Newcastle Disease Genotype VII Prevalence in Poultry and Wild Birds in Egypt
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.1.1. Domestic Birds
2.1.2. Migratory and Non-Migratory Wild Birds
2.2. Sample Processing and Virus Isolation
2.3. RNA Extraction and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
2.4. Sequence-Independent Single Primer Amplification (SISPA)
2.5. Genome Sequencing
2.6. Whole Genome Sequencing Data Analysis
2.7. Phylogenetic Analysis
2.8. Pathogenicity Indices
3. Results
3.1. Clinical Signs and Post-Mortem Lesions
3.2. Virus Identification
3.3. Incidence of NDV Based on F Gene
3.4. Phylogenetics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Committee on Taxonomy of Viruses (ICTV). Virus Taxonomy. 2018. Available online: https://talk.ictvonline.org/taxonomy (accessed on 20 December 2020).
- Diel, D.G.; da Silva, L.H.; Liu, H.; Wang, Z.; Miller, P.J.; Afonso, C.L. Genetic diversity of avian paramyxovirus type 1: Proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes. Infect. Genet. Evol. 2012, 12, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, K.M.; Abolnik, C.; Afonso, C.L.; Albina, E.; Bahl, J.; Berg, M.; Briand, F.X.; Brown, I.H.; Choi, K.S.; Chvala, I.; et al. Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. Infect. Genet. Evol. 2019, 74, 103917. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Wu, S.; Hu, S.; Peng, Y.; Xue, F.; Liu, X. Surveillance for avirulent Newcastle disease viruses in domestic ducks (Anas platyrhynchos and Cairina moschata) at live bird markets in Eastern China and characterization of the viruses isolated. Avian Pathol. 2009, 38, 377–391. [Google Scholar] [CrossRef]
- Snoeck, C.J.; Owoade, A.A.; Couacy-Hymann, E.; Alkali, B.R.; Okwen, M.P.; Adeyanju, A.T.; Komoyo, G.F.; Nakoune, E.; Le Faou, A.; Muller, C.P. High genetic diversity of Newcastle disease virus in poultry in West and Central Africa: Cocirculation of genotype XIV and newly defined genotypes XVII and XVIII. J. Clin. Microbiol. 2013, 51, 2250–2260. [Google Scholar] [CrossRef]
- Daubney, R.; Mansy, W. The occurrence of Newcastle disease in Egypt. J. Comp. Pathol. Ther. 1948, 58, 189–200. [Google Scholar] [CrossRef]
- Moharam, I.; Razik, A.A.E.; Sultan, H.; Ghezlan, M.; Meseko, C.; Franzke, K.; Harder, T.; Beer, M. Investigation of suspected Newcastle disease (ND) outbreaks in Egypt uncovers a high virus velogenic ND virus burden in small-scale holdings and the presence of multiple pathogens. Avian Pathol. 2019, 48, 406–415. [Google Scholar] [CrossRef]
- Abd El-Hamid, H.S.; Hatem, S.; Shafi, M.E.; Albaqami, N.M.; Ellakany, H.F.; Abdelaziz, N.M.; Abdelaziz, M.N.; Abd El-Hack, M.E.; Taha, A.E.; Alanazi, K.M.; et al. Sequence analysis and pathogenicity of Avian Orthoavulavirus 1 strains isolated from poultry flocks during 2015–2019. BMC Vet. Res. 2020, 16, 253. [Google Scholar] [CrossRef]
- El-Bagoury, G.F.; El-Habbaa, A.S.; El-Adaway, S.F.; El-Mahdy, S.S. Isolation, identification and pathotyping of Newcastle disease virus from chickens in Egypt. BVMJ 2015, 29, 196–204. [Google Scholar] [CrossRef][Green Version]
- Megahed, M.M.; Eid, A.A.; Mohamed, W.; Hassanin, O. Genetic characterization of Egyptian Newcastle disease virus strains isolated from flocks vaccinated against Newcastle disease virus, 2014–2015. Slov. Vet. Res. 2018, 55, 17–29. [Google Scholar]
- El-Shall, N.A.; Sedeik, M.E.; El-Nahas, A.F.; Abdel-salam, R.A.; Awad, A.M. Epidemiological surveillance of some avian respiratory viral diseases in broiler chickens. AJVS 2019, 61, 85–94. [Google Scholar]
- Mouhamed, A.A.; Mohamed, M.A.; Bakheet, B.M.; Aziz El-Din, K.A.; Song, C. Molecular studies on Newcastle disease virus isolates in relation to field vaccine strains in Egypt (2012–2015). Int. J. Poult. Sci. 2020, 19, 193–209. [Google Scholar] [CrossRef]
- Sabban, M.; Zied, A.A.; Basyouni, A.; Nadiem, S.; Barhouma, N.; Habashi, Y. Susceptibility and possible role of doves in transmission of Newcastle disease in Egypt. Zent. Vet. Reihe B 1982, 29, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Mayo, M.A. Virus taxonomy—Houston 2002. Arch. Virol. 2002, 147, 1071–1076. [Google Scholar] [PubMed]
