Evolution Characterization and Pathogenicity of a Porcine Reproductive and Respiratory Syndrome Virus Isolate from a Pig Farm in Shandong Province, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Virus Isolation
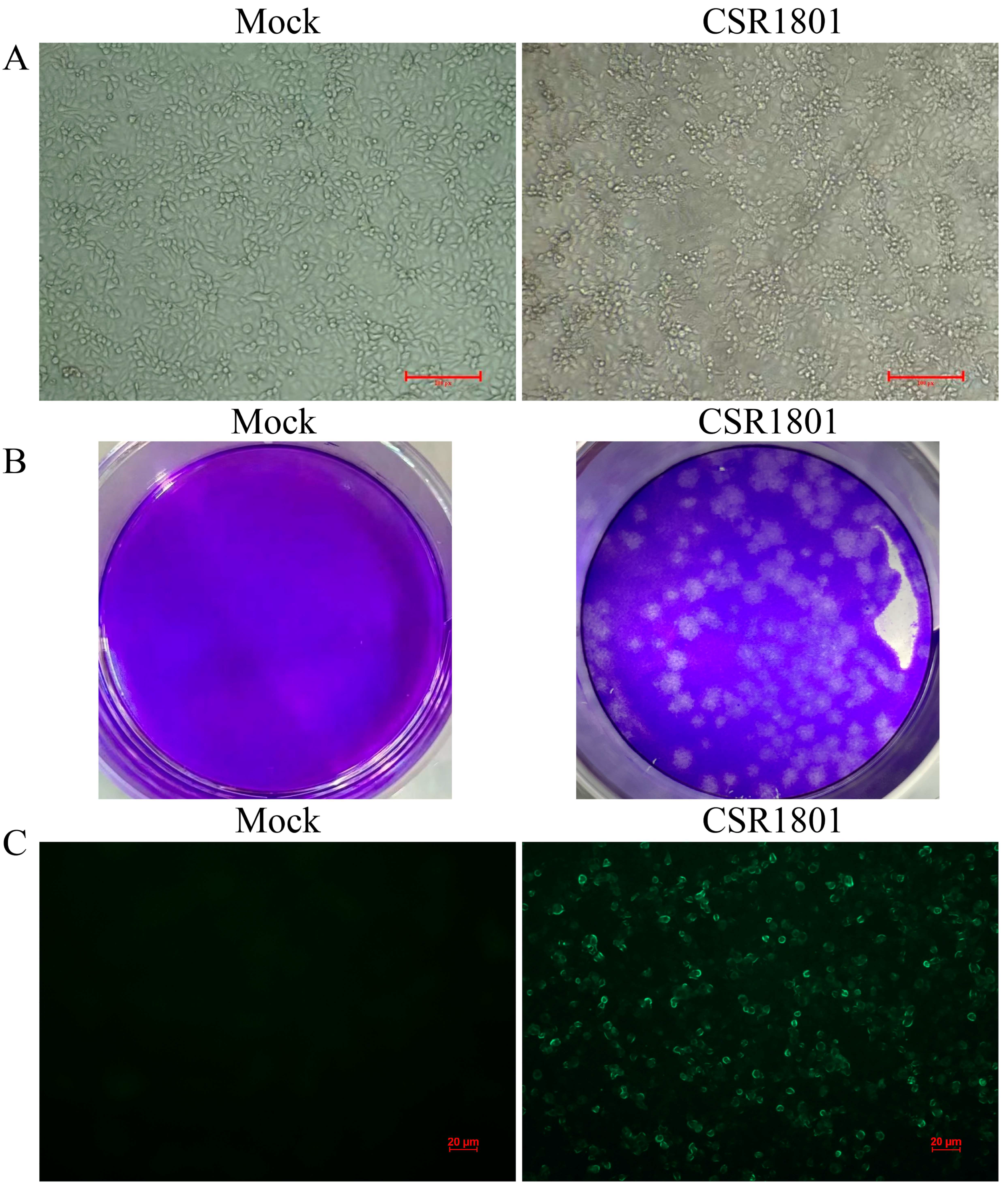
2.2. Plaque Assay
2.3. Immunofluorescence Assay (IFA)
2.4. Viral Genome Extraction and RT-PCR Amplification
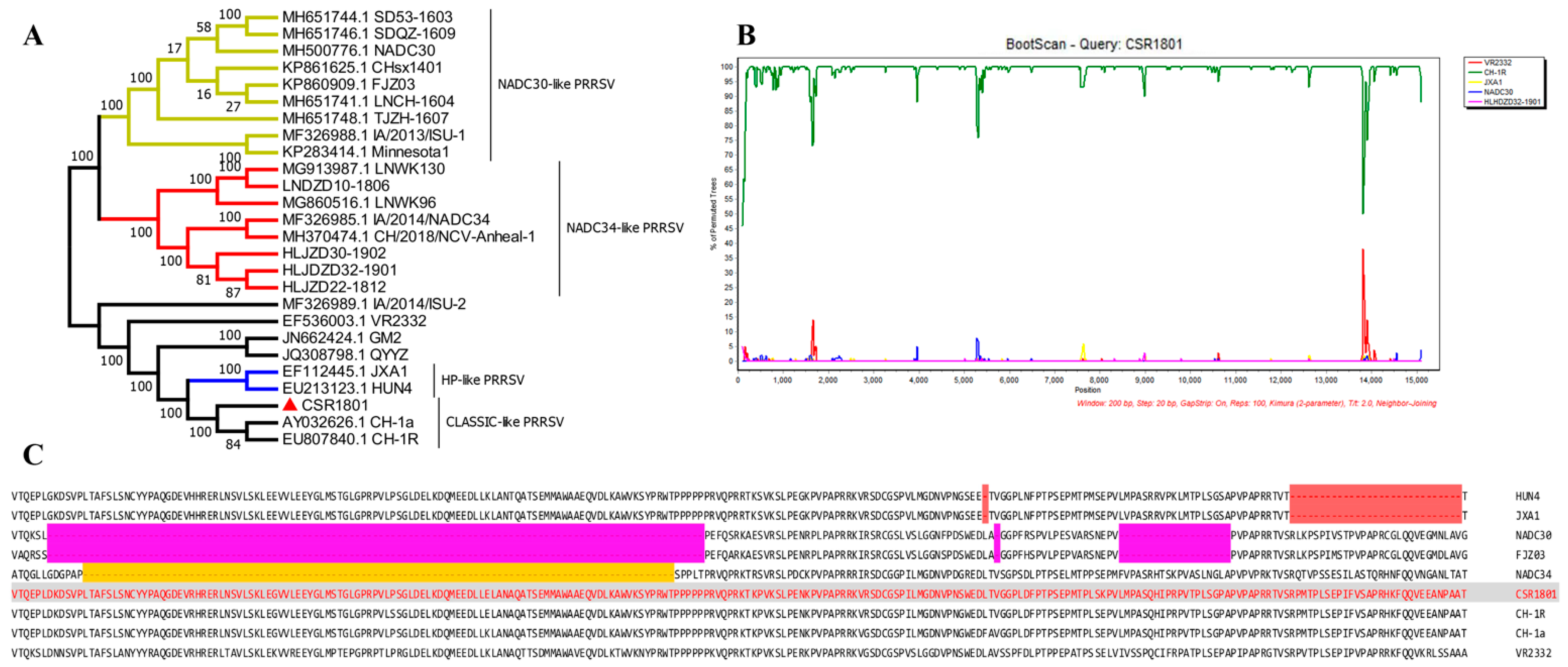
2.5. Phylogenetic and Recombination Analysis
2.6. Animal Experiments
2.7. Real-Time Quantitative PCR (RT-qPCR)
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Histopathological Examination
2.10. Data Analysis
3. Results
3.1. Virus Isolation and Identification
3.2. Analysis of Full-Length Genomic Sequence
3.3. Viral Load in Sera
3.4. Clinical Symptoms and Histopathological Lesion
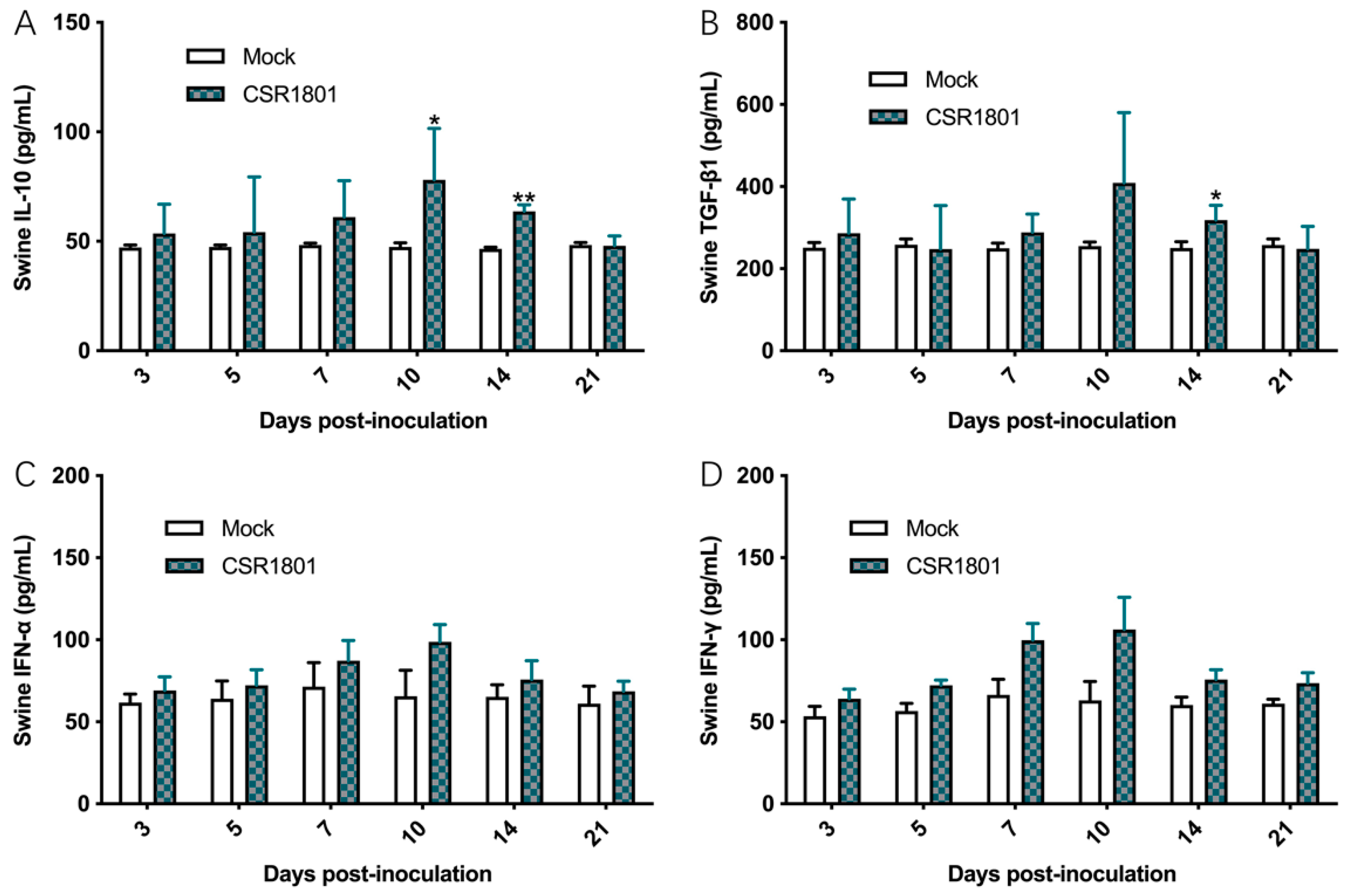
3.5. Concentrations of the Corresponding Cytokines in Sera
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.E.; Zevenhoven-Dobbe, J.C.; Wills, N.M.; Go, Y.Y.; Balasuriya, U.B.R.; Atkins, J.F.; Snijder, E.J.; Posthuma, C.C. Discovery of a small arterivirus gene that overlaps the GP5 coding sequence and is important for virus production. J. Gen. Virol. 2011, 92, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Sun, B.; Mo, J.; Zeng, X.; Zhang, G.; Wang, L.; Zhou, Q.; Zhu, L.; Li, Z.; Xie, Q.; et al. Attenuation and immunogenicity of a live high pathogenic PRRSV vaccine candidate with a 32-amino acid deletion in the nsp2 protein. J. Immunol. Res. 2014, 2014, 810523. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Arch. Virol. 2017, 162, 2505–2538. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Bo, K.; Wang, X.; Tang, B.; Yang, B.; Jiang, W.; Jiang, P. Emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus in the Mid-Eastern region of China. Vet. J. 2007, 174, 577–584. [Google Scholar] [CrossRef]
- Tian, K.; Yu, X.; Zhao, T.; Feng, Y.; Cao, Z.; Wang, C.; Hu, Y.; Chen, X.; Hu, D.; Tian, X.; et al. Emergence of fatal PRRSV variants: Unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE 2007, 2, e526. [Google Scholar] [CrossRef]
