Mosquito-Borne Flaviviruses and Current Therapeutic Advances
Abstract
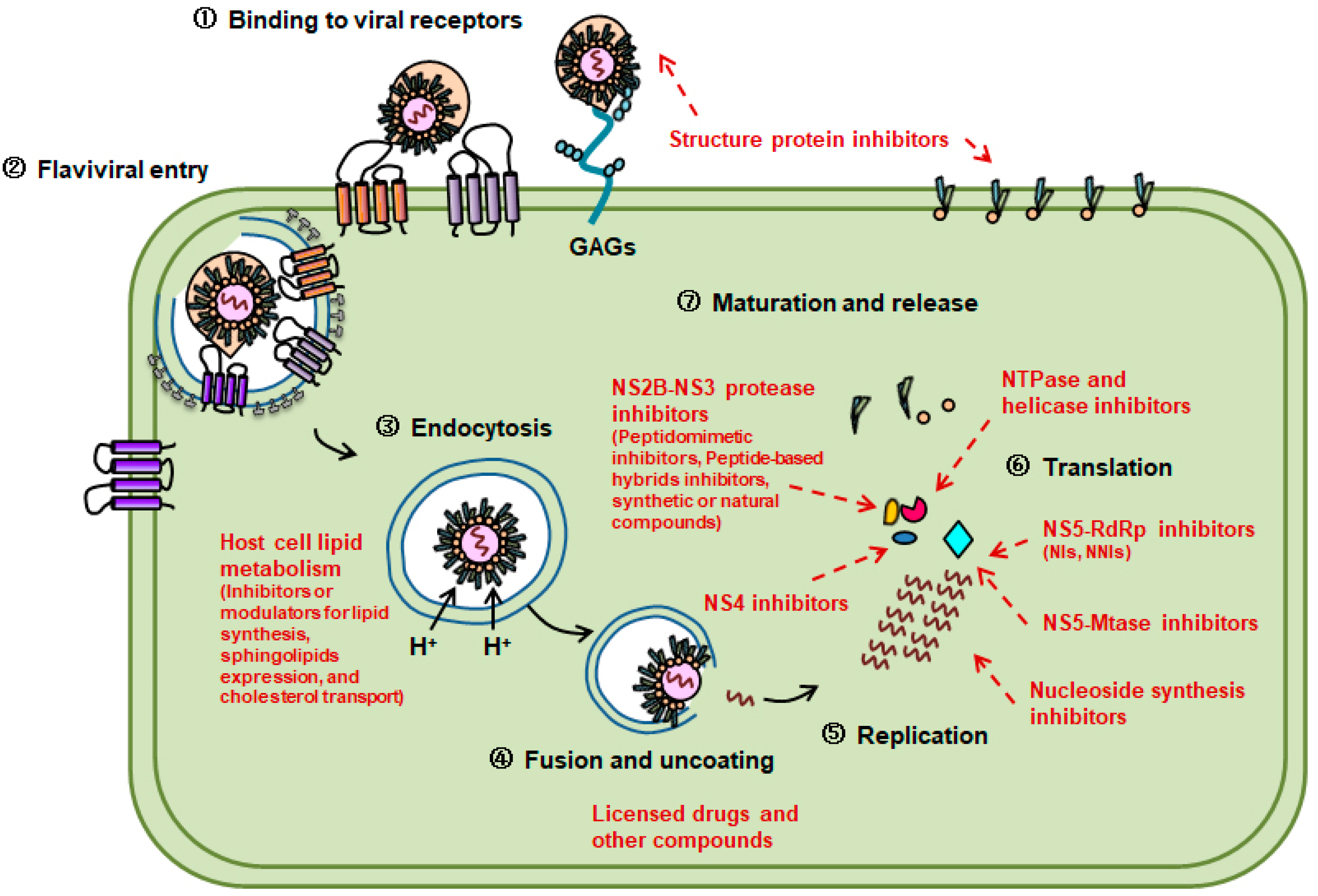
:1. Introduction
2. Inhibitors Targeting Flaviviral NS3
2.1. NS2B–NS3 Protease Inhibitors
2.2. NTPase and Helicase Inhibitors
3. Inhibitors Targeting Flaviviral NS4
4. Inhibitors Targeting Flaviviral NS5
4.1. RdRp Inhibitors
4.1.1. Nucleoside Inhibitors
4.1.2. Non-Nucleoside Inhibitors
4.2. Mtase Inhibitors
4.3. Nucleoside Synthesis Inhibitors
5. Inhibitors Targeting Host Cell Lipid Metabolism
6. Other Antiflaviviral Agents
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kobayashi, N. Impact of Emerging, Re-Emerging and Zoonotic Viral Infectious Diseases, in a Virologist’s Perspective. Open Virol. J. 2018, 12, 131–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, C.J.; Cortese, M.; Acosta, E.G.; Bartenschlager, R. Rewiring cellular networks by members of the Flaviviridae family. Nat. Rev. Microbiol. 2018, 16, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Carro, S.D.; Cherry, S. Beyond the Surface: Endocytosis of Mosquito-Borne Flaviviruses. Viruses 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. Molecular biology of flaviviruses. Adv. Virus Res. 2003, 59, 23–61. [Google Scholar] [PubMed]
- Morita, E.; Suzuki, Y. Membrane-Associated Flavivirus Replication Complex-Its Organization and Regulation. Viruses 2021, 13, 1060. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Rossmann, M.G. Molecular mechanisms involved in the early steps of flavivirus cell entry. Microbes Infect. 2011, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef]
- da Silva-Junior, E.F.; de Araujo-Junior, J.X. Peptide derivatives as inhibitors of NS2B-NS3 protease from Dengue, West Nile, and Zika flaviviruses. Bioorg. Med. Chem. 2019, 27, 3963–3978. [Google Scholar] [CrossRef]
- Luo, D.; Vasudevan, S.G.; Lescar, J. The flavivirus NS2B-NS3 protease-helicase as a target for antiviral drug development. Antivir. Res. 2015, 118, 148–158. [Google Scholar] [CrossRef]
- Nitsche, C.; Schreier, V.N.; Behnam, M.A.; Kumar, A.; Bartenschlager, R.; Klein, C.D. Thiazolidinone-peptide hybrids as dengue virus protease inhibitors with antiviral activity in cell culture. J. Med. Chem. 2013, 56, 8389–8403. [Google Scholar] [CrossRef] [PubMed]
- Hammamy, M.Z.; Haase, C.; Hammami, M.; Hilgenfeld, R.; Steinmetzer, T. Development and characterization of new peptidomimetic inhibitors of the West Nile virus NS2B-NS3 protease. ChemMedChem 2013, 8, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitsche, C.; Behnam, M.A.; Steuer, C.; Klein, C.D. Retro peptide-hybrids as selective inhibitors of the Dengue virus NS2B-NS3 protease. Antivir. Res. 2012, 94, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.A.; Joy, J.; Hill, J.; San Brian Chia, C. Novel agmatine and agmatine-like peptidomimetic inhibitors of the West Nile virus NS2B/NS3 serine protease. Eur. J. Med. Chem. 2011, 46, 3130–3134. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Shao, X.; Graf, D.; Wang, C.; Klein, C.D.; Wang, J.; Zhou, G.C. Identification of fused bicyclic derivatives of pyrrolidine and imidazolidinone as dengue virus-2 NS2B-NS3 protease inhibitors. Eur. J. Med. Chem. 2017, 125, 751–759. [Google Scholar] [CrossRef]
- Prusis, P.; Junaid, M.; Petrovska, R.; Yahorava, S.; Yahorau, A.; Katzenmeier, G.; Lapins, M.; Wikberg, J.E. Design and evaluation of substrate-based octapeptide and non substrate-based tetrapeptide inhibitors of dengue virus NS2B-NS3 proteases. Biochem. Biophys. Res. Commun. 2013, 434, 767–772. [Google Scholar] [CrossRef]
- Takagi, Y.; Matsui, K.; Nobori, H.; Maeda, H.; Sato, A.; Kurosu, T.; Orba, Y.; Sawa, H.; Hattori, K.; Higashino, K.; et al. Discovery of novel cyclic peptide inhibitors of dengue virus NS2B-NS3 protease with antiviral activity. Bioorg. Med. Chem. Lett. 2017, 27, 3586–3590. [Google Scholar] [CrossRef]
- Nitsche, C.; Passioura, T.; Varava, P.; Mahawaththa, M.C.; Leuthold, M.M.; Klein, C.D.; Suga, H.; Otting, G. De Novo Discovery of Nonstandard Macrocyclic Peptides as Noncompetitive Inhibitors of the Zika Virus NS2B-NS3 Protease. ACS Med. Chem. Lett. 2019, 10, 168–174. [Google Scholar] [CrossRef]
- Pinkham, A.M.; Yu, Z.; Cowan, J.A. Broad-spectrum catalytic metallopeptide inactivators of Zika and West Nile virus NS2B/NS3 proteases. Chem. Commun. 2018, 54, 12357–12360. [Google Scholar] [CrossRef]
- Behnam, M.A.; Graf, D.; Bartenschlager, R.; Zlotos, D.P.; Klein, C.D. Discovery of Nanomolar Dengue and West Nile Virus Protease Inhibitors Containing a 4-Benzyloxyphenylglycine Residue. J. Med. Chem. 2015, 58, 9354–9370. [Google Scholar] [CrossRef]