- Alexander, D.J.; Senne, D.A. Newcastle disease. In Diseases of Poultry, 12th ed.; Saif, Y.M., Barnes, H.J., Glisson, J.R., Fadly, A.M., McDougald, L.R., Swayne, D.E., Eds.; Blackwell Publishing: Ames, IA, USA, 2008; pp. 75–100. [Google Scholar]
- Alexander, D.J.; Aldous, E.W.; Fuller, C.M. The long view: A selective review of 40 years of Newcastle disease research the long view. Avian Path. 2012, 41, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.P.; Handberg, K.; Ahrens, P.; Therkildsen, O.; Manvell, R.; Alexander, D. Strains of avian paramyxovirus type 1 of low pathogenicity for chickens isolated from poultry and wild birds in Denmark. Vet. Record. 2004, 154, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, K.; Ramey, A.; Qiu, X.; Bahl, J.; Afonso, C. Temporal, geographic, and host distribution of avian paramyxovirus 1 (Newcastle disease virus). Infect. Genet. Evol. 2016, 39, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Mosad, S.M.; El-Gohary, F.A.; Ali, H.S.; El-Sharkawy, H.; Elmahallawy, E.K. Pathological and Molecular Characterization of H5 Avian Influenza Virus in Poultry Flocks from Egypt over a Ten-Year Period (2009–2019). Animals 2020, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.; ElBakrey, R.M.; Mohamed, F.F.; Hamouda, E.E.; Abdallah, M.S.; Elbestawy, A.R.; Ismail, M.M.; Abdien, H.; Eid, A. Avian Paramyxovirus Type 1 in Egypt: Epidemiology, Evolutionary Perspective, and Vaccine Approach. Front. Vet. Sci. 2021, 8, 647462. [Google Scholar] [CrossRef]
- Miller, P.; Koch, G. Newcastle disease, other avian paramyxoviruses, and avian metapneumovirus infections. In Diseases of Poultry; Swayne, D.E., Ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 89–138. [Google Scholar]
- Higgins, D.A. Nine disease outbreaks associated with myxoviruses among ducks in Hong Kong. Trop Anim Health Prod. 1971, 3, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.; Zhao, C.; Liu, D.; Hu, Y.; Zhao, J.; Zhang, G. Phylogenetic and pathotypical analysis of two virulent Newcastle disease viruses isolated from domestic ducks in China. PLoS ONE 2011, 6, e25000. [Google Scholar] [CrossRef]
- Abd Elsalaam, T. Migratory Birds in Egypt. Egyptian Geographic. Available online: https://egyptiangeographic.com/ar/news/show/439 (accessed on 11 July 2021).
- Takakuwa, H.; Ito, T.; Takada, A.; Okazaki, K.; Kida, H. Potentially virulent Newcastle disease viruses are maintained in migratory waterfowl populations. Jpn. J. Vet. Res. 1998, 45, 207–215. [Google Scholar] [PubMed]
- Kaleta, E.F.; Kummerfeld, N. Isolation of herpesvirus and Newcastle disease virus from White Storks (Ciconia ciconia) maintained at four re-habilitation centres in northern Germany during 1983 to 2001 and failure to detect antibodies against avian influenza A viruses of subtypes H5 and H7 in these birds. Avian Pathol. 2012, 41, 383–389. [Google Scholar] [PubMed]
- Yuan, X.; Wang, Y.; Li, J.; Yu, K.; Yang, J.; Xu, H.; Zhang, Y.; Ai, H.; Wang, J. Surveillance and molecular characterization of Newcastle disease virus in seafowl from coastal areas of China in 2011. Virus Genes 2013, 46, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Wajid, A.; Dimitrov, K.M.; Wasim, M.; Rehmani, S.F.; Basharat, A.; Bibi, T.; Arif, S.; Yaqub, T.; Tayyab, M.; Mustafa Ababneh, M. Repeated isolation of virulent Newcastle disease viruses in poultry and captive non-poultry avian species in Pakistan from 2011 to 2016. Prev. Vet. Med. 2017, 142, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.H.; Kandeil, A.; Alkhazindar, M.; Abd ElSalam, E.T.; Ali, M.A. Isolation of Newcastle Disease Virus from Wild Migratory Birds in Egypt. J. World Poult. Res. 2020, 10, 520–526. [Google Scholar] [CrossRef]
- Abd Elfatah, K.S.A.; Elabasy, M.A.; El-Khyate, F.; Elmahallawy, E.K.; Mosad, S.M.; El-Gohary, F.A.; Abdo, W.; Al-Brakati, A.; Seadawy, M.G.; Tahoon, A.E.; et al. Molecular Characterization of Velogenic Newcastle Disease Virus (Sub-Genotype VII. 1.1) from Wild Birds, with Assessment of Its Pathogenicity in Susceptible Chickens. Animals 2021, 11, 505. [Google Scholar] [CrossRef]
- OIE Terrestrial Manual. Newcastle Disease (Infection with Newcastle Disease Virus). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2021; Chapter 3.3.14; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.03.14_NEWCASTLE_DIS.pdf (accessed on 15 March 2022).