- Tong, G.Z.; Zhou, Y.J.; Hao, X.F.; Tian, Z.J.; An, T.Q.; Qiu, H.J. Highly pathogenic porcine reproductive and respiratory syndrome, China. Emerg. Infect. Dis. 2007, 13, 1434–1436. [Google Scholar] [CrossRef]
- Yu, L.X.; Wang, X.; Yu, H.; Jiang, Y.F.; Gao, F.; Tong, W.; Li, L.W.; Li, H.C.; Yang, S.; Chen, P.F.; et al. The emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus with additional 120aa deletion in Nsp2 region in Jiangxi, China. Transbound. Emerg. Dis. 2018, 65, 1740–1748. [Google Scholar] [CrossRef]
- Song, S.; Xu, H.; Zhao, J.; Leng, C.; Xiang, L.; Li, C.; Fu, J.; Tang, Y.D.; Peng, J.; Wang, Q.; et al. Pathogenicity of NADC34-like PRRSV HLJDZD32-1901 isolated in China. Vet. Microbiol. 2020, 246, 108727. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, J.; Bai, X.; Ji, G.; Yan, H.; Li, Y.; Wang, Y.; Tan, F.; Xiao, Y.; Li, X.; et al. Pathogenicity comparison between highly pathogenic and NADC30-like porcine reproductive and respiratory syndrome virus. Arch. Virol. 2016, 161, 2257–2261. [Google Scholar] [CrossRef]
- Wang, H.M.; Liu, Y.G.; Tang, Y.D.; Liu, T.X.; Zheng, L.L.; Wang, T.Y.; Liu, S.G.; Wang, G.; Cai, X.H. A natural recombinant PRRSV between HP-PRRSV JXA1-like and NADC30-like strains. Transbound. Emerg. Dis. 2018, 65, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Xiao, Y.; Ye, M.; Li, X.; Li, S.; Xie, N.; Wei, Y.; Wang, J.; Zhu, J. High genetic diversity of Chinese porcine reproductive and respiratory syndrome viruses from 2016 to 2019. Res. Vet. Sci. 2020, 131, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, X.; Xiao, Y.; Li, S.; Zhu, J. Characterization of four types of MLV-derived porcine reproductive and respiratory syndrome viruses isolated in unvaccinated pigs from 2016 to 2020. Res. Vet. Sci. 2021, 134, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Saif, L.J.; Marthaler, D.; Esseili, M.A.; Meulia, T.; Lin, C.M.; Vlasova, A.N.; Jung, K.; Zhang, Y.; Wang, Q. Cell culture isolation and sequence analysis of genetically diverse US porcine epidemic diarrhea virus strains including a novel strain with a large deletion in the spike gene. Vet. Microbiol. 