Grapevine Leafroll-Associated Virus 3 Genotype Influences Foliar Symptom Development in New Zealand Vineyards
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Propagation
2.2. Site Location and Study Configuration
2.3. Planting and Maintenance of Each Field Trial Plot
2.4. Climate Data
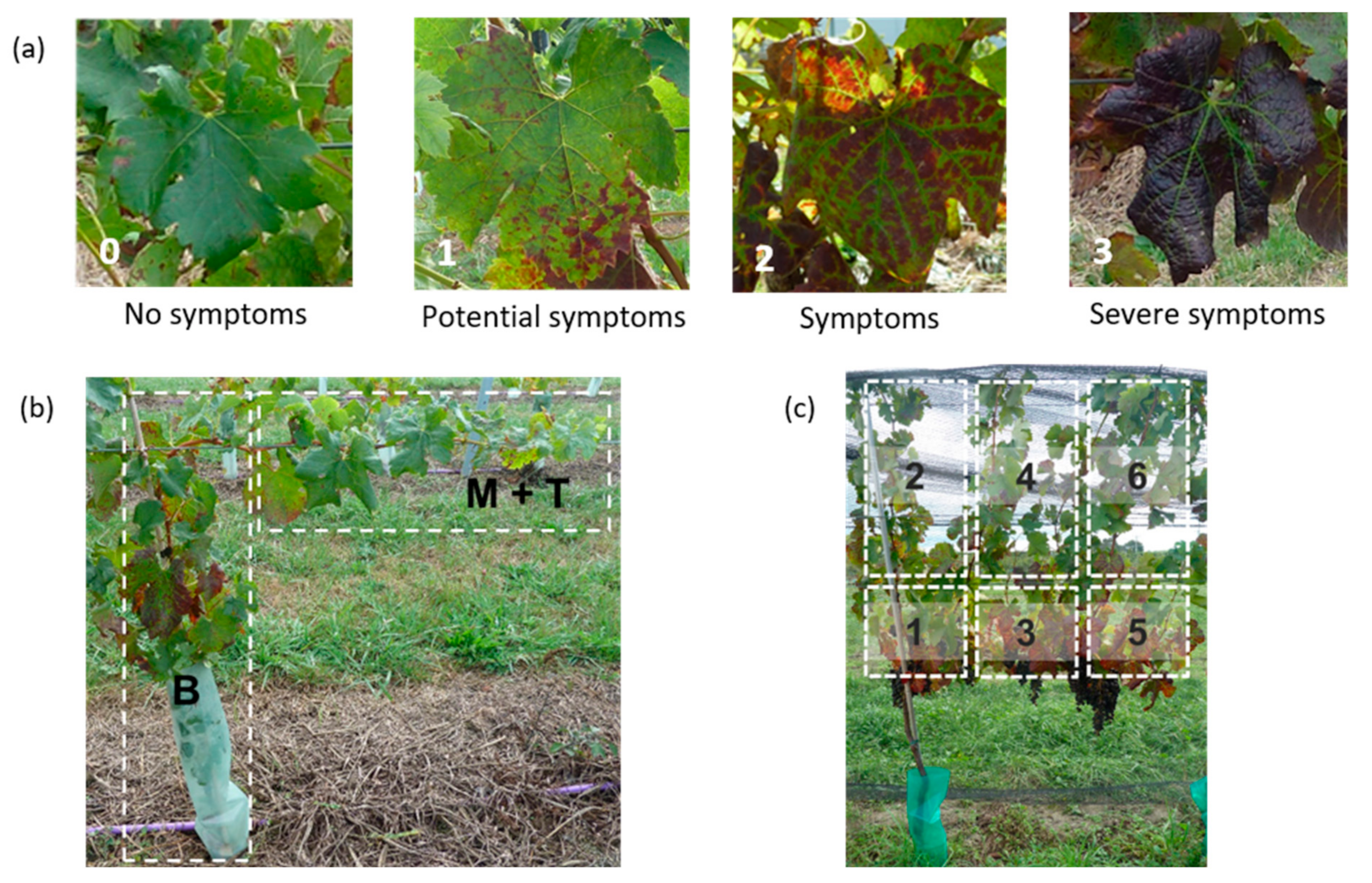
2.5. Visual Symptom Identification (VSI)
2.6. Quantitative Real-Time RT-PCR (RT-qPCR) and High-Resolution Melting Curve (HRM) Analysis
3. Results
3.1. Site Climate Based on Air Temperature and Rainfall
