Serological Evidence of Severe Fever with Thrombocytopenia Syndrome Virus and IgM Positivity Were Identified in Healthy Residents in Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement of Anti-SFTSV IgG and IgM Using Immunofluorescence Antibody Assay (IFA)
2.2. Measurement of Anti-SFTSV IgG and IgM Using Enzyme-Linked Immunosorbent Assays (ELISAs)
2.3. The 50% Focus Reduction Neutralization Test (FRNT50) Assay
2.4. Real-Time RT-PCR for Molecular Diagnosis
3. Results
3.1. Seropositive Rate of IgG and IgM Based on IFA and ELISA
3.2. The Titers of Neutralizing Antibody to SFTSV
3.3. Molecular Diagnostic of SFTSV
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.R.; Heo, S.T.; Park, D.; Kim, H.; Fukuma, A.; Fukushi, S.; Shimojima, M.; Lee, K.H. Family Cluster Analysis of Severe Fever with Thrombocytopenia Syndrome Virus Infection in Korea. Am. J. Trop. Med. Hyg. 2017, 95, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Cheng, N.; Li, Y.; Wang, H.; You, A.; Su, J.; Nie, Y.; Ma, H.; Xu, B.; Huang, X. Seroprevalance of antibodies specific for severe fever with thrombocytopenia syndrome virus and the discovery of asymptomatic infections in Henan Province, China. PLoS Negl. Trop. Dis. 2019, 13, e0007242. [Google Scholar] [CrossRef]
- Xiong, W.Y.; Feng, Z.J.; Matsui, T.; Foxwell, A.R. Risk assessment of human infection with a novel bunyavirus in China. West. Pac. Surveill. Response J. 2012, 3, 61–66. [Google Scholar] [CrossRef]
- Liu, S.; Chai, C.; Wang, C.; Amer, S.; Lv, H.; He, H.; Sun, J.; Lin, J. Systematic review of severe fever with thrombocytopenia syndrome: Virology, epidemiology, and clinical characteristics. Rev. Med. Virol. 2014, 24, 90–102. [Google Scholar] [CrossRef]
- Ra, S.H.; Kim, M.J.; Kim, M.C.; Park, S.Y.; Park, S.Y.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Lee, K.H.; et al. Kinetics of Serological Response in Patients with Severe Fever with Thrombocytopenia Syndrome. Viruses 2021, 1, 6. [Google Scholar] [CrossRef]
- Yen, N.T.; Kim, C.; Jeong, S.; Jeon, K.; Choi, H.; Ro, H.J.; Kim, H.I.; Kim, Y.; Kang, J.G.; Park, D.; et al. Severe Fever with Thrombocytopenia Syndrome Virus Infection, South Korea, 2010. Emerg. Infect. Dis. 2018, 24, 2103–2105. [Google Scholar]
- Takahashi, T.; Maeda, K.; Suzuki, T.; Ishido, A.; Shigeoka, T.; Tominaga, T.; Kamei, T.; Honda, M.; Ninomiya, D.; Sakai, T.; et al. The First Identification and Retrospective Study of Severe Fever with Thrombocytopenia Syndrome in Japan. J. Infect. Dis. 2014, 209, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.C.; Yun, Y.; Kim, S.H.; Thao, N.T.; Man, P.K.; Yoo, J.R.; Heo, S.T.; Cho, N.H.; Lee, K.H. Endemic severe fever with thrombocytopenia syn drome, Vietnam. Emerg. Infect. Dis. 2019, 25, 1029–1031. [Google Scholar] [CrossRef]
