Serum Markers Associated with Disease Severity in a Bosnian Hemorrhagic Fever with Renal Syndrome Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Ethics
2.2. Sample Selection
2.3. Retrospective Diagnostics
2.4. Multiplex Immunoassays
2.5. Statistical Analyses
3. Results
3.1. Patient Cohort Characteristics
3.2. Severe Thrombocytopenia
3.3. Acute Kidney Injury
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laenen, L.; Vergote, V.; Vanmechelen, B.; Tersago, K.; Baele, G.; Lemey, P.; Leirs, H.; Dellicour, S.; Vrancken, B.; Maes, P. Identifying the patterns and drivers of Puumala hantavirus enzootic dynamics using reservoir sampling. Virus Evol. 2019, 5, vez009. [Google Scholar] [CrossRef]
- Hukic, M.; Nikolic, J.; Valjevac, A.; Seremet, M.; Tesic, G.; Markotic, A. A serosurvey reveals Bosnia and Herzegovina as a Europe’s hotspot in hantavirus seroprevalence. Epidemiol. Infect. 2010, 138, 1185–1193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Markotić, A.; Nichol, S.; Kuzman, I.; Sanchez, A.; Ksiazek, T.; Gagro, A.; Rabatić, S.; Zgorelec, R.; Avšič-Županc, T.; Beus, I.; et al. Characteristics of puumala and Dobrava infections in Croatia. J. Med. Virol. 2002, 66, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Županc, T.A.; Korva, M.; Markotić, A. HFRS and hantaviruses in the Balkans/South-East Europe. Virus Res. 2014, 187, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Noack, D.; Goeijenbier, M.; Reusken, C.B.E.M.; Koopmans, M.P.G.; Rockx, B.H.G. Orthohantavirus Pathogenesis and Cell Tropism. Front. Cell. Infect. Microbiol. 2020, 10, 399. [Google Scholar] [CrossRef]
- Khaiboullina, S.F.; Levis, S.; Morzunov, S.P.; Martynova, E.V.; Anokhin, V.A.; Gusev, O.A.; Jeor, S.C.S.; Lombardi, V.C.; Rizvanov, A.A. Serum Cytokine Profiles Differentiating Hemorrhagic Fever with Renal Syndrome and Hantavirus Pulmonary Syndrome. Front. Immunol. 2017, 8, 567. [Google Scholar] [CrossRef]
- Martynova, E.V.; Maksudova, A.N.; Shakirova, V.G.; Abdulkhakov, S.R.; Khaertynova, I.M.; Anokhin, V.A.; Ivanova, V.V.; Abiola, I.M.; Garanina, E.E.; Tazetdinova, L.G.; et al. Urinary Clusterin Is Upregulated in Nephropathia Epidemica. Dis. Markers 2018, 2018, 8658507. [Google Scholar] [CrossRef]
- Korva, M.; Rus, K.R.; Pavletič, M.; Saksida, A.; Knap, N.; Jelovšek, M.; Smrdel, K.S.; Jakupi, X.; Humolli, I.; Dedushaj, J.; et al. Characterization of Biomarker Levels in Crimean–Congo Hemorrhagic Fever and Hantavirus Fever with Renal Syndrome. Viruses 2019, 11, 686. [Google Scholar] [CrossRef]
- Hoornweg, T.E.; Zutt, I.; De Vries, A.; Maas, M.; Hoogerwerf, M.N.; Avšič-Županc, T.; Korva, M.; Reimerink, J.H.J.; Reusken, C.B.E.M. Development of a Comparative European Orthohantavirus Microneutralization Assay with Multi-Species Validation and Evaluation in a Human Diagnostic Cohort. Front. Cell. Infect. Microbiol. 2020, 10, 580478. [Google Scholar] [CrossRef]
- Avsic-Zupanc, T.; Saksida, A.; Korva, M. Hantavirus infections. Clin. Microbiol. Infect. 2019, 21, e6–e16. [Google Scholar] [CrossRef]
- UKidney. eGFR Calculator. 2007–2022. Available online: https://www.ukidney.com/nephrology-resources/egfr-calculator (accessed on 15 April 2021).
