Investigating COVID-19 Vaccine Impact on the Risk of Hospitalisation through the Analysis of National Surveillance Data Collected in Belgium
Abstract
:1. Introduction
2. Data Collection
3. Data Analyses
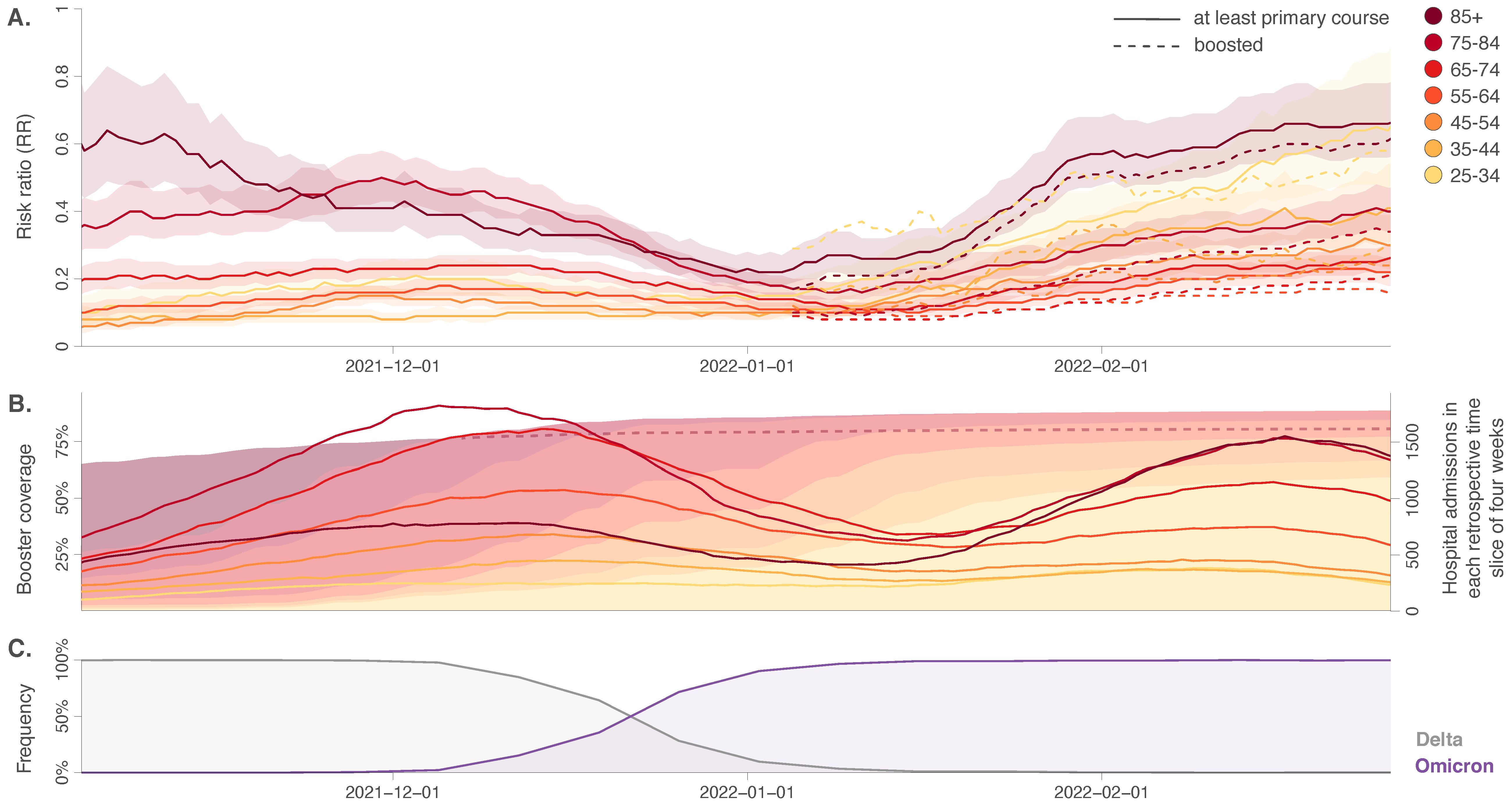
4. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Yu, Q.; Wen, H.; Shi, F.; Wang, F.; Zhao, Y.; Hong, Q.; Yu, C. What matters: Non-pharmaceutical interventions for COVID-19 in Europe. Antimicrob. Resist. Infect. Control 2022, 11, 3. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group; Horby, P.W.; Mafham, M.; Peto, L.; Campbell, M.; Pessoa-Amorim, G.; Spata, E.; Staplin, N.; Emberson, J.R.; Prudon, B.; et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Zuil, M.; Benítez, I.D.; Cabo-Gambín, R.; Manzano Senra, C.; Moncusí-Moix, A.; Gort-Paniello, C.; de Gonzalo-Calvo, D.; Molinero, M.; Vengoechea Aragoncillo, J.J.; Comella, T.; et al. Clinical management and outcome differences between first and second waves among COVID-19 hospitalized patients: A regional prospective observational cohort. PLoS ONE 2021, 16, e0258918. [Google Scholar] [CrossRef]
- Salasc, F.; Lahlali, T.; Laurent, E.; Rosa-Calatrava, M.; Pizzorno, A. Treatments for COVID-19: Lessons from 2020 and new therapeutic options. Curr. Opin. Pharmacol. 2022, 62, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Veerdonk, F.L.V.D.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Bloomberg.com. More Than 11.3 Billion Shots Given: Covid-19 Tracker. Available online: https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/ (accessed on 5 April 2022).