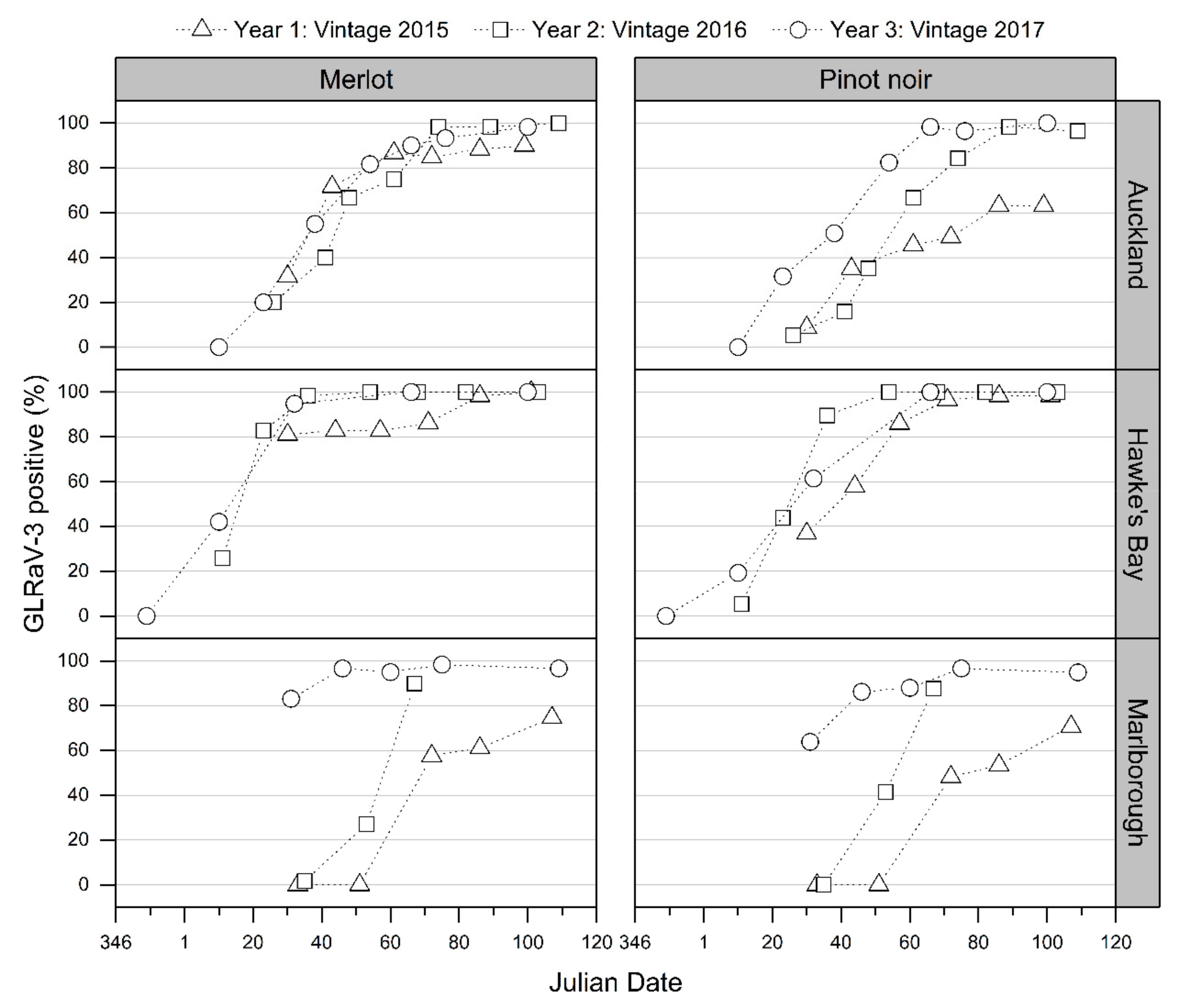
3.2. Visual Symptom Identification (VSI) of Grapevines for Grapevine Leafroll Disease (GLD)
3.2.1. Visual Symptom Identification of Grapevines Infected with One GLRaV-3 Genotype
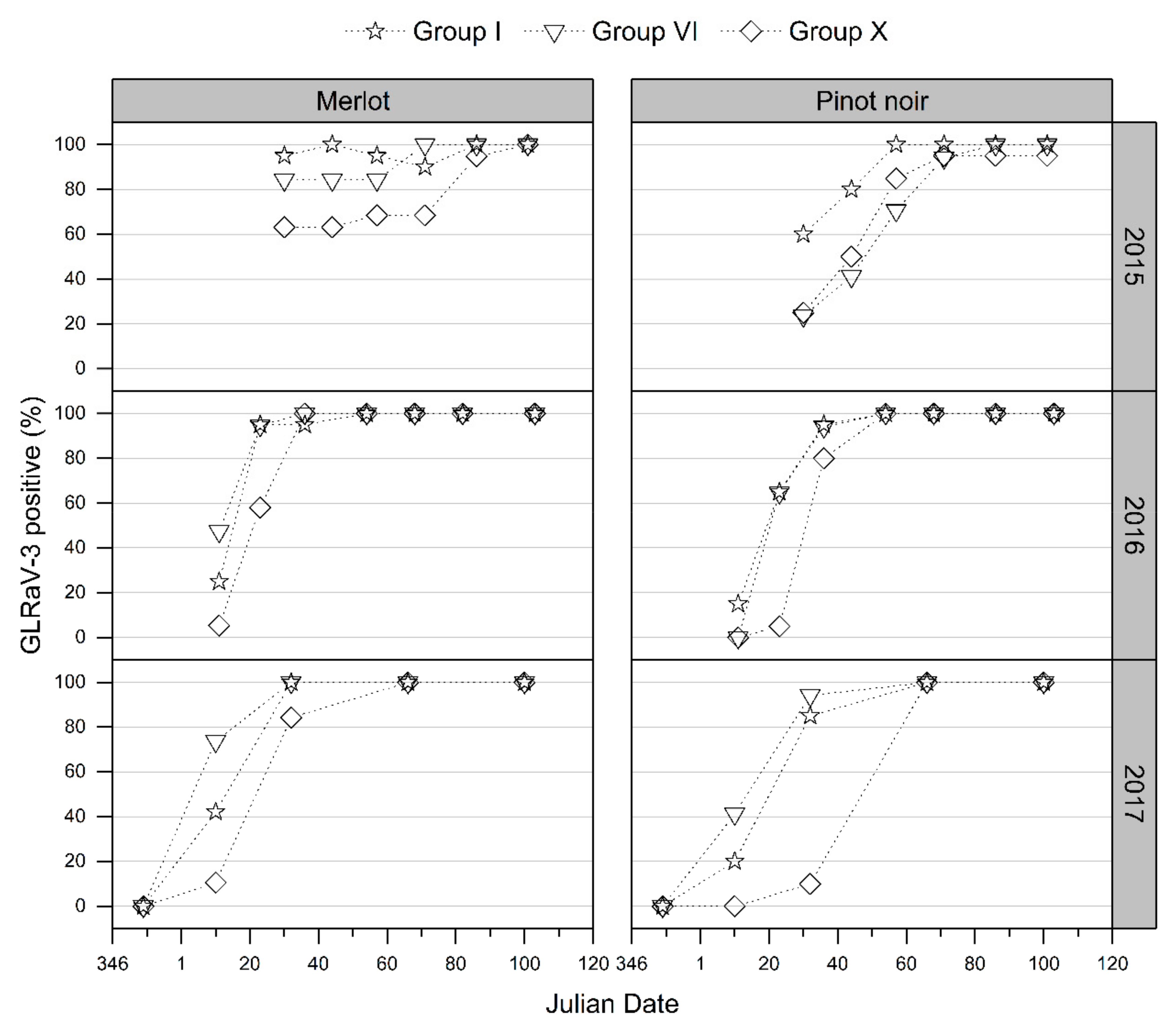
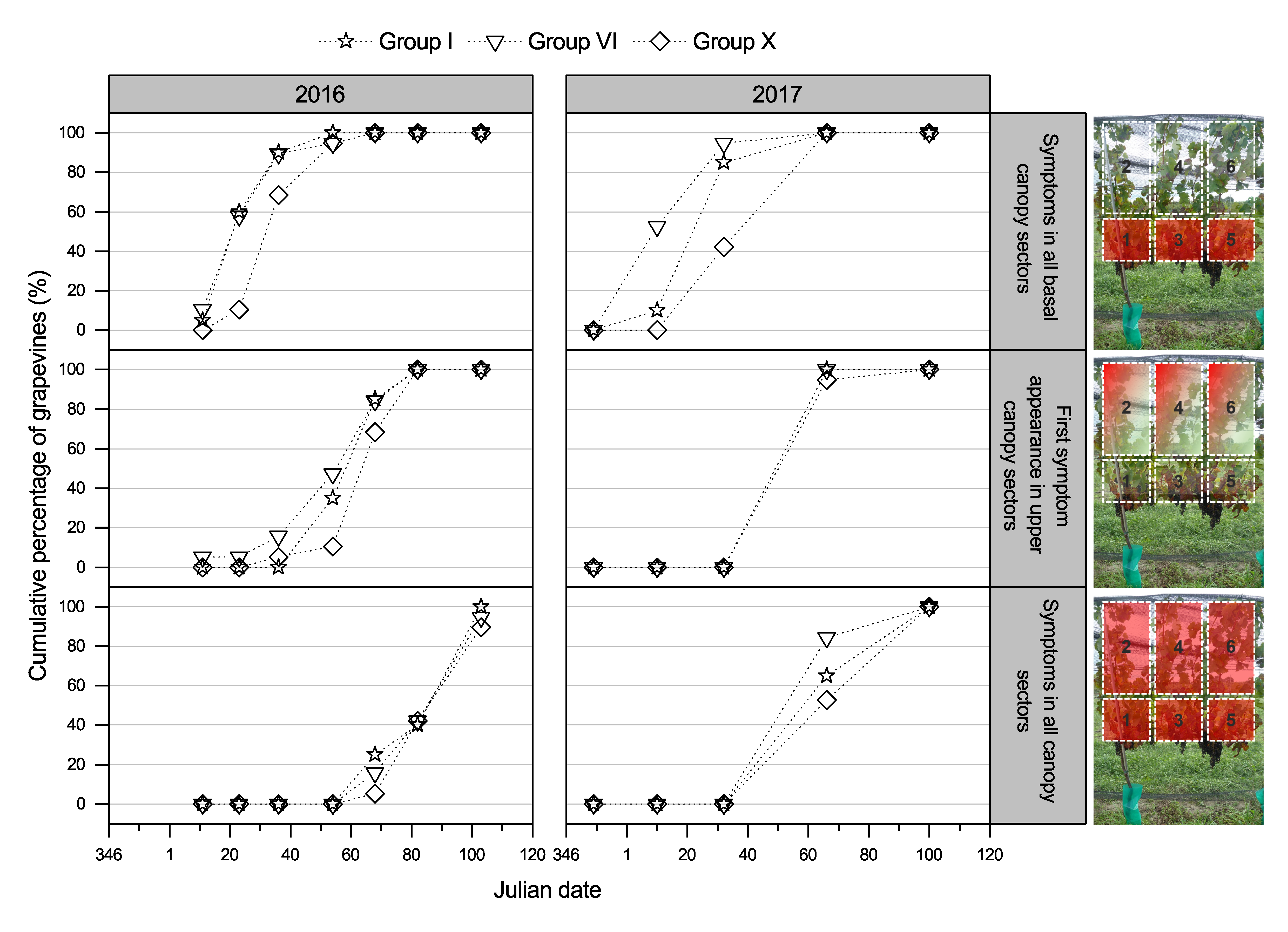
3.2.2. GLD Symptoms Based on Virus Genotype and Spread of Symptoms over the Canopy
3.3. Visual Symptom Identification of Grapevines Infected with Two GLRaV-3 Genotypes
4. Discussion
4.1. Véraison Coincides with GLD Foliar Symptoms in Red-Berry Grapevines
4.2. GLRaV-3 Genotypes, Foliar Symptom Development, and Potential Plant–Virus Interactions
4.3. Dual Infections of Different GLRaV-3 Genotypes Influenced the Onset of GLD Foliar Symptom Expression Compared with Single Infections of the Same GLRaV-3 Genotype
4.4. Visual Symptom Identification for GLD Foliar Symptoms in Red-Berry Grapevines Is an Effective Method for GLD Management
5. Conclusions
Supplementary Materials
 ” points) genotypes at the Auckland site, for the 2016 and 2017 vintages.