- Maleki, K.T.; Tauriainen, J.; García, M.; Kerkman, P.F.; Christ, W.; Dias, J.; Byström, J.W.; Leeansyah, E.; Forsell, M.N.; Ljunggren, H.-G.; et al. MAIT cell activation is associated with disease severity markers in acute hantavirus infection. Cell Rep. Med. 2021, 2, 100220. [Google Scholar] [CrossRef] [PubMed]
- Martynova, E.; Davidyuk, Y.; Kabwe, E.; Garanina, E.; Shakirova, V.; Pavelkina, V.; Uskova, Y.; Stott, R.; Foster, T.; Markelova, M.; et al. Cytokine, Chemokine, and Metalloprotease Activation in the Serum of Patients with Nephropathia Epidemica from the Republic of Tatarstan and the Republic of Mordovia, Russia. Pathogens 2021, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Vangeti, S.; Strandin, T.; Liu, S.; Tauriainen, J.; Räisänen-Sokolowski, A.; Cabrera, L.; Hassinen, A.; Mäkelä, S.; Mustonen, J.; Vaheri, A.; et al. Monocyte subset redistribution from blood to kidneys in patients with Puumala virus caused hemorrhagic fever with renal syndrome. PLoS Pathog. 2021, 17, e1009400. [Google Scholar]
- Shakirova, V.; Khaertynova, I.; Markelova, M.; Tarlinton, R.; Behnke, J.; Martynova, E.; Garanina, E.; Rizvanov, A.; Khaiboullina, S. Serum Cytokine Alterations Associated with Age of Patients with Nephropathia Epidemica. BioMed Res. Int. 2022, 2022, 4685288. [Google Scholar] [CrossRef]
- Raadsen, M.; Du Toit, J.; Langerak, T.; van Bussel, B.; van Gorp, E.; Goeijenbier, M. Thrombocytopenia in Virus Infections. J. Clin. Med. 2021, 10, 877. [Google Scholar] [CrossRef]
- Laine, O.K.; Koskela, S.M.; Outinen, T.K.; Joutsi-Korhonen, L.; Huhtala, H.; Vaheri, A.; Hurme, M.A.; Jylhävä, J.; Mäkelä, S.M.; Mustonen, J.T. Plasma pentraxin-3 and coagulation and fibrinolysis variables during acute Puumala hantavirus infection and associated thrombocytopenia. Blood Coagul. Fibrinolysis 2014, 25, 612–617. [Google Scholar] [CrossRef]
- Outinen, T.K.; Mäkelä, S.; Huhtala, H.; Hurme, M.; Meri, S.; Pörsti, I.; Sane, J.; Vaheri, A.; Syrjänen, J.; Mustonen, J. High pentraxin-3 plasma levels associate with thrombocytopenia in acute Puumala hantavirus-induced nephropathia epidemica. Eur. J. Clin. Microbiol. 2012, 31, 957–963. [Google Scholar] [CrossRef]