2014, 173, 258–269. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhang, H.L.; Zhang, W.L.; Xiang, L.R.; Li, Z.; Leng, C.L.; Chen, J.Z.; Peng, J.M.; Wang, Q.; An, T.Q.; et al. Isolation, characterization and complete genome analysis of classical North American type-porcine reproductive and respiratory syndrome virus in a large-scale pig farm in North China during 2016–2017. Chin. J. Prev. Vet. Med. 2017, 39, 691–696. (In Chinese) [Google Scholar] [CrossRef]
- Halbur, P.G.; Paul, P.S.; Meng, X.J.; Lum, M.A.; Andrews, J.J.; Rathje, J.A. Comparative pathogenicity of nine US porcine reproductive and respiratory syndrome virus (PRRSV) isolates in a five-week-old cesarean-derived, colostrum-deprived pig model. J. Vet. Diagn. Invest. 1996, 8, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.L.; Wu, S.P.; Li, Y.G.; Sun, F.X.; Wang, Q.J.; Zhao, Q.; Yu, J.; Tian, F.L.; Wu, J.Q.; Zhu, R.L.; et al. A porcine alveolar macrophage cell line stably expressing CD163 demonstrates virus replication and cytokine secretion characteristics similar to primary alveolar macrophages following PRRSV infection. Vet. Microbiol. 2020, 244, 108690. [Google Scholar] [CrossRef]
- Han, Z.; Liu, Y.; Wang, G.; He, Y.; Hu, S.; Li, Y.; Shi, W.; Wu, J.; Wang, S.; Liu, H.; et al. Comparative Analysis of Immune Responses in Pigs to High and Low Pathogenic Porcine Reproductive and Respiratory Syndrome Viruses Isolated in China. Transbound. Emerg. Dis. 2015, 62, e1–e10. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Ding, Y.; Ge, X.; Guo, X.; Yang, H. NADC30-like Strain of Porcine Reproductive and Respiratory Syndrome Virus, China. Emerg. Infect. Dis. 2015, 21, 2256–2257. [Google Scholar] [CrossRef]
- Zhao, K.; Ye, C.; Chang, X.B.; Jiang, C.G.; Wang, S.J.; Cai, X.H.; Tong, G.Z.; Tian, Z.J.; Shi, M.; An, T.Q. Importation and Recombination Are Responsible for the Latest Emergence of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus in China. J. Virol. 2015, 89, 10712–10716. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Yang, B.; Xu, L.; Jin, H.; Ge, X.; Guo, X.; Han, J.; Yang, H. Efficacy evaluation of three modified-live virus vaccines against a strain of porcine reproductive and respiratory syndrome virus NADC30-like. Vet. Microbiol. 2017, 207, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Xie, W.; Chen, X.X.; Qiao, S.; Zhao, M.; Gu, Y.; Zhao, B.L.; Zhang, G. Molecular epidemiology of porcine reproductive and respiratory syndrome virus in Central China since 2014: The prevalence of NADC30-like PRRSVs. Microb. Pathog. 2017, 109, 20–28. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Cao, Z.; Tang, Y.D.; Xia, D.; Wang, G.; Shan, H. Recent Advances in Porcine Reproductive and Respiratory Syndrome Virus NADC30-Like Research in China: Molecular Characterization, Pathogenicity, and Control. Front. Microbiol. 2022, 12, 791313. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, C.; Lin, Z.; Xia, W.; Ma, Y.; Dai, A.; Yang, X. Full genome sequence analysis of a 1-7-4-like PRRSV strain in Fujian Province, China. Peer. J. 2019, 7, e7859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Zhang, W.L.; Xiang, L.R.; Leng, C.L.; Tian, Z.J.; Tang, Y.D.; Cai, X.H. Emergence of novel porcine reproductive and respiratory syndrome viruses (ORF5 RFLP 1-7-4 viruses) in China. Vet. Microbiol. 2018, 222, 105–108. [Google Scholar] [CrossRef]
- Xu, H.; Li, C.; Li, W.; Zhao, J.; Gong, B.; Sun, Q.; Tang, Y.D.; Xiang, L.; Leng, C.; Peng, J.; et al. Novel characteristics of Chinese NADC34-like PRRSV during 2020–2021. Transbound. Emerg. Dis. 2022. [Google Scholar] [CrossRef]
- Gao, J.C.; Xiong, J.Y.; Ye, C.; Chang, X.B.; Guo, J.C.; Jiang, C.G.; Zhang, G.H.; Tian, Z.J.; Cai, X.H.; Tong, G.Z.; et al. Genotypic and geographical distribution of porcine reproductive and respiratory syndrome viruses in mainland China in 1996–2016. Vet. Microbiol. 2017, 208, 164–172. [Google Scholar] [CrossRef]
- Liu, J.K.; Zhou, X.; Zhai, J.Q.; Li, B.; Wei, C.H.; Dai, A.L.; Yang, X.Y.; Luo, M.L. Emergence of a novel highly pathogenic porcine reproductive and respiratory syndrome virus in China. Transbound. Emerg. Dis. 2017, 64, 2059–2074. [Google Scholar] [CrossRef]
