The Impact of Early Antiretroviral Treatment (ART) for HIV on the Sensitivity of the Latest Generation of Blood Screening and Point of Care Assays
Abstract
:1. Introduction
2. Methods
2.1. Setting
2.2. Routine Screening
2.3. MATHS Cohort
2.4. HIV Positive Donations Tested for ART Drugs
2.5. Assays
2.6. Statistical Analysis
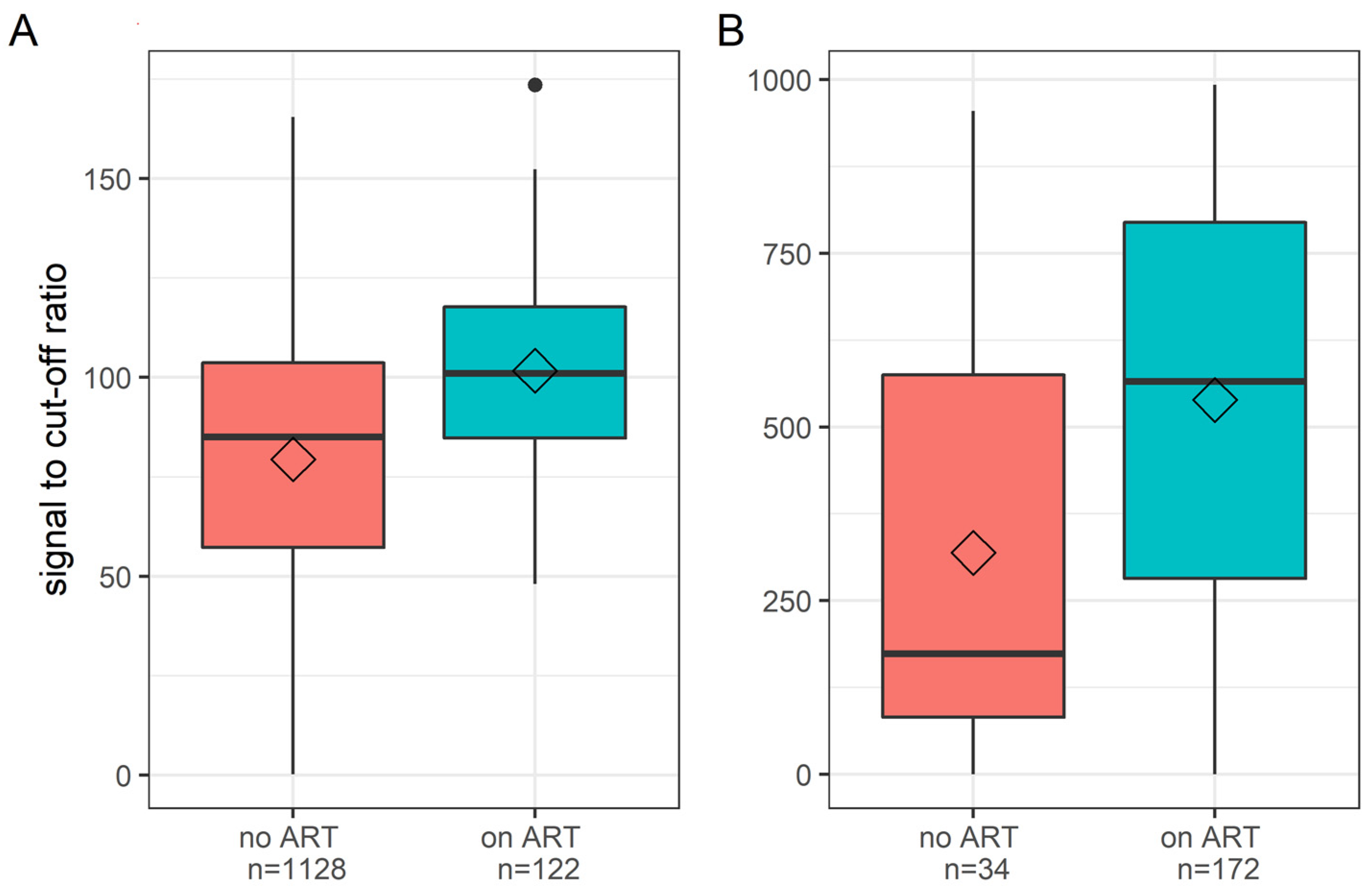
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ananworanich, J.; Schuetz, A.; Vandergeeten, C.; Sereti, I.; de Souza, M.; Rerknimitr, R.; Dewar, R.; Marovich, M.; van Griensven, F.; Sekaly, R.; et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS ONE 2012, 7, e33948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananworanich, J.; Sacdalan, C.P.; Pinyakorn, S.; Chomont, N.; Souzade, M.; Luekasemsuk, T.; Schuetz, A.; Krebs, S.J.; Dewar, R.; Jagodzinski, L.; et al. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J. Virus Erad. 2016, 2, 43–48. [Google Scholar] [CrossRef]
- Schuetz, A.; Deleage, C.; Sereti, I.; Rerknimitr, R.; Phanuphak, N.; Phuang-Ngern, Y.; Estes, J.D.; Sandler, N.G.; Sukhumvittaya, S.; Marovich, M.; et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014, 10, e1004543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrich, T.J.; Hatano, H.; Bacon, O.; Hogan, L.E.; Rutishauser, R.; Hill, A.; Kearney, M.F.; Anderson, E.M.; Buchbinder, S.P.; Cohen, S.E.; et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med. 2017, 14, e1002417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, M.S.; Pinyakorn, S.; Akapirat, S.; Pattanachaiwit, S.; Fletcher, J.L.; Chomchey, N.; Kroon, E.D.; Ubolyam, S.; Michael, N.L.; Robb, M.L.; et al. Initiation of Antiretroviral Therapy During Acute HIV-1 Infection Leads to a High Rate of Nonreactive HIV Serology. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Keating, S.M.; Pilcher, C.D.; Busch, M.P. Editorial Commentary: Timing Is Everything: Shortcomings of Current HIV Diagnostics in the Early Treatment Era. Clin. Infect. Dis. 2016, 63, 562–564. [Google Scholar] [CrossRef] [Green Version]
- Stefic, K.; Novelli, S.; Mahjoub, N.; Seng, R.; Molina, J.M.; Cheneau, C.; Barin, F.; Chaix, M.L.; Meyer, L.; Delaugerre, C.; et al. Nonreactive Human Immunodeficiency Virus Type 1 Rapid Tests After Sustained Viral Suppression Following Antiretroviral Therapy Initiation During Primary Infection. J. Infect. Dis. 2018, 217, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Hare, C.B.; Pappalardo, B.L.; Busch, M.P.; Karlsson, A.C.; Phelps, B.H.; Alexander, S.S.; Bentsen, C.; Ramstead, C.A.; Nixon, D.F.; Levy, J.A.; et al. Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin. Infect. Dis. 2006, 42, 700–708. [Google Scholar] [CrossRef]