” points) genotypes at the Auckland site, for the 2016 and 2017 vintages.Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pathogen-Tested Material of Grapevine Varieties and Rootstocks. EPPO Bull. 2008, 38, 422–429. [CrossRef]
- Bell, V.A.; Blouin, A.G.; Cohen, D.; Hedderley, D.I.; Oosthuizen, T.; Spreeth, N.; Lester, P.J.; Pietersen, G. Visual Symptom Identification of Grapevine Leafroll-Associated Virus 3 in Red Berry Cultivars Supports Virus Management by Roguing. J. Plant Pathol. 2017, 99, 477–482. [Google Scholar]
- Bell, V.A.; Lester, P.J.; Pietersen, G.; Hall, A.J. The Management and Financial Implications of Variable Responses to Grapevine Leafroll Disease. J. Plant Pathol. 2021, 103, 5–15. [Google Scholar] [CrossRef]
- Song, Y.; Hanner, R.H.; Meng, B. Probing into the Effects of Grapevine Leafroll-Associated Viruses on the Physiology, Fruit Quality and Gene Expression of Grapes. Viruses 2021, 13, 593. [Google Scholar] [CrossRef]
- Naidu, R.; Rowhani, A.; Fuchs, M.; Golino, D.; Martelli, G.P. Grapevine Leafroll: A Complex Viral Disease Affecting a High-Value Fruit Crop. Plant Dis. 2014, 98, 1172–1185. [Google Scholar] [CrossRef]
- Naidu, R.A.; Maree, H.J.; Burger, J.T. Grapevine Leafroll Disease and Associated Viruses: A Unique Pathosystem. Annu. Rev. Phytopathol. 2015, 53, 613–634. [Google Scholar] [CrossRef]
- Over de Linden, A.J.; Chamberlain, E.E. Effect of Grapevine Leafroll Virus on Vine Growth and Fruit Yield and Quality. N. Z. J. Agric. Res. 1970, 13, 689–698. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Segura, A.; García-Berrios, J.J. Effects of Grapevine Leafroll-Associated Virus 3 on the Physiology and Must of Vitis Vinifera L. Cv. Albariño Following Contamination in the Field. Am. J. Enol. Vitic. 1999, 50, 40–44. [Google Scholar]
- Montero, R.; Mundy, D.; Albright, A.; Grose, C.; Trought, M.C.T.; Cohen, D.; Chooi, K.M.; MacDiarmid, R.; Flexas, J.; Bota, J. Effects of Grapevine Leafroll Associated Virus 3 (GLRaV-3) and Duration of Infection on Fruit Composition and Wine Chemical Profile of Vitis Vinifera L. Cv. Sauvignon Blanc. Food Chem. 2016, 197, 1177–1183. [Google Scholar] [CrossRef]
- Martelli, G.P.; Agranovsky, A.A.; Bar-Joseph, M.; Boscia, D.; Candresse, T.; Coutts, R.H.A.; Dolja, V.V.; Falk, B.W.; Gonsalves, D.; Jelkmann, W.; et al. The Family Closteroviridae Revised. Arch. Virol. 2002, 147, 2039–2044. [Google Scholar] [CrossRef]
- Martelli, G.P.; Boudon-Padieu, E. Directory of Infectious Diseases of Grapevines and Viroses and Virus-like Diseases of the Grapevine: Bibliographic Report 1998–2004; Martelli, G.P., Boudon-Padieu, E., Eds.; Options Méditerranéennes Série B: Etudes et Recherches; Ciheam: Paris, France, 2006; ISBN 978-2-85352-338-7. [Google Scholar]
- Maree, H.; Almeida, R.; Bester, R.; Chooi, K.M.; Cohen, D.; Dolja, V.; Fuchs, M.; Golino, D.; Jooste, A.; Martelli, G.; et al. Grapevine Leafroll-Associated Virus 3. Front. Microbiol. 2013, 4, 82. [Google Scholar] [CrossRef]
- Gutha, L.R.; Casassa, L.F.; Harbertson, J.F.; Naidu, R.A. Modulation of Flavonoid Biosynthetic Pathway Genes and Anthocyanins Due to Virus Infection in Grapevine (Vitis Vinifera L.) Leaves. BMC Plant Biol. 2010, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Sultanova, N.; Bayramova, N.; Aliyeva, D.; Rastgou, M.; Huseynova, I. Induced Changes in Metabolic Constituents of Grapevine (Vitis Vinifera L.) Leaves Infected with Grapevine Leafroll-Associated Virus-3. Physiol. Mol. Plant Pathol. 2019, 106, 57–63. [Google Scholar] [CrossRef]