- Cano, J.P.; Dee, S.A.; Murtaugh, M.P.; Trincado, C.A.; Pijoan, C.B. Effect of vaccination with a modified-live porcine reproductive and respiratory syndrome virus vaccine on dynamics of homologous viral infection in pigs. Am. J. Vet. Res. 2007, 68, 565–571. [Google Scholar] [CrossRef]
- Tian, Z.J.; An, T.Q.; Zhou, Y.J.; Peng, J.M.; Hu, S.P.; Wei, T.C.; Jiang, Y.F.; Xiao, Y.; Tong, G.Z. An attenuated live vaccine based on highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) protects piglets against HP-PRRS. Vet. Microbiol. 2009, 138, 34–40. [Google Scholar] [CrossRef]
- Leng, X.; Li, Z.; Xia, M.; He, Y.; Wu, H. Evaluation of the efficacy of an attenuated live vaccine against highly pathogenic porcine reproductive and respiratory syndrome virus in young pigs. Clin. Vaccine Immunol. 2012, 19, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Gong, W.; Zhang, D.; She, R.; Xu, B.; Ning, Y. Comparative Respiratory Pathogenicity and Dynamic Tissue Distribution of Chinese Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus and its Attenuated Strain in Piglets. J. Comp. Pathol. 2015, 153, 38–49. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, Z.; Cao, Z.; Wu, J.; Zhang, Z.; Xu, B.; Wang, C.; Hu, D.; Deng, X.; Han, W.; et al. Assessment of the safety and efficacy of an attenuated live vaccine based on highly pathogenic porcine reproductive and respiratory syndrome virus. Clin. Vaccine Immunol. 2015, 22, 493–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Wang, Y.; Xu, X.; Sun, Z.; Xiao, Y.; Ji, G.; Li, Y.; Tan, F.; Li, X.; Tian, K. Commercial vaccines provide limited protection to NADC30-like PRRSV infection. Vaccine 2016, 34, 5540–5545. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Zhou, L.; Bian, T.; Tian, X.X.; Ren, W.K.; Lu, C.; Zhang, L.; Li, X.L.; Cui, M.S.; Yang, H.C.; et al. Efficacy evaluation of two commercial modified-live virus vaccines against a novel recombinant type 2 porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2018, 216, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, M.; Wang, W.; Ju, D.; Cao, L.; Wu, B.; Wang, X.; Wu, Y.; Song, N.; Hu, J.; et