- Sykes, W.; Van den Berg, K.; Jacobs, G.; Jauregui, A.; Roubinian, N.; Wiesner, L.; Maartens, G.; Swanevelder, R.; Custer, B.; Busch, M.; et al. Discovery of False Elite Controllers: HIV Antibody-Positive RNA-Negative Blood Donors Found To Be on Antiretroviral Therapy. J. Infect. Dis. 2019, 220, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Custer, B.; Quiner, C.; Haaland, R.; Martin, A.; Stone, M.; Reik, R.; Steele, W.R.; Kessler, D.; Williamson, P.C.; Anderson, S.A.; et al. HIV antiretroviral therapy and prevention use in US blood donors: A new blood safety concern. Blood 2020, 136, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Lelie, N.; Sykes, W.; Crookes, R.; Swanevelder, J.; Gaggia, L.; Le Roux, M.; Kuun, E.; Gulube, S.; Reddy, R. Impact of individual-donation nucleic acid testing on risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission by blood transfusion in South Africa. Transfusion 2009, 49, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, S.; Busch, M.P.; Murphy, E.L.; Shan, H.; Ness, P.; Glynn, S.A.; National Heart, L.; Blood Institute Recipient, E.; Donor Evaluation, S. The National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): A research program striving to improve blood donor and transfusion recipient outcomes. Transfusion 2014, 54, 942–955. [Google Scholar] [CrossRef] [Green Version]
- Fiebig, E.W.; Wright, D.J.; Rawal, B.D.; Garrett, P.E.; Schumacher, R.T.; Peddada, L.; Heldebrant, C.; Smith, R.; Conrad, A.; Kleinman, S.H.; et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. Aids 2003, 17, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, X.; Dobbs, T.; Kuehl, D.; Nkengasong, J.N.; Hu, D.J.; Parekh, B.S. Development of two avidity-based assays to detect recent HIV type 1 seroconversion using a multisubtype gp41 recombinant protein. AIDS Res. Hum. Retrovir. 2010, 26, 61–71. [Google Scholar] [CrossRef]
- van den Berg, K.; Vermeulen, M.; Louw, V.J.; Murphy, E.L.; Maartens, G. Undisclosed HIV status and antiretroviral therapy use among South African blood donors. Transfusion 2021, 61, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Grebe, E.; Facente, S.N.; Bingham, J.; Pilcher, C.D.; Powrie, A.; Gerber, J.; Priede, G.; Chibawara, T.; Busch, M.P.; Murphy, G.; et al. Interpreting HIV diagnostic histories into infection time estimates: Analytical framework and online tool. BMC Infect. Dis. 2019, 19, 894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manak, M.M.; Jagodzinski, L.L.; Shutt, A.; Malia, J.A.; Leos, M.; Ouellette, J.; Akapirat, S.; Colby, D.L.; Phanuphak, N.; Eller, L.A.; et al. Decreased Seroreactivity in Individuals Initiating Antiretroviral Therapy during Acute HIV Infection. J. Clin. Microbiol. 2019, 57, e00757-19. [Google Scholar] [CrossRef] [Green Version]