- Nitsche, C.; Zhang, L.; Weigel, L.F.; Schilz, J.; Graf, D.; Bartenschlager, R.; Hilgenfeld, R.; Klein, C.D. Peptide-Boronic Acid Inhibitors of Flaviviral Proteases: Medicinal Chemistry and Structural Biology. J. Med. Chem. 2017, 60, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Weigel, L.F.; Nitsche, C.; Graf, D.; Bartenschlager, R.; Klein, C.D. Phenylalanine and Phenylglycine Analogues as Arginine Mimetics in Dengue Protease Inhibitors. J. Med. Chem. 2015, 58, 7719–7733. [Google Scholar] [CrossRef] [PubMed]
- Stoermer, M.J.; Chappell, K.J.; Liebscher, S.; Jensen, C.M.; Gan, C.H.; Gupta, P.K.; Xu, W.J.; Young, P.R.; Fairlie, D.P. Potent cationic inhibitors of West Nile virus NS2B/NS3 protease with serum stability, cell permeability and antiviral activity. J. Med. Chem. 2008, 51, 5714–5721. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.E.; Ma, N.L.; Yin, Z.; Patel, S.J.; Wang, W.L.; Chan, W.L.; Ranga Rao, K.R.; Wang, G.; Ngew, X.; Patel, V.; et al. Peptide inhibitors of West Nile NS3 protease: SAR study of tetrapeptide aldehyde inhibitors. J. Med. Chem. 2006, 49, 6585–6590. [Google Scholar] [CrossRef] [PubMed]
- Schuller, A.; Yin, Z.; Brian Chia, C.S.; Doan, D.N.; Kim, H.K.; Shang, L.; Loh, T.P.; Hill, J.; Vasudevan, S.G. Tripeptide inhibitors of dengue and West Nile virus NS2B-NS3 protease. Antivir. Res. 2011, 92, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Phoo, W.W.; Loh, Y.R.; Wang, W.; Liu, S.; Chen, M.W.; Hung, A.W.; Keller, T.H.; Luo, D.; et al. Structural Dynamics of Zika Virus NS2B-NS3 Protease Binding to Dipeptide Inhibitors. Structure 2017, 25, 1242–1250 e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Huo, T.; Lin, Y.L.; Nie, S.; Wu, F.; Hua, Y.; Wu, J.; Kneubehl, A.R.; Vogt, M.B.; Rico-Hesse, R.; et al. X-ray Crystallography and Antiviral Activity of Allosteric Inhibitors of Flavivirus NS2B-NS3 Protease. J. Am. Chem. Soc. 2019, 141, 6832–6836. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, S.M.; Watowich, S.J. Use of parallel validation high-throughput screens to reduce false positives and identify novel dengue NS2B-NS3 protease inhibitors. Antivir. Res. 2012, 93, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yang, K.; Wu, C.; Chen, C.; Hu, C.; Buzovetsky, O.; Wang, Z.; Ji, X.; Xiong, Y.; Yang, H. Mechanisms of activation and inhibition of Zika virus NS2B-NS3 protease. Cell Res. 2016, 26, 1260–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, D.; Schroder, K.; White, H.; Fang, N.X.; Stoermer, M.J.; Abbenante, G.; Martin, J.L.; Young, P.R.; Fairlie, D.P. Activity of recombinant dengue 2 virus NS3 protease in the presence of a truncated NS2B co-factor, small peptide substrates, and inhibitors. J. Biol. Chem. 2001, 276, 45762–45771. [Google Scholar] [CrossRef] [Green Version]
- Yao, N.; Hesson, T.; Cable, M.; Hong, Z.; Kwong, A.D.; Le, H.V.; Weber, P.C. Structure of the hepatitis C virus RNA helicase domain. Nat. Struct. Biol. 1997, 4, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Basavannacharya, C.; Vasudevan, S.G. Suramin inhibits helicase activity of NS3 protein of dengue virus in a fluorescence-based high throughput assay format. Biochem. Biophys. Res. Commun. 2014, 453, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, N.L.; Hanson, A.M.; Mukherjee, S.; Ndjomou, J.; Geiss, B.J.; Steel, J.J.; Frankowski, K.J.; Li, K.; Schoenen, F.J.; Frick, D.N. Benzothiazole and Pyrrolone Flavivirus Inhibitors Targeting the Viral Helicase. ACS Infect. Dis. 2015, 1, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Bretner, M.; Schalinski, S.; Borowski, P.; Kulikowski, T. 5′-O-fluorosulfonylbenzoyl esters of purine nucleosides as potential inhibitors of NTPase/helicase and polymerase of Flaviviridae viruses. Nucleosides Nucleotides Nucleic Acids 2003, 22, 1531–1533. [Google Scholar] [CrossRef] [PubMed]
- Bretner, M.; Baier, A.; Kopanska, K.; Najda, A.; Schoof, A.; Reinholz, M.; Lipniacki, A.; Piasek, A.; Kulikowski, T.; Borowski, P. Synthesis and biological activity of 1H-benzotriazole and 1H-benzimidazole analogues--inhibitors of the NTpase/helicase of HCV and of some related Flaviviridae. Antivir. Chem. Chemother. 2005, 16, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Bretner, M.; Schalinski, S.; Haag, A.; Lang, M.; Schmitz, H.; Baier, A.; Behrens, S.E.; Kulikowski, T.; Borowski, P. Synthesis and evaluation of ATP-binding site directed potential inhibitors of nucleoside triphosphatases/helicases and polymerases of hepatitis C and other selected Flaviviridae viruses. Antivir. Chem. Chemother. 2004, 15, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Apte-Sengupta, S.; Sirohi, D.; Kuhn, R.J. Coupling of replication and assembly in flaviviruses. Curr. Opin. Virol. 2014, 9, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Wang, Q.Y.; Xu, H.Y.; Qing, M.; Kramer, L.; Yuan, Z.; Shi, P.Y. Inhibition of dengue virus by targeting viral NS4B protein. J. Virol. 2011, 85, 11183–11195. [Google Scholar] [CrossRef] [Green Version]