- Halldorson, M.M.; Keller, M. Grapevine Leafroll Disease Alters Leaf Physiology but Has Little Effect on Plant Cold Hardiness. Planta 2018, 248, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Pietersen, G.; Bell, V.A.; Krüger, K. Management of Grapevine Leafroll Disease and Associated Vectors in Vineyards. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 531–560. ISBN 978-3-319-57706-7. [Google Scholar]
- Cui, Z.-H.; Bi, W.-L.; Hao, X.-Y.; Li, P.-M.; Duan, Y.; Walker, M.A.; Xu, Y.; Wang, Q.-C. Drought Stress Enhances Up-Regulation of Anthocyanin Biosynthesis in Grapevine Leafroll-Associated Virus 3- Infected in Vitro Grapevine (Vitis Vinifera) Leaves. Plant Dis. 2017, 101, 1606–1615. [Google Scholar] [CrossRef]
- Walker, A.R.; Lee, E.; Bogs, J.; McDavid, D.A.J.; Thomas, M.R.; Robinson, S.P. White Grapes Arose through the Mutation of Two Similar and Adjacent Regulatory Genes. Plant J. 2007, 49, 772–785. [Google Scholar] [CrossRef]
- Kobayashi, S.; Goto-Yamamoto, N.; Hirochika, H. Retrotransposon-Induced Mutations in Grape Skin Color. Science 2004, 304, 982. [Google Scholar] [CrossRef]
- This, P.; Lacombe, T.; Cadle-Davidson, M.; Owens, C.L. Wine Grape (Vitis Vinifera L.) Color Associates with Allelic Variation in the Domestication Gene VvmybA1. Theor. Appl. Genet. 2007, 114, 723–730. [Google Scholar] [CrossRef]
- Vondras, A.M.; Lerno, L.; Massonnet, M.; Minio, A.; Rowhani, A.; Liang, D.; Garcia, J.; Quiroz, D.; Figueroa-Balderas, R.; Golino, D.A.; et al. Rootstocks Influence the Response of Ripening Grape Berries to Leafroll Associated Viruses. bioRxiv 2021, 3, 434319. [Google Scholar] [CrossRef]
- Lee, R.; Keremane, M. Mild Strain Cross Protection of Tristeza: A Review of Research to Protect against Decline on Sour Orange in Florida. Front. Microbiol. 2013, 4, 259. [Google Scholar] [CrossRef]
- Diaz-Lara, A.; Klaassen, V.; Stevens, K.; Sudarshana, M.R.; Rowhani, A.; Maree, H.J.; Chooi, K.M.; Blouin, A.G.; Habili, N.; Song, Y.; et al. Characterization of Grapevine Leafroll-Associated Virus 3 Genetic Variants and Application towards RT-QPCR Assay Design. PLoS ONE 2018, 13, e0208862. [Google Scholar] [CrossRef] [PubMed]
- Chooi, K.M.; Cohen, D.; Pearson, M.N. Molecular Characterisation of Two Divergent Variants of Grapevine Leafroll-Associated Virus 3 in New Zealand. Arch. Virol. 2013, 158, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Chooi, K.M. Molecular Characterisation of Grapevine Leafroll-Associated Virus 3 and Implications for Diagnostic Testing and Pathogenicity. Ph.D. Thesis, University of Auckland, Auckland, New Zealand, 2013. [Google Scholar]
- Chooi, K.M.; Cohen, D.; Pearson, M.N. Generic and Sequence-Variant Specific Molecular Assays for the Detection of the Highly Variable Grapevine Leafroll-Associated Virus 3. J. Virol. Methods 2013, 189, 20–29. [Google Scholar] [CrossRef]
- Chooi, K.M.; Cohen, D.; Pearson, M.N. Differential Distribution and Titre of Selected Grapevine Leafroll-Associated Virus 3 Genetic Variants within Grapevine Rootstocks. Arch. Virol. 2016, 161, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, P.; Nolasco, G. The P19.7 RNA Silencing Suppressor from Grapevine Leafroll-Associated Virus 3 Shows Different Levels of Activity across Phylogenetic Groups. Virus Genes 2012, 45, 333–339. [Google Scholar] [CrossRef]
- Gouveia, P.; Dandlen, S.; Costa, Â.; Marques, N.; Nolasco, G. Identification of an RNA Silencing Suppressor Encoded by Grapevine Leafroll-Associated Virus 3. Eur. J. Plant Pathol. 2012, 133, 237–245. [Google Scholar] [CrossRef]
- Habili, N.; Nutter, F.W. Temporal and Spatial Analysis of Grapevine Leafroll-Associated Virus 3 in Pinot Noir Grapevines in Australia. Plant Dis. 1997, 81, 625–628. [Google Scholar] [CrossRef]