- Davis, C.; Logan, N.; Tyson, G.; Orton, R.; Harvey, W.T.; Perkins, J.S.; Mollett, G.; Blacow, R.M.; The COVID-19 Genomics UK (COG-UK) Consortium; Peacock, T.P.; et al. Reduced neutralisation of the Delta (B.1.617.2) SARS-CoV-2 variant of concern following vaccination. PLoS Path. 2021, 17, e1010022. [Google Scholar] [CrossRef]
- Lu, L.; Mok, B.W.-Y.; Chen, L.; Chan, J.M.-C.; Tsang, O.T.-Y.; Lam, B.H.-S.; Chuang, V.W.-M.; Chu, A.W.-H.; Chan, W.-M.; Ip, J.D.; et al. Neutralization of severe acute respiratory syndrome coronavirus 2 Omicron variant by sera from BNT162b2 or CoronaVac vaccine recipients. Clin. Infect. Dis. 2021, 2021, ciab1041. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Federal Platform of SARS-CoV-2 Genomic Surveillance, Genomic Surveillance of SARS-CoV-2 in Belgium. Available online: https://www.uzleuven.be/nl/laboratoriumgeneeskunde/genomic-surveillance-sars-cov-2-belgium (accessed on 28 March 2022).
- Cele, S.; Karim, F.; Lustig, G.; San, J.E.; Hermanus, T.; Tegally, H.; Snyman, J.; Moyo-Gwete, T.; Wilkinson, E.; Bernstein, M.; et al. SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell Host Microbe 2022, 30, 154–162. [Google Scholar] [CrossRef]
- Newman, J.; Thakur, N.; Peacock, T.P.; Bialy, D.; Elreafey, A.M.E.; Bogaardt, C.; Horton, D.L.; Ho, S.; Kankeyan, T.; Carr, C.; et al. Neutralising antibody activity against SARS-CoV-2 variants, including Omicron, in an elderly cohort vaccinated with BNT162b2. medRxiv 2021. [Google Scholar] [CrossRef]
- Straten, K.V.D.; Guerra, D.; Gils, M.J.V.; Bontjer, I.; Caniels, T.G.; van Willigen, H.D.G.; Wynberg, E.; Poniman, M.; Burger, J.A.; Bouhuijs, J.H.; et al. Mapping the antigenic diversification of SARS-CoV-2. medRxiv 2022. [Google Scholar] [CrossRef]
- Goethem, N.V.; Vilain, A.; Wyndham-Thomas, C.; Deblonde, J.; Bossuyt, N.; Lernout, T.; Gonzalez, J.R.; Quoilin, S.; Melis, V.; Beckhoven, D.V. Rapid establishment of a national surveillance of COVID-19 hospitalizations in Belgium. Arch. Public Health 2020, 78, 121. [Google Scholar] [CrossRef] [PubMed]
- Goethem, N.V.; Serrien, B.; Vandromme, M.; Wyndham-Thomas, C.; Catteau, L.; Brondeel, R.; Klamer, S.; Meurisse, M.; Cuypers, L.; André, E.; et al. Conceptual causal framework to assess the effect of SARS-CoV-2 variants on COVID-19 disease severity among hospitalized patients. Arch. Public Health 2021, 79, 185. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; He, S.; Nasreen, S.; Sundaram, M.E.; Buchan, S.A.; Wilson, S.E.; Chen, B.; Calzavara, A.; Fell, D.B.; Austin, P.C.; et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada: Test negative design study. BMJ 2021, 374, n1943. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Hitchings, M.D.T.; Dorion, M.; D’Agostini, T.L.; Paula, R.C.D.; Paula, O.F.P.D.; Villela, E.F.D.M.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: Test negative case-control study. BMJ 2021, 374, n2015. [Google Scholar] [CrossRef]
- Morris, J.A.; Gardner, M.J. Statistics in medicine: Calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br. Med. J. 1988, 296, 1313–1316. [Google Scholar] [CrossRef] [Green Version]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Chand, M.; Brown, K.; Ladhani, S.N.; et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef]
- Starrfelt, J.; Skyrud Danielsen, A.; Buanes, E.A.; Juvet, L.K.; Lyngstad, T.M.; Rø, G.Ø.I.; Veneti, L.; Viksmoen Watle, S.; Meijerink, H. Age and product dependent vaccine effectiveness against SARS-CoV-2 infection and hospitalisation among adults in Norway: A national cohort study, July–November 2021. medRxiv 2022. [Google Scholar] [CrossRef]
- Vitek, M.G.; Klavs, I.; Učakar, V.; Serdt, M.; Mrzel, M.; Vrh, M.; Fafangel, M. Vaccine effectiveness against severe acute respiratory infections (SARI) COVID-19 hospitalisations estimated from real-world surveillance data, Slovenia, October 2021. Eurosurveillance 2022, 27, 2101110. [Google Scholar]
- Pulliam, J.R.C.; Schalkwyk, C.V.; Govender, N.; Gottberg, A.V.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science 2022. [Google Scholar] [CrossRef]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef] [PubMed]
- HKUMed. HKUMed Reports that SARS-CoV-2 Omicron Infection Recalls mRNA-Vaccine-Induced Immunity for Broad Protection. Available online: http://www.med.hku.hk/en/news/press/20220127-omicron-infection-recalls-mrna-vaccine-induced-immunity-for-broad-protection?utm_medium=social&utm_source=twitter&utm_campaign=press_release (accessed on 20 April 2022).