- Peng, S.-H.; Yang, S.-L.; Tang, S.-E.; Wang, T.-C.; Hsu, T.-C.; Su, C.-L.; Chen, M.-Y.; Shimojima, M.; Yoshikawa, T.; Shu, P.-Y. Human Case of Severe Fever with Thrombocytopenia Syndrome Virus Infection, Taiwan 2019. Emerg. Infect. Dis. 2020, 26, 1612–1614. [Google Scholar] [CrossRef]
- Win, A.M.; Nguyen, Y.T.H.; Kim, Y.; Ha, N.-Y.; Kang, J.-G.; Kim, H.; San, B.; Kyaw, O.; Htike, W.W.; Choi, D.-O.; et al. Genotypic Heterogeneity of Orientia tsutsugamushi in Scrub Typhus Patients and Thrombocytopenia Syndrome Co-infection, Myanmar. Emerg. Infect. Dis. 2020, 26, 1878–1881. [Google Scholar] [CrossRef] [PubMed]
- Ongkittikul, S.; Ruedeerat Watanawong, R.; Photchana Rompho, P. Severe Fever with Thrombocytopenia Syndrome Virus: The First Case Report in Thailand. Bangk. Med. J. 2020, 16, 204–206. [Google Scholar]
- Zohaib, A.; Zhang, J.; Saqib, M.; Athar, M.A.; Hussain, M.H.; Chen, J.; Tayyab, M.H.; Batool, M.; Khan, S.; Luo, Y.; et al. Serologic Evidence of Severe Fever with Thrombocytopenia Syndrome Virus and Related Viruses in Pakistan. Emerg. Infect. Dis. 2020, 26, 1513–1516. [Google Scholar] [CrossRef]
- Yamanaka, A.; Kirino, Y.; Fujimoto, S.; Ueda, N.; Himeji, D.; Miura, M.; Sudaryatma, P.E.; Sato, Y.; Tanaka, H.; Mekata, H.; et al. Direct Transmission of Severe Fever with Thrombocytopenia Syndrome Virus from Domestic Cat to Veterinary Personnel. Emerg. Infect. Dis. 2020, 26, 2994–2998. [Google Scholar] [CrossRef]
- Yoo, J.R.; Kim, J.Y.; Heo, S.T.; Kim, J.; Park, H.J.; Lee, J.Y.; Lim, H.Y.; Park, W.J.; Cho, N.H.; Kim, J.M.; et al. Neutralizing Antibodies to Severe Fever With Thrombocytopenia Syndrome Virus Among Survivors, Non-Survivors and Healthy Residents in South Korea. Front. Cell Infect. Microbiol. 2021, 11, 649570. [Google Scholar] [CrossRef]
- Li, P.; Tong, Z.D.; Li, K.F.; Tang, A.; Dai, Y.X.; Yan, J.B. Seroprevalence of severe fever with thrombocytopenia syndrome virus in China: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0175592. [Google Scholar] [CrossRef]
- Han, M.A.; Kim, C.M.; Kim, D.M.; Yun, N.R.; Park, S.W.; Han, M.G.; Lee, W.J. Seroprevalence of severe fever with thrombocytepenia syndrome virus antibodies in rural areas, South Korea. Emerg. Infect. Dis. 2018, 24, 872–874. [Google Scholar] [CrossRef]
- Kim, K.H.; Ko, M.K.; Kim, N.; Kim, H.H.; Yi, J. Seroprevalence of severe fever with thrombocytopenia syndrome in south. eastern Korea, 2015. J. Korean Med. Sci. 2017, 32, 29–32. [Google Scholar] [CrossRef]