- Jooste, A.E.C.; Pietersen, G.; Burger, J.T. Distribution of Grapevine Leafroll Associated Virus-3 Variants in South African Vineyards. Eur. J. Plant Pathol. 2011, 131, 371–381. [Google Scholar] [CrossRef]
- Sharma, A.M.; Wang, J.; Duffy, S.; Zhang, S.; Wong, M.K.; Rashed, A.; Cooper, M.L.; Daane, K.M.; Almeida, R.P.P. Occurrence of Grapevine Leafroll-Associated Virus Complex in Napa Valley. PLoS ONE 2011, 6, e26227. [Google Scholar] [CrossRef]
- Rast, H.E.; James, D.; Habili, N.; Masri, S.A. Genome Organization and Characterization of a Novel Variant of Grapevine Leafroll-Associated Virus 3. In Proceedings of the 17th Meeting of the International Council for the Study of Virus and Virus-like Diseases of the Grapevine, Davis, CA, USA, 7–14 October 2012; pp. 61–63. [Google Scholar]
- Habili, N.; Cameron, I.; Randles, J. A Mild Strain of Grapevine Leafroll-Associated Virus 3 Is Present in Desirable Clones of Crimson Seedless Table Grapes in Western Australia. In Proceedings of the 16th Meeting of the International Council for the Study of Virus and Virus-like Diseases of the Grapevine, Dijon, France, 31 August–4 September 2009; pp. 237–238. [Google Scholar]
- Blouin, A.G.; Chooi, K.M.; Cohen, D.; MacDiarmid, R.M. Serological Methods for the Detection of Major Grapevine Viruses. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 409–429. ISBN 978-3-319-57706-7. [Google Scholar]
- Roossinck, M.J.; Saha, P.; Wiley, G.B.; Quan, J.; White, J.D.; Lai, H.; Chavarría, F.; Shen, G.; Roe, B.A. Ecogenomics: Using Massively Parallel Pyrosequencing to Understand Virus Ecology. Mol. Ecol. 2010, 19, 81–88. [Google Scholar] [CrossRef]
- Blouin, A.G.; Ross, H.A.; Hobson-Peters, J.; O’Brien, C.A.; Warren, B.; MacDiarmid, R. A New Virus Discovered by Immunocapture of Double-Stranded RNA, a Rapid Method for Virus Enrichment in Metagenomic Studies. Mol. Ecol. Resour. 2016, 16, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J. Experimental Designs Balanced for the Estimation of Residual Effects of Treatments. Aust. J. Chem. 1949, 2, 149–168. [Google Scholar] [CrossRef]
- Jackson, D.I.; Cherry, N.J. Prediction of a District’s Grape-Ripening Capacity Using a Latitude-Temperature Index (LTI). Am. J. Enol. Vitic. 1988, 39, 19–28. [Google Scholar]
- White, E.J.; Venter, M.; Hiten, N.F.; Burger, J.T. Modified Cetyltrimethylammonium Bromide Method Improves Robustness and Versatility: The Benchmark for Plant RNA Extraction. Biotechnol. J. 2008, 3, 1424–1428. [Google Scholar] [CrossRef]
- Tyerman, S.D.; Tilbrook, J.; Pardo, C.; Kotula, L.; Sullivan, W.; Steudle, E. Direct Measurement of Hydraulic Properties in Developing Berries of Vitis Vinifera L. Cv Shiraz and Chardonnay. Aust. J. Grape Wine Res. 2004, 10, 170–181. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Gambetta, G.A.; Wada, H.; Krasnow, M.N.; Cramer, G.R.; Peterlunger, E.; Shackel, K.A.; Matthews, M.A. Characterization of Major Ripening Events during Softening in Grape: Turgor, Sugar Accumulation, Abscisic Acid Metabolism, Colour Development, and Their Relationship with Growth. J. Exp. Bot. 2016, 67, 709–722. [Google Scholar] [CrossRef]
- Lang, A.; Thorpe, M.R. Xylem, Phloem and Transpiration Flows in a Grape: Application of a Technique for Measuring the Volume of Attached Fruits to High Resolution Using Archimedes’ Principle. J. Exp. Bot. 1989, 40, 1069–1078. [Google Scholar] [CrossRef]
- Greenspan, M.; Schultz, H.; Matthews, M. Field Evaluation of Water Transport in Grape Berries during Water Deficits. Physiol. Plant. 1996, 97, 55–62. [Google Scholar] [CrossRef]