- Patkar, C.G.; Larsen, M.; Owston, M.; Smith, J.L.; Kuhn, R.J. Identification of inhibitors of yellow fever virus replication using a replicon-based high-throughput assay. Antimicrob. Agents Chemother. 2009, 53, 4103–4114. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.P.; Wang, Q.Y.; Noble, C.G.; Chen, Y.L.; Dong, H.; Zou, B.; Yokokawa, F.; Nilar, S.; Smith, P.; Beer, D.; et al. Ten years of dengue drug discovery: Progress and prospects. Antivir. Res. 2013, 100, 500–519. [Google Scholar] [CrossRef]
- Zou, G.; Puig-Basagoiti, F.; Zhang, B.; Qing, M.; Chen, L.; Pankiewicz, K.W.; Felczak, K.; Yuan, Z.; Shi, P.Y. A single-amino acid substitution in West Nile virus 2K peptide between NS4A and NS4B confers resistance to lycorine, a flavivirus inhibitor. Virology 2009, 384, 242–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Liu, W.; Gong, P. A Structural Overview of RNA-Dependent RNA Polymerases from the Flaviviridae Family. Int. J. Mol. Sci. 2015, 16, 12943–12957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boldescu, V.; Behnam, M.A.M.; Vasilakis, N.; Klein, C.D. Broad-spectrum agents for flaviviral infections: Dengue, Zika and beyond. Nat. Rev. Drug Discov. 2017, 16, 565–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deval, J.; Symons, J.A.; Beigelman, L. Inhibition of viral RNA polymerases by nucleoside and nucleotide analogs: Therapeutic applications against positive-strand RNA viruses beyond hepatitis C virus. Curr. Opin. Virol. 2014, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Neyts, J. Antiviral agents acting as DNA or RNA chain terminators. Handb. Exp. Pharmacol. 2009, 189, 53–84. [Google Scholar]
- Poveda, E.; Wyles, D.L.; Mena, A.; Pedreira, J.D.; Castro-Iglesias, A.; Cachay, E. Update on hepatitis C virus resistance to direct-acting antiviral agents. Antivir. Res. 2014, 108, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Eyer, L.; Fojtikova, M.; Nencka, R.; Rudolf, I.; Hubalek, Z.; Ruzek, D. Viral RNA-Dependent RNA Polymerase Inhibitor 7-Deaza-2′-C-Methyladenosine Prevents Death in a Mouse Model of West Nile Virus Infection. Antimicrob. Agents Chemother. 2019, 63, e02093-18. [Google Scholar] [CrossRef] [Green Version]
- Cho, A.; Saunders, O.L.; Butler, T.; Zhang, L.; Xu, J.; Vela, J.E.; Feng, J.Y.; Ray, A.S.; Kim, C.U. Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg. Med. Chem. Lett. 2012, 22, 2705–2707. [Google Scholar] [CrossRef]
- Lee, J.C.; Tseng, C.K.; Wu, Y.H.; Kaushik-Basu, N.; Lin, C.K.; Chen, W.C.; Wu, H.N. Characterization of the activity of 2′-C-methylcytidine against dengue virus replication. Antivir. Res. 2015, 116, 1–9. [Google Scholar] [CrossRef]
- Eyer, L.; Nencka, R.; de Clercq, E.; Seley-Radtke, K.; Ruzek, D. Nucleoside analogs as a rich source of antiviral agents active against arthropod-borne flaviviruses. Antivir. Chem. Chemother. 2018, 26, 2040206618761299. [Google Scholar] [CrossRef]
- Yeo, K.L.; Chen, Y.L.; Xu, H.Y.; Dong, H.; Wang, Q.Y.; Yokokawa, F.; Shi, P.Y. Synergistic suppression of dengue virus replication using a combination of nucleoside analogs and nucleoside synthesis inhibitors. Antimicrob. Agents Chemother. 2015, 59, 2086–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuigan, C.; Serpi, M.; Slusarczyk, M.; Ferrari, V.; Pertusati, F.; Meneghesso, S.; Derudas, M.; Farleigh, L.; Zanetta, P.; Bugert, J. Anti-flavivirus Activity of Different Tritylated Pyrimidine and Purine Nucleoside Analogues. ChemistryOpen 2016, 5, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanko, K.; Eggermont, K.; Patel, A.; Kaptein, S.; Delang, L.; Verfaillie, C.M.; Neyts, J. Replication of the Zika virus in different iPSC-derived neuronal cells and implications to assess efficacy of antivirals. Antivir. Res. 2017, 145, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Yokokawa, F.; Shi, P.Y. The search for nucleoside/nucleotide analog inhibitors of dengue virus. Antivir. Res. 2015, 122, 12–19. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yin, Z.; Duraiswamy, J.; Schul, W.; Lim, C.C.; Liu, B.; Xu, H.Y.; Qing, M.; Yip, A.; Wang, G.; et al. Inhibition of dengue virus RNA synthesis by an adenosine nucleoside. Antimicrob. Agents Chemother. 2010, 54, 2932–2939. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Chen, Y.L.; Schul, W.; Wang, Q.Y.; Gu, F.; Duraiswamy, J.; Kondreddi, R.R.; Niyomrattanakit, P.; Lakshminarayana, S.B.; Goh, A.; et al. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. USA 2009, 106, 20435–20439. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.Q.; Zhang, N.N.; Li, C.F.; Tian, M.; Hao, J.N.; Xie, X.P.; Shi, P.Y.; Qin, C.F. Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus. Open Forum Infect. Dis. 2016, 3, ofw175. [Google Scholar] [CrossRef]