- Outinen, T.K.; Mäkelä, S.M.; O Ala-Houhala, I.; Huhtala, H.S.; Hurme, M.; Paakkala, A.S.; Pörsti, I.H.; Syrjänen, J.T.; Mustonen, J.T. The severity of Puumala hantavirus induced nephropathia epidemica can be better evaluated using plasma interleukin-6 than C-reactive protein determinations. BMC Infect. Dis. 2010, 10, 132. [Google Scholar] [CrossRef]
- Hansson, M.; Gustafsson, R.; Jacquet, C.; Chebaane, N.; Satchell, S.; Thunberg, T.; Ahlm, C.; Connolly, A.-M.F. Cystatin C and α-1-Microglobulin Predict Severe Acute Kidney Injury in Patients with Hemorrhagic Fever with Renal Syndrome. Pathogens 2020, 9, 666. [Google Scholar] [CrossRef]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef]
- Nusshag, C.; Stütz, A.; Hägele, S.; Speer, C.; Kälble, F.; Eckert, C.; Brenner, T.; Weigand, M.A.; Morath, C.; Reiser, J.; et al. Glomerular filtration barrier dysfunction in a self-limiting, RNA virus-induced glomerulopathy resembles findings in idiopathic nephrotic syndromes. Sci. Rep. 2020, 10, 19117. [Google Scholar] [CrossRef] [PubMed]
- Son, G.H.; Kwon, J.Y.; Lee, S.; Park, J.; Kim, Y.-J.; Yun, B.; Park, J.H. Comparison of serum and urinary nephrin levels between normal pregnancies and severe preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 166, 139–144. [Google Scholar] [CrossRef]
- Du, T.-Y.; Luo, H.-M.; Qin, H.-C.; Wang, F.; Wang, Q.; Xiang, Y.; Zhang, Y. Circulating Serum Trefoil Factor 3 (TFF3) Is Dramatically Increased in Chronic Kidney Disease. PLoS ONE 2013, 8, e80271. [Google Scholar] [CrossRef] [PubMed]
- Gunasekara, T.; De Silva, P.M.C.; Herath, C.; Siribaddana, S.; Siribaddana, N.; Jayasumana, C.; Jayasinghe, S.; Cardenas-Gonzalez, M.; Jayasundara, N. The Utility of Novel Renal Biomarkers in Assessment of Chronic Kidney Disease of Unknown Etiology (CKDu): A Review. Int. J. Environ. Res. Public Health 2020, 17, 9522. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.; A Bruijn, J.; Gerding, M.N.; Jordans, J.G.; Van Charante, A.W.M.; Osterhaus, A.D. Hantavirus antigen detection in kidney biopsies from patients with nephropathia epidemica. Clin. Nephrol. 1996, 46, 379–383. [Google Scholar]
- Krautkrämer, E.; Grouls, S.; Stein, N.; Reiser, J.; Zeier, M. Pathogenic Old World Hantaviruses Infect Renal Glomerular and Tubular Cells and Induce Disassembling of Cell-to-Cell Contacts. J. Virol. 2011, 85, 9811–9823. [Google Scholar] [CrossRef]