- Keller, M.; Zhang, Y.; Shrestha, P.M.; Biondi, M.; Bondada, B.R. Sugar Demand of Ripening Grape Berries Leads to Recycling of Surplus Phloem Water via the Xylem. Plant Cell Environ. 2015, 38, 1048–1059. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Wang, X.-L.; Wang, X.-F.; Xia, G.-H.; Pan, Q.-H.; Fan, R.-C.; Wu, F.-Q.; Yu, X.-C.; Zhang, D.-P. A Shift of Phloem Unloading from Symplasmic to Apoplasmic Pathway Is Involved in Developmental Onset of Ripening in Grape Berry. Plant Physiol. 2006, 142, 220–232. [Google Scholar] [CrossRef]
- Koseki, M.; Goto, K.; Masuta, C.; Kanazawa, A. The Star-Type Color Pattern in Petunia Hybrida “red Star” Flowers Is Induced by Sequence-Specific Degradation of Chalcone Synthase RNA. Plant Cell Physiol. 2005, 46, 1879–1883. [Google Scholar] [CrossRef] [PubMed]
- Teycheney, P.-Y.; Tepfer, M. 2001 Virus-Specific Spatial Differences in the Interference with Silencing of the Chs-A Gene in Non-Transgenic Petunia. J. Gen. Virol. 2001, 82, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-D.; Folimonova, S.Y. The P33 Protein of Citrus Tristeza Virus Affects Viral Pathogenicity by Modulating a Host Immune Response. New Phytol. 2019, 221, 2039–2053. [Google Scholar] [CrossRef]
- Díaz-Pendón, J.A.; Ding, S.-W. Direct and Indirect Roles of Viral Suppressors of RNA Silencing in Pathogenesis. Annu. Rev. Phytopathol. 2008, 46, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.C.; Vega, J.; Oliveira, J.G.; Gomes, M.M.A. Sugarcane Yellow Leaf Virus Infection Leads to Alterations in Photosynthetic Efficiency and Carbohydrate Accumulation in Sugarcane Leaves. Fitopatol. Bras. 2005, 30, 10–16. [Google Scholar] [CrossRef]
- Hyodo, K.; Hashimoto, K.; Kuchitsu, K.; Suzuki, N.; Okuno, T. Harnessing Host ROS-Generating Machinery for the Robust Genome Replication of a Plant RNA Virus. Proc. Natl. Acad. Sci. USA 2017, 114, E1282–E1290. [Google Scholar] [CrossRef]
- Espinoza, C.; Medina, C.; Somerville, S.; Arce-Johnson, P. Senescence-Associated Genes Induced during Compatible Viral Interactions with Grapevine and Arabidopsis. J. Exp. Bot. 2007, 58, 3197–3212. [Google Scholar] [CrossRef]
- Pandey, N.; Ahmad, W.; Chooi, K.M.; Karunairetnam, S.; Blouin, A.G.; Ziebell, H.; MacDiarmid, R.M. Characterising the Suppressors of Silencing Encoded by Grapevine Leafroll-Associated Virus 3 and Their Activity in New Zealand Genetic Variants. In Proceedings of the 18th Meeting of the International Council for the Study of Virus and Virus-like Diseases of the Grapevine, Ankara, Turkey, 7–11 September 2015; pp. 159–160. [Google Scholar]
- Qu, F.; Morris, T.J. Efficient Infection of Nicotiana Benthamiana by Tomato Bushy Stunt Virus Is Facilitated by the Coat Protein and Maintained by P19 through Suppression of Gene Silencing. Mol. Plant Microbe Interact. 2002, 15, 193–202. [Google Scholar] [CrossRef]
- Manabayeva, S.A.; Shamekova, M.; Park, J.-W.; Ding, X.S.; Nelson, R.S.; Hsieh, Y.-C.; Omarov, R.T.; Scholthof, H.B. Differential Requirements for Tombusvirus Coat Protein and P19 in Plants Following Leaf versus Root Inoculation. Virology 2013, 439, 89–96. [Google Scholar] [CrossRef]
- Bester, R.; Pepler, P.T.; Burger, J.T.; Maree, H.J. Relative Quantitation Goes Viral: An RT-QPCR Assay for a Grapevine Virus. J. Virol. Methods 2014, 210, 67–75. [Google Scholar] [CrossRef]
- Chooi, K.M.; Blouin, A.G.; Cohen, D.; Bell, V.A.; Mundy, D.; Nobilo, S.; Taylor, T.; Vanga, B.; Albright, A.; MacDiarmid, R.M. The Effect of Leafroll 3 Genetic Variants on Grapevines; New Zealand Winegrowers: Auckland, New Zealand, 2017; pp. 98–99. [Google Scholar]
 ” points) genotype at the Auckland site, for the vintages 2015, 2016, and 2017.
” points) genotype at the Auckland site, for the vintages 2015, 2016, and 2017.
 ” points) genotype at the Auckland site, for the vintages 2015, 2016, and 2017.