- Warren, T.K.; Wells, J.; Panchal, R.G.; Stuthman, K.S.; Garza, N.L.; Van Tongeren, S.A.; Dong, L.; Retterer, C.J.; Eaton, B.P.; Pegoraro, G.; et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 2014, 508, 402–405. [Google Scholar] [CrossRef] [Green Version]
- Eyer, L.; Zouharova, D.; Sirmarova, J.; Fojtikova, M.; Stefanik, M.; Haviernik, J.; Nencka, R.; de Clercq, E.; Ruzek, D. Antiviral activity of the adenosine analogue BCX4430 against West Nile virus and tick-borne flaviviruses. Antivir. Res. 2017, 142, 63–67. [Google Scholar] [CrossRef]
- Julander, J.G.; Siddharthan, V.; Evans, J.; Taylor, R.; Tolbert, K.; Apuli, C.; Stewart, J.; Collins, P.; Gebre, M.; Neilson, S.; et al. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antivir. Res. 2017, 137, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Julander, J.G.; Bantia, S.; Taubenheim, B.R.; Minning, D.M.; Kotian, P.; Morrey, J.D.; Smee, D.F.; Sheridan, W.P.; Babu, Y.S. BCX4430, a novel nucleoside analog, effectively treats yellow fever in a Hamster model. Antimicrob. Agents Chemother. 2014, 58, 6607–6614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julander, J.G.; Shafer, K.; Smee, D.F.; Morrey, J.D.; Furuta, Y. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob. Agents Chemother. 2009, 53, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuta, Y.; Takahashi, K.; Shiraki, K.; Sakamoto, K.; Smee, D.F.; Barnard, D.L.; Gowen, B.B.; Julander, J.G.; Morrey, J.D. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antivir. Res. 2009, 82, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Matz, K.; Emanuel, J.; Callison, J.; Gardner, D.; Rosenke, R.; Mercado-Hernandez, R.; Williamson, B.N.; Feldmann, H.; Marzi, A. Favipiravir (T-705) Protects IFNAR(-/-) Mice against Lethal Zika Virus Infection in a Sex-Dependent Manner. Microorganisms 2021, 9, 1178. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M.; Vaidya, A. Sofosbuvir: First global approval. Drugs 2014, 74, 273–282. [Google Scholar] [CrossRef]
- de Freitas, C.S.; Higa, L.M.; Sacramento, C.Q.; Ferreira, A.C.; Reis, P.A.; Delvecchio, R.; Monteiro, F.L.; Barbosa-Lima, G.; James Westgarth, H.; Vieira, Y.R.; et al. Yellow fever virus is susceptible to sofosbuvir both in vitro and in vivo. PLoS Negl. Trop. Dis. 2019, 13, e0007072. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.T.; Colby-Germinario, S.P.; Hassounah, S.A.; Fogarty, C.; Osman, N.; Palanisamy, N.; Han, Y.; Oliveira, M.; Quan, Y.; Wainberg, M.A. Evaluation of Sofosbuvir (beta-D-2′-deoxy-2′-alpha-fluoro-2′-beta-C-methyluridine) as an inhibitor of Dengue virus replication. Sci. Rep. 2017, 7, 6345. [Google Scholar] [CrossRef]
- Bullard-Feibelman, K.M.; Govero, J.; Zhu, Z.; Salazar, V.; Veselinovic, M.; Diamond, M.S.; Geiss, B.J. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antivir. Res. 2017, 137, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.C.; Zaverucha-do-Valle, C.; Reis, P.A.; Barbosa-Lima, G.; Vieira, Y.R.; Mattos, M.; Silva, P.P.; Sacramento, C.; de Castro Faria Neto, H.C.; Campanati, L.; et al. Sofosbuvir protects Zika virus-infected mice from mortality, preventing short- and long-term sequelae. Sci. Rep. 2017, 7, 9409. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.M.; Tran, C.N.; Phung, L.K.; Duong, K.T.; Huynh Hle, A.; Farrar, J.; Nguyen, Q.T.; Tran, H.T.; Nguyen, C.V.; Merson, L.; et al. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J. Infect. Dis. 2013, 207, 1442–1450. [Google Scholar] [CrossRef]
- Obi, J.O.; Gutierrez-Barbosa, H.; Chua, J.V.; Deredge, D.J. Current Trends and Limitations in Dengue Antiviral Research. Trop. Med. Infect. Dis. 2021, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, D.; Cannalire, R.; Mastrangelo, E.; Croci, R.; Querat, G.; Barreca, M.L.; Bolognesi, M.; Manfroni, G.; Cecchetti, V.; Milani, M. Targeting flavivirus RNA dependent RNA polymerase through a pyridobenzothiazole inhibitor. Antivir. Res. 2016, 134, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, C.G.; Lim, S.P.; Chen, Y.L.; Liew, C.W.; Yap, L.; Lescar, J.; Shi, P.Y. Conformational flexibility of the Dengue virus RNA-dependent RNA polymerase revealed by a complex with an inhibitor. J. Virol. 2013, 87, 5291–5295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.P.; Noble, C.G.; Seh, C.C.; Soh, T.S.; El Sahili, A.; Chan, G.K.; Lescar, J.; Arora, R.; Benson, T.; Nilar, S.; et al. Potent Allosteric Dengue Virus NS5 Polymerase Inhibitors: Mechanism of Action and Resistance Profiling. PLoS Pathog. 2016, 12, e1005737. [Google Scholar] [CrossRef] [Green Version]