- Sane, J.; Laine, O.; Mäkelä, S.; Paakkala, A.; Jarva, H.; Mustonen, J.; Vapalahti, O.; Meri, S.; Vaheri, A. Complement activation in Puumala hantavirus infection correlates with disease severity. Ann. Med. 2012, 44, 468–475. [Google Scholar] [CrossRef]
- Outinen, T.K.; Mantula, P.; Jaatinen, P.; Hämäläinen, M.; Moilanen, E.; Vaheri, A.; Huhtala, H.; Mäkelä, S.; Mustonen, J. Glycoprotein YKL-40 Is Elevated and Predicts Disease Severity in Puumala Hantavirus Infection. Viruses 2019, 11, 767. [Google Scholar] [CrossRef]
- Outinen, T.K.; Mäkelä, S.; Pörsti, I.; Vaheri, A.; Mustonen, J. Severity Biomarkers in Puumala Hantavirus Infection. Viruses 2021, 14, 45. [Google Scholar] [CrossRef]

| Clinical or Laboratory Variable | Median or Number | Range |
|---|---|---|
| Patients, no. | 40 | N/A |
| Sex, female/male, no. | 6/34 | N/A |
| Age, median (range) | 42 | (16–69) |
| Sample collection on days post-onset of symptoms, median (range) | 6 | (3-10) |
| Mortality, yes/no, no. | 0/40 | N/A |
| Thrombocyte levels (<100 × 109/L), median (range) | 73.5 | (39–435) |
| Clinically reported bleeding, yes/no, no. | 16/14 | N/A |
| Hemodialysis, yes/no, no. | 4/36 | N/A |
| Intensive care unit, yes/no, no. | 2/38 | N/A |
| Minimal estimated glomerular filtration rate (m/min/1.73 m2), median (range) | 49 | (5–136) |
| Urine output, anuric first day/oliguric/regular, no. | 4/31/5 | N/A |
| Pulse at admission, median (range) | 67.5 | (60–90) |
| Mean arterial pressure, median (range) | 96.7 | (70–173.3) |
| Reported diabetes mellitus 2, yes/no, no. | 3/37 | N/A |
| Reported hypertension, yes/no, no. | 5/35 | N/A |
| Serum ID | RT-qPCR | Ct-value | MNT PUUV | MNT DOBV | Diagnosis | Serum ID | RT-qPCR | Ct-Value | MNT PUUV | MNT DOBV | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 274 | - | - | 1600 | <50 | PUUV | 527 | PUUV | 33.8 | 400 | <50 | PUUV |
| 334 | - | - | 800 | 100 | PUUV | 530 | - | - | 400 | <50 | PUUV |
| 338 | PUUV | 34.9 | 3200 | <50 | PUUV | 536 | PUUV | 36.7 | 800 | 50 | PUUV |
| 357 | PUUV | 36.9 | 800 | 50 | PUUV | 542 | PUUV | 35.4 | 800 | <50 | PUUV |