- Kimura, T.; Fukuma, A.; Shimojima, M.; Yamashita, Y.; Mizota, F.; Yamashita, M.; Otsuka, Y.; Kan, M.; Fukushi, S.; Tani, H.; et al. Seroprevalence of severe fever. with thrombocytopenia syndrome (SFTS) virus antibodies in humans and animals in Ehime prefecture, Japan, an endemic region of SFTS. J. Infect. Chemother. 2018, 24, 802–806. [Google Scholar] [CrossRef]
- Gokuden, M.; Fukushi, S.; Saijo, M.; Nakadouzono, F.; Iwamoto, Y.; Yamamoto, M.; Hozumi, N.; Nakayama, K.; Ishitani, K.; Nishi, N.; et al. Low seroprevalence of severe fever with thrombocytopenia syndrome virus antibodies in individuals living in an endemic area in Japan. Jpn. J. Infect. Dis. 2018, 71, 225–228. [Google Scholar] [CrossRef]
- Yoo, J.R.; Heo, S.T.; Kim, M.; Song, S.W.; Boo, J.W.; Lee, K.H. Seroprevalence of severe fever with thrombocytopenia syndrome in the agricultural population of Jeju Island, Korea, 2015–2017. Infect. Chemother. 2019, 51, 337–344. [Google Scholar] [CrossRef]
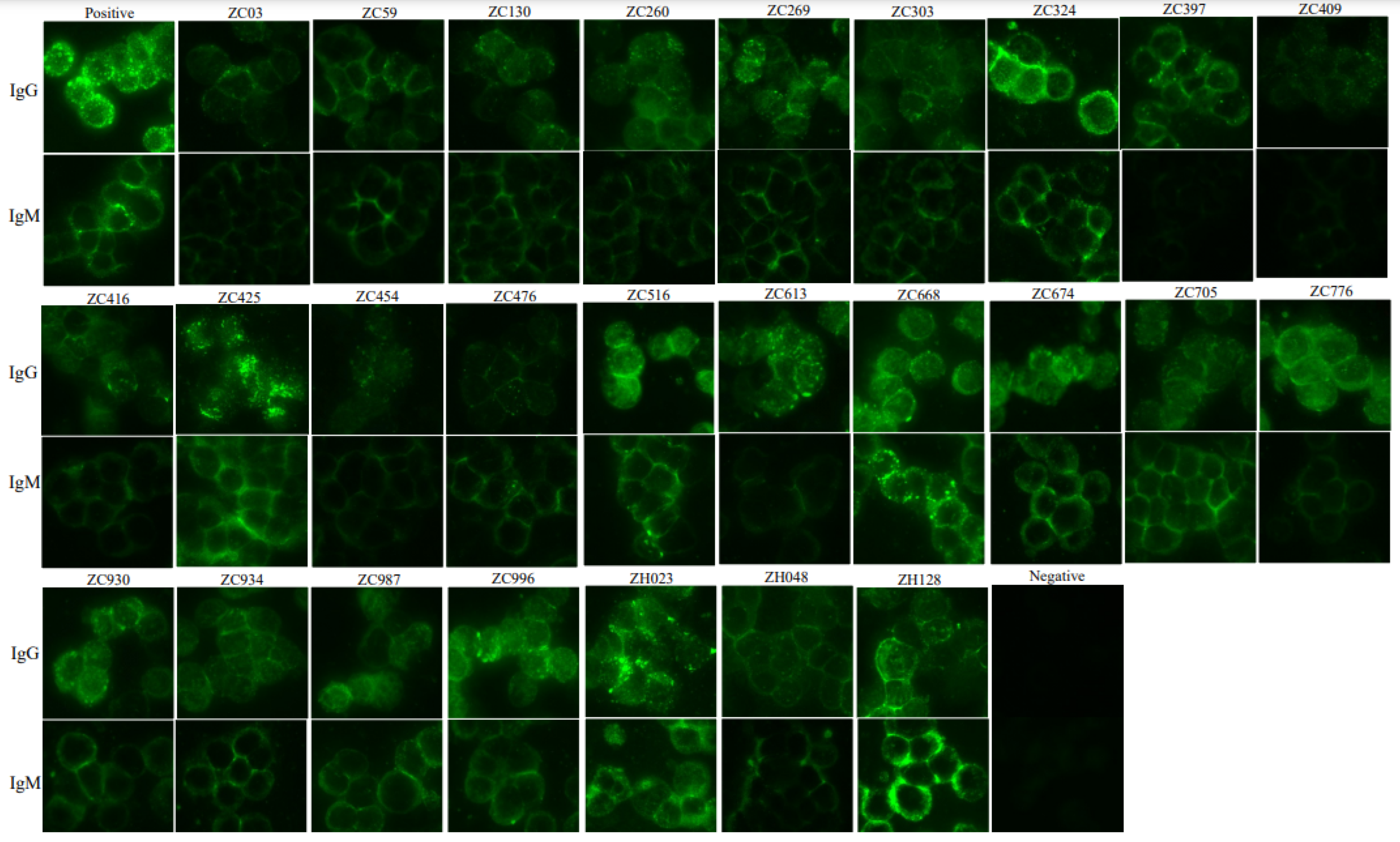
| No. | Age (Year)/Sex | Occupation | Date of Sampling | Status | IFA (IgM) | ELISA (IgM) | IFA (IgG) | ELISA (IgG) | FRNT50 Titer * | RT-PCR for SFTSV | Study Site |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZC03 | 57/F | Small trader | 25 August 2018 | Healthy | P | N | 60 | P | N/A | N | Thua Thien Hue Province |
| ZC59 | 36/F | Farmer | 25 August 2018 | Healthy | P | P | 10 | P | N/A | N | Thua Thien Hue Province |
| ZC130 | 30/F | Farmer | 25 August 2018 | Healthy | P | N | 10 | P | N/A | N | Thua Thien Hue Province |
| ZC260 | 76/F | Retired | 25 August 2018 | Healthy | P | N | 10 | P | N/A | N | Thua Thien Hue Province |
| ZC269 | 16/F | Student | 25 August 2018 | Healthy | P | N | 40 | P | 1:10 (15.8) | N | Thua Thien Hue Province |
| ZC303 | 64/M | Farmer | 25 August 2018 | Healthy | P | N | 10 | P | N/A | N | Thua Thien Hue Province |
| ZC324 | 55/F | Farmer | 25 August 2018 | Healthy | P | P | 160 | P | 1:20 (29.6) | N | Thua Thien Hue Province |
| ZC397 | 46/M | Teacher | 25 August 2018 | Healthy | N | P | 10 | P | N/A | N | Thua Thien Hue Province |
| ZC409 | 50/M | Small trader | 25 August 2018 | Healthy | N | N | P | P | N/A | N | Thua Thien Hue Province |
| ZC416 | 25/M | Worker | 25 August 2018 | Healthy | P | N | 10 | P | N/A | N | Thua Thien Hue Province |
| ZC425 | 56/F | Farmer | 25 August 2018 | Healthy | 10 | N | 2560 | P | 1:40 (49.0) | N | Thua Thien Hue Province |
| ZC454 | 23/M | Driver | 25 August 2018 | Healthy | P | N | 10 | P | N/A | N | Thua Thien Hue Province |
| ZC476 | 33/F | Farmer | 25 August 2018 | Healthy | P | P | 10 | P | N/A | N | Thua Thien Hue Province |
| ZC516 | 25/M | Farmer | 8 September 2018 | Healthy | 40 | N | P | P | 1:20 (20.6) | N | Quang Nam Province |
| ZC613 | 63/M | Free worker | 8 September 2018 | Healthy | P | N | 160 | P | 1:10 (20.6) | N | Quang Nam Province |