- Noble, C.G.; Lim, S.P.; Arora, R.; Yokokawa, F.; Nilar, S.; Seh, C.C.; Wright, S.K.; Benson, T.E.; Smith, P.W.; Shi, P.Y. A Conserved Pocket in the Dengue Virus Polymerase Identified through Fragment-based Screening. J. Biol. Chem. 2016, 291, 8541–8548. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.P.; Noble, C.G.; Nilar, S.; Shi, P.Y.; Yokokawa, F. Discovery of Potent Non-nucleoside Inhibitors of Dengue Viral RNA-Dependent RNA Polymerase from Fragment Screening and Structure-Guided Design. Dengue Zika Control. Antivir. Treat. Strateg. 2018, 1062, 187–198. [Google Scholar]
- Qian, X.; Wu, B.; Tang, H.; Luo, Z.; Xu, Z.; Ouyang, S.; Li, X.; Xie, J.; Yi, Z.; Leng, Q.; et al. Rifapentine is an entry and replication inhibitor against yellow fever virus both in vitro and in vivo. Emerg. Microbes Infect. 2022, 11, 873–884. [Google Scholar] [CrossRef]
- Lim, S.P.; Noble, C.G.; Shi, P.Y. The dengue virus NS5 protein as a target for drug discovery. Antivir. Res. 2015, 119, 57–67. [Google Scholar] [CrossRef]
- Chen, H.; Liu, L.; Jones, S.A.; Banavali, N.; Kass, J.; Li, Z.; Zhang, J.; Kramer, L.D.; Ghosh, A.K.; Li, H. Selective inhibition of the West Nile virus methyltransferase by nucleoside analogs. Antivir. Res. 2013, 97, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Vernekar, S.K.; Qiu, L.; Zhang, J.; Kankanala, J.; Li, H.; Geraghty, R.J.; Wang, Z. 5′-Silylated 3′-1,2,3-triazolyl Thymidine Analogues as Inhibitors of West Nile Virus and Dengue Virus. J. Med. Chem. 2015, 58, 4016–4028. [Google Scholar] [CrossRef] [Green Version]
- Seley, K.L.; Zhang, L.; Hagos, A.; Quirk, S. “Fleximers”. Design and synthesis of a new class of novel shape-modified nucleosides (1). J. Org. Chem. 2002, 67, 3365–3373. [Google Scholar] [CrossRef]
- Yates, M.K.; Chatterjee, P.; Flint, M.; Arefeayne, Y.; Makuc, D.; Plavec, J.; Spiropoulou, C.F.; Seley-Radtke, K.L. Probing the Effects of Pyrimidine Functional Group Switches on Acyclic Fleximer Analogues for Antiviral Activity. Molecules 2019, 24, 3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thames, J.E.; Waters, C.D., 3rd; Valle, C.; Bassetto, M.; Aouadi, W.; Martin, B.; Selisko, B.; Falat, A.; Coutard, B.; Brancale, A.; et al. Synthesis and biological evaluation of novel flexible nucleoside analogues that inhibit flavivirus replication in vitro. Bioorg. Med. Chem. 2020, 28, 115713. [Google Scholar] [CrossRef] [PubMed]
- Leyssen, P.; Balzarini, J.; De Clercq, E.; Neyts, J. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J. Virol. 2005, 79, 1943–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Guillen, J.; Rabah, N.; Blanjoie, A.; Debart, F.; Vasseur, J.J.; Canard, B.; Decroly, E.; Coutard, B. mRNA Capping by Venezuelan Equine Encephalitis Virus nsP1: Functional Characterization and Implications for Antiviral Research. J. Virol. 2015, 89, 8292–8303. [Google Scholar] [CrossRef] [Green Version]
- McDowell, M.; Gonzales, S.R.; Kumarapperuma, S.C.; Jeselnik, M.; Arterburn, J.B.; Hanley, K.A. A novel nucleoside analog, 1-beta-d-ribofuranosyl-3-ethynyl-[1,2,4]triazole (ETAR), exhibits efficacy against a broad range of flaviviruses in vitro. Antivir. Res. 2010, 87, 78–80. [Google Scholar] [CrossRef] [Green Version]
- Crance, J.M.; Scaramozzino, N.; Jouan, A.; Garin, D. Interferon, ribavirin, 6-azauridine and glycyrrhizin: Antiviral compounds active against pathogenic flaviviruses. Antivir. Res. 2003, 58, 73–79. [Google Scholar] [CrossRef]
- Morrey, J.D.; Smee, D.F.; Sidwell, R.W.; Tseng, C. Identification of active antiviral compounds against a New York isolate of West Nile virus. Antivir. Res. 2002, 55, 107–116. [Google Scholar] [CrossRef]
- Adcock, R.S.; Chu, Y.K.; Golden, J.E.; Chung, D.H. Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay. Antivir. Res. 2017, 138, 47–56. [Google Scholar] [CrossRef]
- Dukhan, D.; Leroy, F.; Peyronnet, J.; Bosc, E.; Chaves, D.; Durka, M.; Storer, R.; La Colla, P.; Seela, F.; Gosselin, G. Synthesis of 5-aza-7-deazaguanine nucleoside derivatives as potential anti-flavivirus agents. Nucleosides Nucleotides Nucleic Acids 2005, 24, 671–674. [Google Scholar] [CrossRef]
- Martin-Acebes, M.A.; Vazquez-Calvo, A.; Saiz, J.C. Lipids and flaviviruses, present and future perspectives for the control of dengue, Zika, and West Nile viruses. Prog. Lipid Res. 2016, 64, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Heaton, N.S.; Randall, G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 2010, 8, 422–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, R.; Riley, C.; Isaac, G.; Hopf-Jannasch, A.S.; Moore, R.J.; Weitz, K.W.; Pasa-Tolic, L.; Metz, T.O.; Adamec, J.; Kuhn, R.J. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 2012, 8, e1002584. [Google Scholar] [CrossRef] [PubMed]