- Simpson, E.H. The interpretation of interaction in contingency tables. J. R. Stat. Soc. Ser. B 1951, 13, 238–241. [Google Scholar] [CrossRef]
- Wagner, C.H. Simpson’s Paradox in real life. Am. Stat. 1982, 36, 46–48. [Google Scholar]
- European Centre for Disease Prevention and Control. Interim Analysis of COVID-19 Vaccine Effectiveness against Severe Acute Respiratory Infection due to Laboratory-Confirmed SARS-CoV-2 Among Individuals Aged 30 Years and Older. ECDC Multi-Country Study—Second Update. Available online: https://www.ecdc.europa.eu/en/publications-data/interim-analysis-covid-19-vaccine-effectiveness-against-severe-acute-respiratory (accessed on 20 April 2022).
- Lin, D.-Y.; Gu, Y.; Wheeler, B.; Young, H.; Holloway, S.; Sunny, S.-K.; Moore, Z.; Zeng, D. Effectiveness of Covid-19 vaccines over a 9-month period in North Carolina. N. Engl. J. Med. 2022, 386, 933–941. [Google Scholar] [CrossRef]
- Bager, P.; Wohlfahrt, J.; Fonager, J.; Rasmussen, M.; Albertsen, M.; Michaelsen, T.Y.; Holter Møller, C.; Ethelberg, S.; Legarth, R.; Fisher Button, M.S.; et al. Risk of hospitalisation associated with infection with SARS-CoV-2 lineage B.1.1.7 in Denmark: An observational cohort study. Lancet Infect. Dis. 2021, 21, 1507–1517. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erazo, D.; Vincenti-Gonzalez, M.F.; van Loenhout, J.A.F.; Hubin, P.; Vandromme, M.; Maes, P.; Taquet, M.; Van Weyenbergh, J.; Catteau, L.; Dellicour, S. Investigating COVID-19 Vaccine Impact on the Risk of Hospitalisation through the Analysis of National Surveillance Data Collected in Belgium. Viruses 2022, 14, 1315. https://doi.org/10.3390/v14061315
Erazo D, Vincenti-Gonzalez MF, van Loenhout JAF, Hubin P, Vandromme M, Maes P, Taquet M, Van Weyenbergh J, Catteau L, Dellicour S. Investigating COVID-19 Vaccine Impact on the Risk of Hospitalisation through the Analysis of National Surveillance Data Collected in Belgium. Viruses. 2022; 14(6):1315. https://doi.org/10.3390/v14061315
Chicago/Turabian StyleErazo, Diana, Maria F. Vincenti-Gonzalez, Joris A. F. van Loenhout, Pierre Hubin, Mathil Vandromme, Piet Maes, Maxime Taquet, Johan Van Weyenbergh, Lucy Catteau, and Simon Dellicour. 2022. "Investigating COVID-19 Vaccine Impact on the Risk of Hospitalisation through the Analysis of National Surveillance Data Collected in Belgium" Viruses 14, no. 6: 1315. https://doi.org/10.3390/v14061315
APA StyleErazo, D., Vincenti-Gonzalez, M. F., van Loenhout, J. A. F., Hubin, P., Vandromme, M., Maes, P., Taquet, M., Van Weyenbergh, J., Catteau, L., & Dellicour, S. (2022). Investigating COVID-19 Vaccine Impact on the Risk of Hospitalisation through the Analysis of National Surveillance Data Collected in Belgium. Viruses, 14(6), 1315. https://doi.org/10.3390/v14061315