al. An Attenuated Highly Pathogenic Chinese PRRS Viral Vaccine Confers Cross Protection to Pigs against Challenge with the Emerging PRRSV NADC30-Like Strain. Virol. Sin. 2018, 33, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, X.; Zhai, J.; Wei, C.; Dai, A.; Yang, X.; Luo, M. Recombination in JXA1-R vaccine and NADC30-like strain of porcine reproductive and respiratory syndrome viruses. Vet. Microbiol. 2017, 204, 110–120. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H. Evolutionary analysis of six isolates of porcine reproductive and respiratory syndrome virus from a single pig farm: MLV-evolved and recombinant viruses. Infect. Genet. Evol. 2018, 66, 111–119. [Google Scholar] [CrossRef]


| Fragments | Sequences of PCR Primers (5′–3′) | Position 1 | Length of Amplicon |
|---|---|---|---|
| PRRSV-1F | ATGACGTATAGGTGTTGGCTCTATG | 1–1894 | 1894 bp |
| PRRSV-1R | AGCGGCTGGGATAGCACTGCTAGGC | ||
| PRRSV-2F | GGTGAGCATTGGACTGTCACTG | 1700–3617 | 1918 bp |
| PRRSV-2R | TCTCGAGGATGCGTGGAACATC | ||
| PRRSV-3F | GTCTGTTTACCAGGCGATTTGC | 3427–5297 | 1871 bp |
| PRRSV-3R | CACAAAGCAACCAGGTAAGAGG | ||
| PRRSV-4F | TTTCCCAACACGGCCTTACCCT | 5103–7007 | 1905 bp |
| PRRSV-4R | TATCAGCAAAAGCTTCAAGTTTGG | ||
| PRRSV-5F | ATCATGAGTCTCTGACTGGTGCCC | 6777–8697 | 1921 bp |
| PRRSV-5R | CTTCTTCCCGCAATACTGTTTCTT | ||
| PRRSV-6F | GAATTCTATGGCTGGAATAAATGG | 8547–10407 | 1861 bp |
| PRRSV-6R | AACATAGCAATGAGAATCAAAACC | ||
| PRRSV-7F | CACCGGTCCGTGGGTTCGCATCCT | 10227–12097 | 1871bp |
| PRRSV-7R | AAAGGCTTTGCATGGACCCCATTT | ||
| PRRSV-8F | GGAGTTCTCGTTGGATGACCCAGT | 11847–13837 | 1991 bp |
| PRRSV-8R | ACAAAGAAAGCAATTGCGAGCAAC | ||
| PRRSV-9F | CATCGTGGCTGTGTGTGTCAATTT | 13607–15409 | 1803 bp |
| PRRSV-9R | AATTTCGGCCGCATGGTTCTCGCC |
| ORFs | aa Position | 155 | 227 | 235 | 402 | 509 | 510 | 560 | 573 | 582 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRRSV-Prototype | |||||||||||
| ORF1a (2503 aa) | CH-1a | S | H | S | S | R | L | L | A | P | |
| CH-1R | S | Y | S | S | R | L | F | V | P | ||