- Samsa, M.M.; Mondotte, J.A.; Iglesias, N.G.; Assuncao-Miranda, I.; Barbosa-Lima, G.; Da Poian, A.T.; Bozza, P.T.; Gamarnik, A.V. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 2009, 5, e1000632. [Google Scholar] [CrossRef]
- Martin-Acebes, M.A.; Jimenez de Oya, N.; Saiz, J.C. Lipid Metabolism as a Source of Druggable Targets for Antiviral Discovery against Zika and Other Flaviviruses. Pharmaceuticals 2019, 12, 97. [Google Scholar] [CrossRef] [Green Version]
- Jimenez de Oya, N.; Blazquez, A.B.; Casas, J.; Saiz, J.C.; Martin-Acebes, M.A. Direct Activation of Adenosine Monophosphate-Activated Protein Kinase (AMPK) by PF-06409577 Inhibits Flavivirus Infection through Modification of Host Cell Lipid Metabolism. Antimicrob. Agents. Chemother. 2018, 62, e00360-18. [Google Scholar] [CrossRef] [Green Version]
- Soto-Acosta, R.; Bautista-Carbajal, P.; Cervantes-Salazar, M.; Angel-Ambrocio, A.H.; Del Angel, R.M. DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: A potential antiviral target. PLoS Pathog. 2017, 13, e1006257. [Google Scholar] [CrossRef]
- Farfan-Morales, C.N.; Cordero-Rivera, C.D.; Osuna-Ramos, J.F.; Monroy-Muñoz, I.E.; de Jesús-González, L.A.; Muñoz-Medina, J.E.; Hurtado-Monzón, A.M.; Reyes-Ruiz, J.M.; Del Ángel, R.M. The antiviral effect of metformin on zika and dengue virus infection. Sci. Rep. 2021, 11, 8743. [Google Scholar] [CrossRef]
- Htun, H.L.; Yeo, T.W.; Tam, C.C.; Pang, J.; Leo, Y.S.; Lye, D.C. Metformin use and severe dengue in diabetic adults. Sci. Rep. 2018, 8, 3344. [Google Scholar] [CrossRef]
- Jimenez de Oya, N.; Esler, W.P.; Huard, K.; El-Kattan, A.F.; Karamanlidis, G.; Blazquez, A.B.; Ramos-Ibeas, P.; Escribano-Romero, E.; Louloudes-Lazaro, A.; Casas, J.; et al. Targeting host metabolism by inhibition of acetyl-Coenzyme A carboxylase reduces flavivirus infection in mouse models. Emerg. Microbes Infect. 2019, 8, 624–636. [Google Scholar] [CrossRef] [Green Version]
- Hitakarun, A.; Khongwichit, S.; Wikan, N.; Roytrakul, S.; Yoksan, S.; Rajakam, S.; Davidson, A.D.; Smith, D.R. Evaluation of the antiviral activity of orlistat (tetrahydrolipstatin) against dengue virus, Japanese encephalitis virus, Zika virus and chikungunya virus. Sci. Rep. 2020, 10, 1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Acebes, M.A.; Merino-Ramos, T.; Blazquez, A.B.; Casas, J.; Escribano-Romero, E.; Sobrino, F.; Saiz, J.C. The composition of West Nile virus lipid envelope unveils a role of sphingolipid metabolism in flavivirus biogenesis. J. Virol. 2014, 88, 12041–12054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Li, Y.; Zhang, H.; Zhao, R.; Jing, R.; Xu, Y.; He, M.; Peer, J.; Kim, Y.C.; Luo, J.; et al. Zika virus propagation and release in human fetal astrocytes can be suppressed by neutral sphingomyelinase-2 inhibitor GW4869. Cell Discov. 2018, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Farfan-Morales, C.N.; Cordero-Rivera, C.D.; Reyes-Ruiz, J.M.; Hurtado-Monzon, A.M.; Osuna-Ramos, J.F.; Gonzalez-Gonzalez, A.M.; De Jesus-Gonzalez, L.A.; Palacios-Rapalo, S.N.; Del Angel, R.M. Anti-flavivirus Properties of Lipid-Lowering Drugs. Front. Physiol. 2021, 12, 749770. [Google Scholar] [CrossRef]
- Fedson, D.S.; Rordam, O.M. Treating Ebola patients: A ‘bottom up’ approach using generic statins and angiotensin receptor blockers. Int. J. Infect. Dis. 2015, 36, 80–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Gutierrez, M.; Correa-Londono, L.A.; Castellanos, J.E.; Gallego-Gomez, J.C.; Osorio, J.E. Lovastatin delays infection and increases survival rates in AG129 mice infected with dengue virus serotype 2. PLoS ONE 2014, 9, e87412. [Google Scholar] [CrossRef]
- Whitehorn, J.; Van Vinh Chau, N.; Truong, N.T.; Tai, L.T.; Van Hao, N.; Hien, T.T.; Wolbers, M.; Merson, L.; Dung, N.T.; Peeling, R.; et al. Lovastatin for adult patients with dengue: Protocol for a randomised controlled trial. Trials 2012, 13, 203. [Google Scholar] [CrossRef] [Green Version]
- Osuna-Ramos, J.F.; Reyes-Ruiz, J.M.; Bautista-Carbajal, P.; Cervantes-Salazar, M.; Farfan-Morales, C.N.; De Jesus-Gonzalez, L.A.; Hurtado-Monzon, A.M.; Del Angel, R.M. Ezetimibe inhibits dengue virus infection in Huh-7 cells by blocking the cholesterol transporter Niemann-Pick C1-like 1 receptor. Antivir. Res. 2018, 160, 151–164. [Google Scholar] [CrossRef]
- Zhou, Z.; Khaliq, M.; Suk, J.E.; Patkar, C.; Li, L.; Kuhn, R.J.; Post, C.B. Antiviral compounds discovered by virtual screening of small-molecule libraries against dengue virus E protein. ACS Chem. Biol. 2008, 3, 765–775. [Google Scholar] [CrossRef]