- Bloch, E.M.; Vermeulen, M.; Murphy, E. Blood transfusion safety in Africa: A literature review of infectious disease and organizational challenges. Transfus. Med. Rev. 2012, 26, 164–180. [Google Scholar] [CrossRef] [Green Version]
- Pruett, C.R.; Vermeulen, M.; Zacharias, P.; Ingram, C.; Tayou Tagny, C.; Bloch, E.M. The use of rapid diagnostic tests for transfusion infectious screening in Africa: A literature review. Transfus. Med. Rev. 2015, 29, 35–44. [Google Scholar] [CrossRef]
- Vermeulen, M.; Lelie, N.; Coleman, C.; Sykes, W.; Jacobs, G.; Swanevelder, R.; Busch, M.; van Zyl, G.; Grebe, E.; Welte, A.; et al. Assessment of HIV transfusion transmission risk in South Africa: A 10-year analysis following implementation of individual donation nucleic acid amplification technology testing and donor demographics eligibility changes. Transfusion 2019, 59, 267–276. [Google Scholar] [CrossRef]
- Ma, Z.M.; Stone, M.; Piatak, M., Jr.; Schweighardt, B.; Haigwood, N.L.; Montefiori, D.; Lifson, J.D.; Busch, M.P.; Miller, C.J. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J. Virol. 2009, 83, 3288–3297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AVAC. Prepwatch. 2021. Available online: https://data.prepwatch.org/ (accessed on 4 March 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vermeulen, M.; van Schalkwyk, C.; Jacobs, G.; van den Berg, K.; Stone, M.; Bakkour, S.; Custer, B.; Jentsch, U.; Busch, M.P.; Murphy, E.; et al. The Impact of Early Antiretroviral Treatment (ART) for HIV on the Sensitivity of the Latest Generation of Blood Screening and Point of Care Assays. Viruses 2022, 14, 1426. https://doi.org/10.3390/v14071426
Vermeulen M, van Schalkwyk C, Jacobs G, van den Berg K, Stone M, Bakkour S, Custer B, Jentsch U, Busch MP, Murphy E, et al. The Impact of Early Antiretroviral Treatment (ART) for HIV on the Sensitivity of the Latest Generation of Blood Screening and Point of Care Assays. Viruses. 2022; 14(7):1426. https://doi.org/10.3390/v14071426
Chicago/Turabian StyleVermeulen, Marion, Cari van Schalkwyk, Genevieve Jacobs, Karin van den Berg, Mars Stone, Sonia Bakkour, Brian Custer, Ute Jentsch, Michael P. Busch, Edward Murphy, and et al. 2022. "The Impact of Early Antiretroviral Treatment (ART) for HIV on the Sensitivity of the Latest Generation of Blood Screening and Point of Care Assays" Viruses 14, no. 7: 1426. https://doi.org/10.3390/v14071426
APA StyleVermeulen, M., van Schalkwyk, C., Jacobs, G., van den Berg, K., Stone, M., Bakkour, S., Custer, B., Jentsch, U., Busch, M. P., Murphy, E., & Grebe, E. (2022). The Impact of Early Antiretroviral Treatment (ART) for HIV on the Sensitivity of the Latest Generation of Blood Screening and Point of Care Assays. Viruses, 14(7), 1426. https://doi.org/10.3390/v14071426