| 365 | - | - | 6400 | <50 | PUUV | 556 | - | - | 800 | <50 | PUUV |
| 368 | - | - | 400 | <50 | PUUV | 559 | - | - | 200 | <50 | PUUV |
| 375 | - | - | 400 | <50 | PUUV | 562 | PUUV | 38.6 | 800 | <50 | PUUV |
| 393 | - | - | 400 | <50 | PUUV | 563 | - | - | 800 | <50 | PUUV |
| 394 | - | - | 800 | <50 | PUUV | 570 | - | - | 6400 | <50 | PUUV |
| 395 | - | - | 3200 | 50 | PUUV | 579 | - | - | 800 | <50 | PUUV |
| 405 | - | - | 1600 | <50 | PUUV | 581 | PUUV | 36.5 | 50 | <50 | PUUV |
| 413 | - | - | 800 | <50 | PUUV | 582 | - | - | 100 | <50 | PUUV |
| 425 | - | - | 400 | 100 | PUUV | 583 | - | - | 200 | <50 | PUUV |
| 497 | - | - | 400 | <50 | PUUV | 586 | PUUV | 37.0 | 400 | <50 | PUUV |
| 498 | - | - | 400 | <50 | PUUV | 588 | PUUV | 37.8 | 100 | <50 | PUUV |
| 504 | PUUV | 38.0 | 400 | <50 | PUUV | 619 | - | - | 100 | <50 | PUUV |
| 505 | PUUV | 35.4 | 800 | <50 | PUUV | 622 | PUUV | 35.0 | 400 | <50 | PUUV |
| 507 | - | - | 1600 | <50 | PUUV | 626 | PUUV | 33.5 | ≥3200 | ≥3200 | PUUV |
| 517 | PUUV | 36.9 | 400 | <50 | PUUV | 627 | PUUV | 37.0 | 1600 | 400 | PUUV |
| 524 | PUUV | 35.7 | 6400 | <50 | PUUV | 5737 | - | - | 1600 | <50 | PUUV |
| Serum Marker | Severe Thrombocytopenia (n = 27) | Low—Normal Platelet Count (n = 13) | p-Value |
|---|---|---|---|
| D-dimer (pg/mL) | 1,463,837 (315,636–7,962,830) | 585,048 (260,203–6,029,023) | 0.0599 |
| E-selectin (pg/mL) | 52,904 (13,972–112,748) | 61,120 (30,304–95,610) | 0.2175 |
| Factor IX (pg/mL) | 1,126,515 (123,805–2,646,300) | 1,230,664 (393,903–2,488,834) | 0.9093 |
| ICAM-1 (pg/mL) | 410,492 (53,625–1,451,000) | 241,146 (90,676–868,792) | 0.5490 |
| IL-6 (pg/mL) | 104.6 (104.6–2487) | 104.6 (104.6–1230) | 0.9307 |
| IL-8 (pg/mL) | 35,876 (35,876–4,703,235) | 35,876 (35,876–1,341,001) | 0.3395 |
| PAI-1 (pg/mL) | 636,183 (233,006–1,870,126) | 537,770 (264,323–3,269,881) | 0.8867 |
| P-selectin (pg/mL) | 40,365 (9156–333,392) | 29,217 (15,141–124,363) | 0.9773 |
| PSGL-1 (pg/mL) | 48,518 (27,093–72,048) | 39,528 (22,163–71,841) | 0.1271 |
| RANTES (pg/mL) | 20,767 (6398–90,191) | 40,056 (11,293-99,276) | 0.0290 |
| sCD40L (pg/mL) | 43,816 (860.0–972,480) | 93,321 (860.0–404,848) | 0.3619 |
| tPA (pg/mL) | 43,589 (256.0–132,727) | 22,108 (905.0–130,382) | 0.3165 |