” points) genotype at the Auckland site, for the vintages 2015, 2016, and 2017.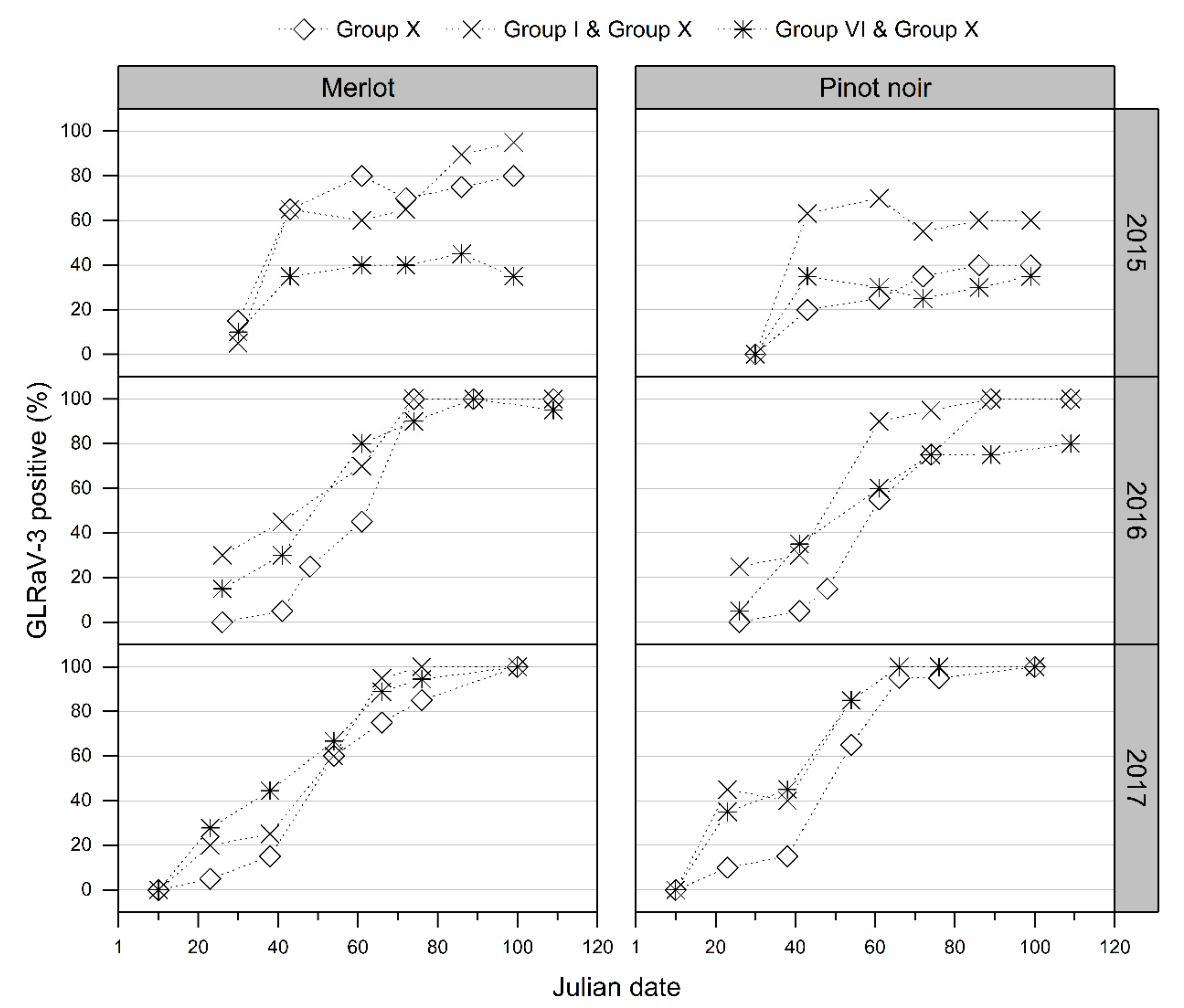
| Auckland | Hawke’s Bay | Marlborough | |
|---|---|---|---|
| 2015 vintage | |||
| MTWM (°C) | n/a | 18.4 | 18.8 |
| MTCM (°C) | n/a | 7.9 | 7.4 |
| Annual rainfall (mm) | n/a | 607.8 | 381.6 |
| GDDs | n/a | 1357.3 | 1367.2 |
| LTI | n/a | 374.4 | 347.8 |
| 2016 vintage | |||
| MTWM (°C) | 19.9 | 20.1 | 20.0 |
| MTCM (°C) | 10.3 | 8.8 | 8.3 |
| Annual rainfall (mm) | 1276 | 661.9 | 590.8 |
| GDDs (from 18 November 2015) | 1277.8 | 1183.6 | 1167.6 |
| GDDs (complete vintage) | n/a | 1357.3 | 1386.5 |
| LTI | 461.1 | 409.0 | 370 |
| 2017 vintage | |||
| MTWM (°C) | 18.2 | 19.7 | 18.6 |
| MTCM (°C) | 10.2 | 8.0 | 7.9 |
| Annual rainfall (mm) | 1538 # | 846.6 | 591.4 |
| GDDs | 1405.6 | 1614.1 | 1368 |
| LTI | 431.0 | 400.9 | 344.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chooi, K.M.; Bell, V.A.; Blouin, A.G.; Cohen, D.; Mundy, D.; Henshall, W.; MacDiarmid, R.M. Grapevine Leafroll-Associated Virus 3 Genotype Influences Foliar Symptom Development in New Zealand Vineyards. Viruses 2022, 14, 1348. https://doi.org/10.3390/v14071348
Chooi KM, Bell VA, Blouin AG, Cohen D, Mundy D, Henshall W, MacDiarmid RM. Grapevine Leafroll-Associated Virus 3 Genotype Influences Foliar Symptom Development in New Zealand Vineyards. Viruses. 2022; 14(7):1348. https://doi.org/10.3390/v14071348
Chicago/Turabian StyleChooi, Kar Mun, Vaughn A. Bell, Arnaud G. Blouin, Daniel Cohen, Dion Mundy, Warwick Henshall, and Robin M. MacDiarmid. 2022. "Grapevine Leafroll-Associated Virus 3 Genotype Influences Foliar Symptom Development in New Zealand Vineyards" Viruses 14, no. 7: 1348. https://doi.org/10.3390/v14071348
APA StyleChooi, K. M., Bell, V. A., Blouin, A. G., Cohen, D., Mundy, D., Henshall, W., & MacDiarmid, R. M. (2022). Grapevine Leafroll-Associated Virus 3 Genotype Influences Foliar Symptom Development in New Zealand Vineyards. Viruses, 14(7), 1348. https://doi.org/10.3390/v14071348