| ZC668 | 50/F | Free worker | 8 September 2018 | Healthy | 160< | P | P | P | 1:40 (55.9) | N | Quang Nam Province |
| ZC674 | 35/M | Farmer | 8 September 2018 | Healthy | P | P | P | P | N/A | N | Quang Nam Province |
| ZC705 | 38/F | Farmer | 8 September 2018 | Healthy | 10 | N | 10 | P | N/A | N | Quang Nam Province |
| ZC776 | 35/F | Worker | 8 September 2018 | Healthy | P | N | 20 | P | 1:20 (31.6) | N | Quang Nam Province |
| ZC930 | 23/M | Student | 8 September 2018 | Healthy | P | N | 10 | P | N/A | N | Quang Nam Province |
| ZC934 | 39/M | Farmer | 8 September 2018 | Healthy | P | N | P | P | N/A | N | Quang Nam Province |
| ZC987 | 47/M | Farmer | 8 September 2018 | Healthy | P | N | P | N | N/A | N | Quang Nam Province |
| ZC996 | 54/M | Farmer | 8 September 2018 | Healthy | 20 | P | 20 | P | 1:20 (33.7) | N | Quang Nam Province |
| ZH023 | 22/F | Farmer | 14 June 2018 | Healthy | P | P | 160 | P | 1:20 (20.0) | N | Thua Thien Hue Province |
| ZH048 | 31/F | Small trader | 14 June 2018 | Healthy | P | N | 10 | P | N/A | N | Thua Thien Hue Province |
| ZH128 | 55/M | Farmer | 25 August 2018 | Healthy | 80 | N | 80 | P | 1:20 (35.2) | N | Thua Thien Hue Province |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, X.C.; Kim, S.H.; Lee, J.-E.; Kim, S.-H.; Kang, S.Y.; Binh, N.D.; Duc, P.V.; Phuong, P.T.K.; Thao, N.T.P.; Lee, W.; et al. Serological Evidence of Severe Fever with Thrombocytopenia Syndrome Virus and IgM Positivity Were Identified in Healthy Residents in Vietnam. Viruses 2022, 14, 2280. https://doi.org/10.3390/v14102280
Tran XC, Kim SH, Lee J-E, Kim S-H, Kang SY, Binh ND, Duc PV, Phuong PTK, Thao NTP, Lee W, et al. Serological Evidence of Severe Fever with Thrombocytopenia Syndrome Virus and IgM Positivity Were Identified in Healthy Residents in Vietnam. Viruses. 2022; 14(10):2280. https://doi.org/10.3390/v14102280
Chicago/Turabian StyleTran, Xuan Chuong, Sung Hye Kim, Jeong-Eun Lee, So-Hee Kim, Su Yeon Kang, Nguyen D. Binh, Pham V. Duc, Phan T. K. Phuong, Nguyen T. P. Thao, Wonwoo Lee, and et al. 2022. "Serological Evidence of Severe Fever with Thrombocytopenia Syndrome Virus and IgM Positivity Were Identified in Healthy Residents in Vietnam" Viruses 14, no. 10: 2280. https://doi.org/10.3390/v14102280
APA StyleTran, X. C., Kim, S. H., Lee, J.-E., Kim, S.-H., Kang, S. Y., Binh, N. D., Duc, P. V., Phuong, P. T. K., Thao, N. T. P., Lee, W., Bae, J.-Y., Park, M.-S., Kim, M., Yoo, J. R., Heo, S. T., An, K. H., Kim, J. M., Cho, N.-H., Kee, S.-H., & Lee, K. H. (2022). Serological Evidence of Severe Fever with Thrombocytopenia Syndrome Virus and IgM Positivity Were Identified in Healthy Residents in Vietnam. Viruses, 14(10), 2280. https://doi.org/10.3390/v14102280