- Gao, Y.; Tai, W.; Wang, N.; Li, X.; Jiang, S.; Debnath, A.K.; Du, L.; Chen, S. Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors. Viruses 2019, 11, 1019. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Deng, Y.Q.; Zou, P.; Wang, Q.; Dai, Y.; Yu, F.; Du, L.; Zhang, N.N.; Tian, M.; Hao, J.N.; et al. A peptide-based viral inactivator inhibits Zika virus infection in pregnant mice and fetuses. Nat. Commun. 2017, 8, 15672. [Google Scholar] [CrossRef] [PubMed]
- Byrd, C.M.; Dai, D.; Grosenbach, D.W.; Berhanu, A.; Jones, K.F.; Cardwell, K.B.; Schneider, C.; Wineinger, K.A.; Page, J.M.; Harver, C.; et al. A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob. Agents Chemother. 2013, 57, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, M.J.; Burtseva, E.I.; Ellery, P.J.; Marsh, G.A.; Lew, A.M.; Slepushkin, A.N.; Crowe, S.M.; Tannock, G.A. Antiviral activity of arbidol, a broad-spectrum drug for use against respiratory viruses, varies according to test conditions. J. Med. Virol. 2012, 84, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Haviernik, J.; Stefanik, M.; Fojtikova, M.; Kali, S.; Tordo, N.; Rudolf, I.; Hubalek, Z.; Eyer, L.; Ruzek, D. Arbidol (Umifenovir): A Broad-Spectrum Antiviral Drug That Inhibits Medically Important Arthropod-Borne Flaviviruses. Viruses 2018, 10, 184. [Google Scholar] [CrossRef] [Green Version]
- Beck, S.; Zhu, Z.; Oliveira, M.F.; Smith, D.M.; Rich, J.N.; Bernatchez, J.A.; Siqueira-Neto, J.L. Mechanism of Action of Methotrexate against Zika Virus. Viruses 2019, 11, 338. [Google Scholar] [CrossRef] [Green Version]
- Scroggs, S.L.P.; Andrade, C.C.; Chinnasamy, R.; Azar, S.R.; Schirtzinger, E.E.; Garcia, E.I.; Arterburn, J.B.; Hanley, K.A.; Rossi, S.L. Old Drugs with New Tricks: Efficacy of Fluoroquinolones to Suppress Replication of Flaviviruses. Viruses 2020, 12, 1022. [Google Scholar] [CrossRef]
- Puschnik, A.S.; Marceau, C.D.; Ooi, Y.S.; Majzoub, K.; Rinis, N.; Contessa, J.N.; Carette, J.E. A Small-Molecule Oligosaccharyltransferase Inhibitor with Pan-flaviviral Activity. Cell Rep. 2017, 21, 3032–3039. [Google Scholar] [CrossRef] [Green Version]
- Valencia, H.J.; de Aguiar, M.; Costa, M.A.; Mendonca, D.C.; Reis, E.V.; Arias, N.E.C.; Drumond, B.P.; Bonjardim, C.A. Evaluation of kinase inhibitors as potential therapeutics for flavivirus infections. Arch. Virol. 2021, 166, 1433–1438. [Google Scholar] [CrossRef]
- Eyre, N.S.; Kirby, E.N.; Anfiteatro, D.R.; Bracho, G.; Russo, A.G.; White, P.A.; Aloia, A.L.; Beard, M.R. Identification of Estrogen Receptor Modulators as Inhibitors of Flavivirus Infection. Antimicrob. Agents Chemother. 2020, 64, e00289-20. [Google Scholar] [CrossRef]
- Quintana, V.M.; Selisko, B.; Brunetti, J.E.; Eydoux, C.; Guillemot, J.C.; Canard, B.; Damonte, E.B.; Julander, J.G.; Castilla, V. Antiviral activity of the natural alkaloid anisomycin against dengue and Zika viruses. Antivir. Res. 2020, 176, 104749. [Google Scholar] [CrossRef]
- Carocci, M.; Hinshaw, S.M.; Rodgers, M.A.; Villareal, V.A.; Burri, D.J.; Pilankatta, R.; Maharaj, N.P.; Gack, M.U.; Stavale, E.J.; Warfield, K.L.; et al. The bioactive lipid 4-hydroxyphenyl retinamide inhibits flavivirus replication. Antimicrob. Agents Chemother. 2015, 59, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitts, J.D.; Li, P.C.; de Wispelaere, M.; Yang, P.L. Antiviral activity of N-(4-hydroxyphenyl) retinamide (4-HPR) against Zika virus. Antivir. Res. 2017, 147, 124–130. [Google Scholar] [CrossRef] [PubMed]
| Target | Classification | Representatives of the Compounds | Flaviviruses | Study Stages | References |
|---|---|---|---|---|---|
| NS2B–NS3 protease | Peptidomimetic inhibitors | Agmatine-based peptidomimetics | WNV | Cell culture | [14] |
| Retro-tripeptides | DENV | Cell culture | [13] | ||
| Fused bicyclic peptides of imidazolidinones and pyrrolidines | DENV2 | Cell culture | [15] | ||
| Peptidomimetics consisting of Abz-Arg-Arg-Arg-Arg-X-X-X-X-Tyr(NO2)-NH2 | DENV1, DENV2, DENV3, DENV4 | Cell culture | [16] | ||
| Macrocyclic peptidomimetics | DENV2, ZIKV | Cell culture | [17,18] | ||
| Metallopeptidomimetic compounds | WNV, ZIKV | Cell culture | [19] | ||
| Peptide-based hybrids inhibitors | Peptide hybrids containing thiazolidine or thiophene | WNV, DENV2 | Cell culture | [20] | |
| Dipeptides containing boronic acid or its derivatives | WNV, DENV2, ZIKV | Cell culture | [21] | ||
| Tripeptides composed of Bz-X-Lys-Phg-NH2 or phenacetyl substituents | WNV, DENV3 | Cell culture | [22,23] | ||
| Synthesized tetrapeptide aldehyde compounds | WNV | Cell culture | [24] | ||
