | 8/67 | 9.06 × 10−10 |
| hsa04919 | Thyroid hormone signaling pathway | 9 | 9/67 | 1.51 × 10−9 |
| hsa04062 | Chemokine signaling pathway | 10 | 10/67 | 3.64 × 10−9 |
| hsa04926 | Relaxin signaling pathway | 9 | 9/67 | 4.03 × 10−9 |
| hsa04064 | NF-kB signaling pathway | 8 | 8/67 | 7.15 × 10−9 |
| hsa04072 | Phospholipase D signaling pathway | 9 | 9/67 | 9.09 × 10−9 |
| hsa04664 | Fc epsilon RI signaling pathway | 7 | 7/67 | 1.90 × 10−8 |
| hsa04010 | MAPK signaling pathway | 11 | 11/67 | 1.94 × 10−8 |
| Ingredient–Target | BindingAffinity | Binding Residues | ||
|---|---|---|---|---|
| H-Bonds | Hydrophobic Interaction | π-Stacking/Salt Bridge | ||
| Afzelin–IL6 | −6.7 | TYR-97, ASN-63, THR-137 | ASP-140, GLU-93 | N/A |
| Afzelin–DPP4 | −8.6 | ARG-560, TYR-631, GLY-632, TRP-629, TYR-547, LYS-554 | N/A | VAL-546, ASP-545 |
| Afzelin–ELANE | −6.7 | ARG-23, CYS-136, GLN-122, GLY-207 | PHE-29, LEU-137, TRP-27 | N/A |
| Afzelin–MAPK1 | -9.4 | PHE-129, GLN-132, ASP-106, ILE-84, ASN-158, THR-150 | ILE-133, ASN-82 | N/A |
| Afzelin–HSP90AA1 | −7.6 | GLN-133 | N/A | ARG-46 |
| Afzelin–SERPINE1 | −8.1 | SER-119, ASP-95, THR-94, TYR-79 | PHE-117, ARG-76 | N/A |
| Apigenin–IL6 | −6.7 | GLN-152, ASN-103, ARG-104, ASP-160 | GLN-159, GLN-156 | N/A |
| Apigenin–DPP4 | −8.4 | SER-630, VAL-546, TYR-547 | TRP-629 | N/A |
| Apigenin–ELANE | −7.6 | CYS-168, ARG-178 | PRO-230, AL-181, THR-164 | N/A |
| Apigenin–MAPK1 | −8.1 | ILE-133, ASN-154, GLN-132 | LEU-150, ILE-140, LEU-155, LEU-157 | N/A |
| Apigenin–HSP90AA1 | −7.3 | LYS-58, PHE-138, GLY-135 | THR-184, LEU-107, THR-109, ASN-51 | N/A |
| Apigenin–SERPINE1 | −8.5 | PHE-117, SER-41, TYR-37, TYR-39 | LEU-116, LEU-75, TYR-79 | N/A |
| Kaempferol–IL6 | −6.8 | GLN-156, GLN-159, ARG-104 | GLN-152 | N/A |
| Kaempferol–DPP4 | −8.1 | GLU-205, ASN-710, ARG-125, SER-630, | VAL-711, PHE-357 | TYR-666 |
| Kaempferol–ELANE | −7.2 | ASN-180, THR-164 | VAL-181, LEU-130 | N/A |
| Kaempferol–MAPK1 | −8.3 | GLN-132, LEU-156 | LEU-157, ILE-140, LEU-150 | N/A |
| Kaempferol–HSP90AA1 | −7.4 | LYS-58, ASN-51 | THR-109, LEU-107, PHE-138, THR-184 | N/A |
| Kaempferol–SERPINE1 | −8.5 | ASP-95, PHE-117, LEU-75, ALA-72 | SER-41, TYR-79 | N/A |
| Quercetin–IL6 | −7.2 | GLN-152, ARG-104 | GLN-156, GLN-159 | N/A |
| Quercetin–DPP4 | −8.5 | SER-630, TYR-662, ASN-710, ARG-125, ARG-358 | TYR-666, PHE-357 | N/A |
| Quercetin–ELANE | −7.3 | ARG-128, CYS-168, GLN-233, ARG-129, ARG-176 | THR-164, LEU-130, VAL-181 | N/A |
| Quercetin–MAPK1 | −8.5 | HIS-147, ASN-82 | LEU-155, LEU-156, LEU-157 | N/A |
| Quercetin–HSP90AA1 | −7.4 | GLY-97, THR-184, LEU-107, GLY-135 | ALA-55, ASN-51, ASP-54 | N/A |
| Quercetin–SERPINE1 | −8.7 | SER-41, ASP-95, SER-119, TYR-37, LEU-75, PHE-117 | TYR-79, LEU-116 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yuan, S.; Yao, Y.; Wang, J.; Scalabrino, G.; Jiang, S.; Sheridan, H. Network Pharmacology and Molecular Docking Elucidate the Underlying Pharmacological Mechanisms of the Herb Houttuynia cordata in Treating Pneumonia Caused by SARS-CoV-2. Viruses 2022, 14, 1588. https://doi.org/10.3390/v14071588
Liu J, Yuan S, Yao Y, Wang J, Scalabrino G, Jiang S, Sheridan H. Network Pharmacology and Molecular Docking Elucidate the Underlying Pharmacological Mechanisms of the Herb Houttuynia cordata in Treating Pneumonia Caused by SARS-CoV-2. Viruses. 2022; 14(7):1588. https://doi.org/10.3390/v14071588
Chicago/Turabian StyleLiu, Junying, Shouli Yuan, Yao Yao, Jinfan Wang, Gaia Scalabrino, Shibo Jiang, and Helen Sheridan. 2022. "Network Pharmacology and Molecular Docking Elucidate the Underlying Pharmacological Mechanisms of the Herb Houttuynia cordata in Treating Pneumonia Caused by SARS-CoV-2" Viruses 14, no. 7: 1588. https://doi.org/10.3390/v14071588
APA StyleLiu, J., Yuan, S., Yao, Y., Wang, J., Scalabrino, G., Jiang, S., & Sheridan, H. (2022). Network Pharmacology and Molecular Docking Elucidate the Underlying Pharmacological Mechanisms of the Herb Houttuynia cordata in Treating Pneumonia Caused by SARS-CoV-2. Viruses, 14(7), 1588. https://doi.org/10.3390/v14071588