| CSR1801 | G | H | G | L | C | H | L | V | S | ||
| 764 | 780 | 851 | 860 | 865 | 867 | 964 | 969 | 997 | |||
| CH-1a | A | K | D | R | C | R | V | P | E | ||
| CH-1R | V | R | D | R | D | R | I | L | E | ||
| CSR1801 | A | R | N | Q | D | W | V | P | D | ||
| 1015 | 1023 | 1038 | 1193 | 1245 | 1254 | 1259 | 1307 | 1429 | |||
| CH-1a | N | S | E | R | T | L | E | W | R | ||
| CH-1R | D | S | K | W | I | F | E | W | Q | ||
| CSR1801 | D | A | K | W | T | F | G | . | Q | ||
| 1445 | 1508 | 1522 | 1523 | 1543 | 1549 | 1630 | 1640 | 1643 | |||
| CH-1a | W | P | L | L | H | W | W | L | W | ||
| CH-1R | W | P | F | F | Y | W | R | L | W | ||
| CSR1801 | R | S | F | F | Y | R | W | F | R | ||
| 1705 | 1742 | 1755 | 1797 | 1994 | 2063 | 2067 | 2105 | 2109 | |||
| CH-1a | A | S | W | H | S | W | A | S | S | ||
| CH-1R | V | G | W | Y | R | R | S | F | F | ||
| CSR1801 | V | G | R | Y | R | R | A | F | S | ||
| 2166 | 2241 | 2273 | 2295 | 2341 | 2410 | 2441 | 2511 | ||||
| CH-1a | P | . | R | Q | P | W | W | R | |||
| CH-1R | P | R | R | . | P | W | R | C | |||
| CSR1801 | S | R | . | Q | L | . | W | R | |||
| ORF1b (1457 aa) | 2619 | 2745 | 2837 | 2838 | 2933 | 2959 | 3023 | 3034 | 3130 | ||
| CH-1a | I | P | K | K | C | V | H | L | F | ||
| CH-1R | I | L | E | K | C | A | Y | P | S | ||
| CSR1801 | V | L | K | E | S | V | Y | L | F | ||
| 3197 | 3247 | 3366 | 3424 | 3426 | 3433 | 3537 | 3551 | 3606 | |||
| CH-1a | E | V | L | R | I | D | V | K | S | ||
| CH-1R | N | A | F | Q | I | D | V | E | T | ||
| CSR1801 | K | V | F | Q | V | G | A | K | T | ||
| 3775 | 3783 | 3847 | 3918 | 3926 | 3936 | ||||||
| CH-1a | Y | W | V | K | A | Y | |||||
| CH-1R | H | W | I | E | A | Y | |||||
| CSR1801 | H | . | V | E | T | N | |||||
| 3972 | 4021 | 4028 | 4052 | 4085 | 4102 | 4178 | 4182 | ||||
| ORF2 (257 aa) | CH-1a | N | F | H | R | A | . | H | R | ||
| CH-1R | D | F | Y | L | T | W | H | R | |||
| CSR1801 | N | Y | Y | L | T | W | Y | C | |||
| ORF3 (255 aa) | 4219 | 4252 | 4254 | 4303 | 4312 | 4334 | 4368 | 4384 | 4399 | ||
| CH-1a | N | G | D | Q | F | F | S | T | R | ||