| Peptidyl-aldehyde compounds | WNV, DENV2, ZIKV | Cell culture | [25,26] | ||
| Synthetic or natural compounds | Synthesized compounds targeting histone-modifying enzymes | WNV, DENV, ZIKV | Cell culture, animal model | [27] | |
| Ivermectin, tyrothricin and selamectin alexidine | WNV, DENV | Cell culture | [28] | ||
| Aprotinin | DENV, ZIKV | Cell culture | [29,30] | ||
| NTPase and helicase | □ | Suramin | DENV | Cell culture | [32] |
| Benzothiazole and pyrrolone | WNV, DENV | Cell culture | [33] | ||
| FSBI, 1H-benzotriazole and 1H-benzimidazole analogs | WNV, DENV, JEV | Cell culture | [34,35,36] | ||
| NS4 | □ | NITD-618 | DENV | Cell culture | [38] |
| CCG-3394 and CCG-4088 | DENV | Cell culture | [39,40] | ||
| Lycorine | WNV, YFV, DENV | Cell culture | [41] | ||
| NS5-RdRp | Nucleoside inhibitors | R-1479 and RO-9187 | WNV | Cell culture | [47] |
| GS-441524 | YFV, DENV | Cell culture | [48] | ||
| 2′-C-Methylated nucleosides | DENV | Cell culture | [49,50] | ||
| INX-08189 | DENV2 | Cell culture | [51] | ||
| Tritylated pyrimidine nucleosides | YFV, DENV2 | Cell culture | [52] | ||
| 2′-C-Methylated nucleosides | ZIKV | Cell culture | [53] | ||
| 2′-C-Methylcytidine, 7-deaza-2′-C-methyladenosine | WNV, YFV, DENV | Animal model | [47] | ||
| 2′-C-Ethynyl-substituted nucleosides and derivatives | WNV, YFV, DENV, ZIKV | Cell culture, animal model | [54,55,56,57] | ||
| BCX4430 | WNV, YFV, DENV, ZIKV, JEV | Cell culture | [58,59,60,61] | ||
| T-1106 | YFV | Animal model | [62] | ||
| T-705 | WNV, YFV, ZIKV | Cell culture, animal model | [63,64] | ||
| Sofosbuvir | YFV, DENV, ZIKV | Animal model | [66,67,68,69] | ||
| Balapiravir | DENV | Phase I | [70] | ||
| Non-nucleoside inhibitors | Pyridobenzothiazole-based compounds | WNV, DENV | Cell culture | [72,73] | |
| DENV allosteric N-pocket inhibitors | DENV | Cell culture | [74,75] | ||
| Rifapentine | YFV | Animal model | [77] | ||
| NS5-Mtase | □ | SAH, sinefungin, GMP | DENV, ZIKV | Cell culture | [78] |
| GRL-002 and GRL-003 | WNV | Cell culture | [79] | ||
| 5′-Silylated 3′-azidothymidine substituents | WNV, DENV | Cell culture | [80] | ||
| Fleximers | YFV, DENV, ZIKV | Cell culture | [82,83] | ||
| Nucleoside synthesis | □ | Ribavirin | YFV, DENV | Animal model | [62] |
| Modified ribavirin derivatives of ETAR and IM18 | DENV2 | Cell culture | [86] | ||
| 6-Azauridine and its derivatives | WNV, YFV, DENV, ZIKV, JEV | Cell culture | [87,88,89] | ||
| ZX-2401 | YFV | Cell culture | [90] | ||
| Host cell lipid metabolism | Lipid synthesis | NDGA and its derivatives, PF-429242, fatostatin | WNV, DENV, ZIKV | Cell culture | [95] |
| PF-06409577, metformin, AICAR | WNV, DENV, ZIKV | Cell culture, animal model, clinical evaluation | [96,97,98,99] | ||
| PF-05175157 | WNV | Animal model | [100] | ||
| Orlistat | DENV, ZIKV, JEV | Cell culture | [101] | ||
| Sphingolipids | GW4869 | WNV, ZIKV | Cell culture | [102,103] | |
| Cholesterol | Statins | DENV, ZIKV | Cell culture, animal model, clinical evaluation | [104,105,106,107] | |
| Imipramine, benzamil, ezetimibe | DENV, ZIKV | Cell culture | [108] | ||
| Viral structural proteins | Viral E protein | NITD-448 and D02, D04 and D05 | DENV | Cell culture | [40,109] |
| Gossypol | DENV, ZIKV | Cell culture | [110] | ||
| Z2 | YFV, DENV, ZIKV | Cell culture, animal model | [111] | ||
| Viral C protein | ST-148 | DENV | Cell culture | [112] | |
| Licensed drugs | Licensed drugs | Arbidol | WNV, ZIKV | Cell culture | [114] |
| MTX | ZIKV | Cell culture | [115] | ||
| Enoxacin | DENV, ZIKV | Cell culture, animal model | [116] | ||
| Other compounds | Small-molecule inhibitor | NGI-1 | DENV, ZIKV | Cell culture | [117] |
| MEK inhibitor | Trametinib | YFV, DENV, ZIKV | Cell culture | [118] | |
| Estrogen receptor modulators | Raloxifene hydrochloride and quinestrol | WNV, DENV, ZIKV | Cell culture | [119] | |
| Natural compound | Alkaloid anisomycin | DENV, ZIKV | Cell culture, animal model | [120] | |
| Synthetic compound | Fenretinide | WNV, DENV, ZIKV | Cell culture, animal model | [121,122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Qi, Z. Mosquito-Borne Flaviviruses and Current Therapeutic Advances. Viruses 2022, 14, 1226. https://doi.org/10.3390/v14061226
Qian X, Qi Z. Mosquito-Borne Flaviviruses and Current Therapeutic Advances. Viruses. 2022; 14(6):1226. https://doi.org/10.3390/v14061226
Chicago/Turabian StyleQian, Xijing, and Zhongtian Qi. 2022. "Mosquito-Borne Flaviviruses and Current Therapeutic Advances" Viruses 14, no. 6: 1226. https://doi.org/10.3390/v14061226
APA StyleQian, X., & Qi, Z. (2022). Mosquito-Borne Flaviviruses and Current Therapeutic Advances. Viruses, 14(6), 1226. https://doi.org/10.3390/v14061226