| uPAR (pg/mL) | 1386 (719.0–2727) | 1238 (794.6–2034) | 0.5927 |
| VEGF (pg/mL) | 97.18 (8.88–374.7) | 88.29 (8.88–489.9) | 0.4449 |
| Cystatin C (pg/mL) | 1,818,000 (702,466–5,819,300) | 2,191,400 (440,681–6,472,400) | 0.4539 |
| Galectin-3BP (pg/mL) | 1,378,500 (707,253–4,978,800) | 1,259,500 (877,266–2,124,200) | 0.3912 |
| IGFBP-7 (pg/mL) | 11,022 (2043–29,575) | 15,889 (5729–45,940) | 0.3197 |
| IL-18 (pg/mL) | 677.9 (72.97–2334) | 448.1 (107.4–1010) | 0.0042 |
| IP-10 (pg/mL) | 274.7 (59.97–2416) | 164.5 (63.31–1234) | 0.0559 |
| KIM-1 (pg/mL) | 103.5 (54.41–167.6) | 111.1 (83.43–145.3) | 0.5140 |
| Nephrin (pg/mL) | 834.7 (572.0–1218) | 891.1 (665.0–1027) | 0.1610 |
| NGAL (pg/mL) | 129,637 (45,709–591,532) | 103,241 (41,860–219,892) | 0.7757 |
| Osteopontin (pg/mL) | 35,207 (8486–247,279) | 51,577 (3620–754,860) | 0.8146 |
| Pentraxin 3 (pg/mL) | 8562 (1913–41,062) | 6295 (2579–29,075) | 0.1679 |
| TFF3 (pg/mL) | 1251 (336.6–7611) | 982.5 (312.4–8333) | 0.9319 |
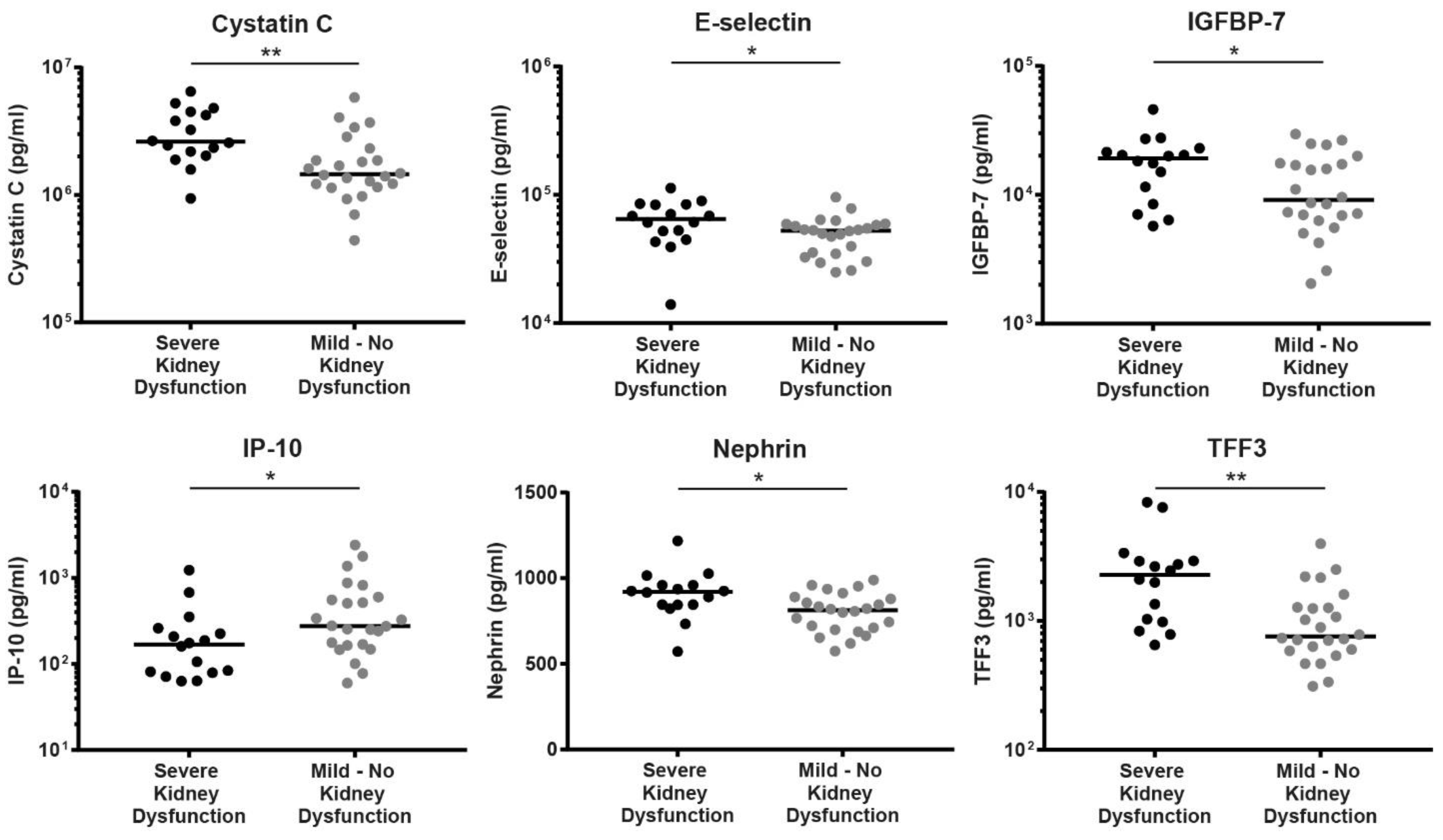
| Serum Marker | Severe Kidney Dysfunction (n = 16) | Mild—No Kidney Dysfunction (n = 24) | p-Value |
|---|---|---|---|
| D-dimer (pg/mL) | 614,979 (260,203–6,029,023) | 1,280,942 (282,273–7,962,830) | 0.8379 |
| E-selectin (pg/mL) | 64,632 (13,972–112,748) | 52,554 (24,964–95,610) | 0.0360 |
| Factor IX (pg/mL) | 1,178,590 (393,903–2,646,300) | 1,087,530 (123,805–2,488,834) | 0.8166 |
| ICAM-1 (pg/mL) | 420,550 (104,141–1,154,500) | 288,051 (53,625–1,451,000) | 0.3175 |
| IL-6 (pg/mL) | 104.6 (104.6–255.1) | 104.6 (104.6–2487) | 0.3349 |
| IL-8 (pg/mL) | 35,876 (35,876–1,341,001) | 35,876 (35,876–4,703,235) | 0.4344 |
| PAI-1 (pg/mL) | 608,817 (264,323–1,929,785) | 567,400 (233,006–3,269,881) | 0.7330 |
| P-selectin (pg/mL) | 59,708 (15,141–333,392) | 29,164 (9156–126,873) | 0.0702 |
| PSGL-1 (pg/mL) | 39,834 (22,163–72,048) | 48,386 (27,093–69,005) | 0.2790 |
| RANTES (pg/mL) | 38,531 (6398–84,374) | 22,974 (6551–99,276) | 0.2017 |
| sCD40L (pg/mL) | 69,466 (860.0–530,310) | 67,983 (860.0–972,480) | 0.8317 |
| tPA (pg/mL) | 30,254 (256.0–132,727) | 41,553 (905.0–92,487) | 0.3887 |
| uPAR (pg/mL) | 1390 (719.0–2727) | 1306 (794.6–2439) | 0.4730 |