| CH-1R | N | S | E | R | V | Y | F | T | T | ||
| CSR1801 | D | S | E | Q | S | Y | F | I | R | ||
| 4402 | |||||||||||
| CH-1a | A | ||||||||||
| CH-1R | A | ||||||||||
| CSR1801 | T | ||||||||||
| ORF4 (179 aa) | 4522 | 4524 | |||||||||
| CH-1a | F | P | |||||||||
| CH-1R | F | Q | |||||||||
| CSR1801 | L | P | |||||||||
| 4538 | 4546 | 4571 | 4606 | 4679 | 4683 | ||||||
| ORF5 (201 aa) | CH-1a | C | R | H | H | L | G | ||||
| CH-1R | Y | R | Q | H | Q | G | |||||
| CSR1801 | Y | Q | Q | N | L | D | |||||
| 4791 | 4806 | 4816 | 4881 | ||||||||
| ORF6 (175 aa) | CH-1a | S | Q | R | K | ||||||
| CH-1R | S | Q | R | K | |||||||
| CSR1801 | L | Q | R | ||||||||
| 4909 | 4944 | 4946 | 4975 | 5017 | |||||||
| ORF7 (124 aa) | CH-1a | R | K | K | C | V | |||||
| CH-1R | Q | K | N | C | V | ||||||
| CSR1801 | Q | I | K | R | A | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Ji, X.; Fu, C.; Hu, D.; Pang, H.; Wang, T.; Li, C.; Wang, G.; Peng, J. Evolution Characterization and Pathogenicity of a Porcine Reproductive and Respiratory Syndrome Virus Isolate from a Pig Farm in Shandong Province, China. Viruses 2022, 14, 1194. https://doi.org/10.3390/v14061194
Xu Y, Ji X, Fu C, Hu D, Pang H, Wang T, Li C, Wang G, Peng J. Evolution Characterization and Pathogenicity of a Porcine Reproductive and Respiratory Syndrome Virus Isolate from a Pig Farm in Shandong Province, China. Viruses. 2022; 14(6):1194. https://doi.org/10.3390/v14061194
Chicago/Turabian StyleXu, Yulin, Xiaojing Ji, Chunyu Fu, Dong Hu, Heng Pang, Tingting Wang, Chuangang Li, Gang Wang, and Jun Peng. 2022. "Evolution Characterization and Pathogenicity of a Porcine Reproductive and Respiratory Syndrome Virus Isolate from a Pig Farm in Shandong Province, China" Viruses 14, no. 6: 1194. https://doi.org/10.3390/v14061194
APA StyleXu, Y., Ji, X., Fu, C., Hu, D., Pang, H., Wang, T., Li, C., Wang, G., & Peng, J. (2022). Evolution Characterization and Pathogenicity of a Porcine Reproductive and Respiratory Syndrome Virus Isolate from a Pig Farm in Shandong Province, China. Viruses, 14(6), 1194. https://doi.org/10.3390/v14061194