| VEGF (pg/mL) | 152.7 (8.88–486.9) | 70 (11.65–489.9) | 0.1804 |
| Cystatin C (pg/mL) | 2,613,100 (938,353–6,472,400) | 1,454,550 (440,681–5,819,300) | 0.0015 |
| Galectin-3BP (pg/mL) | 1,458,250 (707,253–4,644,800) | 1,324,500 (783,212–4,978,800) | 0.6923 |
| IGFBP-7 (pg/mL) | 19,131 (5729–45,940) | 9114 (2043–29,575) | 0.0451 |
| IL-18 (pg/mL) | 478 (72.97–2334) | 649.3 (348.5–1064) | 0.1084 |
| IP-10 (pg/mL) | 168.3 (63.31–1234) | 276.5 (59.97–2416) | 0.0312 |
| KIM-1 (pg/mL) | 107.9 (54.41–145.3) | 98.5 (80.68–167.6) | 0.3184 |
| Nephrin (pg/mL) | 921 (572–1218) | 813.5 (574.3–989.7) | 0.0105 |
| NGAL (pg/mL) | 148,830 (77,732–591,532) | 99,766 (41,860–328,496) | 0.0747 |
| Osteopontin (pg/mL) | 45,389 (3620–754,860) | 31,385 (5622–247,279) | 0.7278 |
| Pentraxin 3 (pg/mL) | 9146 (2579–41,062) | 7847 (1913–25,083) | 0.9023 |
| TFF3 (pg/mL) | 2281 (652.8–8333) | 760.5 (312.4–3967) | 0.0011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noack, D.; Travar, M.; Mrdjen, V.; Voermans, J.J.C.; van de Vijver, D.; Molenkamp, R.; Koopmans, M.P.G.; Goeijenbier, M.; Rockx, B. Serum Markers Associated with Disease Severity in a Bosnian Hemorrhagic Fever with Renal Syndrome Cohort. Viruses 2022, 14, 1377. https://doi.org/10.3390/v14071377
Noack D, Travar M, Mrdjen V, Voermans JJC, van de Vijver D, Molenkamp R, Koopmans MPG, Goeijenbier M, Rockx B. Serum Markers Associated with Disease Severity in a Bosnian Hemorrhagic Fever with Renal Syndrome Cohort. Viruses. 2022; 14(7):1377. https://doi.org/10.3390/v14071377
Chicago/Turabian StyleNoack, Danny, Maja Travar, Visnja Mrdjen, Jolanda J. C. Voermans, David van de Vijver, Richard Molenkamp, Marion P. G. Koopmans, Marco Goeijenbier, and Barry Rockx. 2022. "Serum Markers Associated with Disease Severity in a Bosnian Hemorrhagic Fever with Renal Syndrome Cohort" Viruses 14, no. 7: 1377. https://doi.org/10.3390/v14071377
APA StyleNoack, D., Travar, M., Mrdjen, V., Voermans, J. J. C., van de Vijver, D., Molenkamp, R., Koopmans, M. P. G., Goeijenbier, M., & Rockx, B. (2022). Serum Markers Associated with Disease Severity in a Bosnian Hemorrhagic Fever with Renal Syndrome Cohort. Viruses, 14(7), 1377. https://doi.org/10.3390/v14071377