- World Health Organization. WHO Information for the Molecular Detection of Influenza Viruses. Available online: http://www.who.int/influenza/gisrs_laboratory/collaborating_centres/list/en/index.html (accessed on 22 May 2022).
- Chrzastek, K.; Lee, D.; Smith, D.; Sharma, P.; Suarez, D.L.; Pantin-Jackwood, M.; Kapczynski, D.R. Use of Sequence-Independent, Single-Primer-Amplification (SISPA) for rapid detection, identification, and characterization of avian RNA viruses. Virology 2017, 509, 159–166. [Google Scholar] [CrossRef]
- Chrzastek, K.; Tennakoon, C.; Bialy, D.; Freimanis, G.; Flannery, J.; Shelton, H. A random priming amplification method for whole genome sequencing of SARS-CoV-2 virus. BMC Genom. 2022, 23, 406. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://wwwbioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 22 May 2022).
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Silva, G.G.; Dutilh, B.E.; Matthews, T.D.; Elkins, K.; Schmieder, R.; Dinsdale, E.A.; Edwards, R.A. Combining de novo and reference-guided assembly with scaffold_builder. Source Code Biol. Med. 2013, 8, 23. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Miller, P.J.; Haddas, R.; Simanov, L.; Lublin, A.; Rehmani, S.F.; Wajid, A.; Bibi, T.; Khan, T.A.; Yaqub, T.; Setiyaningsih, S. Identification of new sub-genotypes of virulent Newcastle disease virus with potential panzootic features. Infect. Genet. Evol. 2015, 29, 216–229. [Google Scholar] [CrossRef]
- Xue, C.; Xu, X.; Yin, R.; Qian, J.; Sun, Y.; Wang, C.; Ding, C.; Yu, S.; Hu, S.; Liu, X.; et al. Identification and pathotypical analysis of a novel VIk sub-genotype Newcastle disease virus obtained from pigeon in China. Virus Res. 2017, 238, 1–7. [Google Scholar] [CrossRef]
- Abozaid, K.G.A.; Abdel-Moneim, A.S. Epidemiological surveillance of Newcastle disease virus in Egypt—A 6-year cohort study. Trop. Anim. Health Prod. 2022, 54, 243. [Google Scholar] [CrossRef]
- Ameji, O.N.; Sa’idu, L.; Abdu, P.A. Survey for Newcastle disease viruses in poultry and wild birds in Kogi state, Nigeria. Sokoto J. Vet. Sci. 2016, 14, 47–53. [Google Scholar] [CrossRef][Green Version]
- Boynukara, B.; Gülhan, T.; Çöven, F.; Kiziroğlu, İ.; Durmuş, A. Determination of Newcastle disease virus among wild bird populations in Lake Van basin, Turkey. Turkish J. Vet. Anim. Sci. 2013, 37, 86–93. [Google Scholar] [CrossRef]
- Cappelle, J.; Caron, A.; De Almeida, R.S.; Gil, P.; Pedrono, M.; Mundava, J.; Fofana, B.; Balanca, G.; Dakouo, M.; Ould El Mamy, A.B. Empirical analysis suggests continuous and homogeneous circulation of Newcastle disease virus in a wide range of wild bird species in Africa. Epidemiol. Infect. 2015, 143, 1292–1303. [Google Scholar] [CrossRef]
- Abdisa, T.; Tagesu, T. Review on Newcastle Disease in Poultry and its Public Health Importance. J. Anim. Poult. Sci. 2017, 6, 29–39. [Google Scholar] [CrossRef]
- Seal, B.; Wise, M.; Pedersen, J.; Senne, D.; Alvarez, R.; Scott, M.; King, D.; Yu, Q.; Kapczynski, D. Genomic sequences of low-virulence avian paramyxovirus-1 (Newcastle disease virus) isolates obtained from live-bird markets in North America not related to commonly utilized commercial vaccine strains. Vet. Microbiol. 2005, 106, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Aziz-ul-Rahman, M.H.; Shabbir, M.Z. Suppl-2, M3: Adaptation of Newcastle Disease Virus (NDV) in Feral Birds and their Potential Role in Interspecies Transmission. Open Virol. J. 2018, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.M.; King, D.J.; Curry, P.E.; Suarez, D.L.; Swayne, D.E.; Stallknecht, D.E.; Afonso, C.L. Phylogenetic diversity among low-virulence Newcastle disease viruses from waterfowl and shorebirds and comparison of genotype distributions to those of poultry-origin isolates. J. Virol. 2007, 81, 12641–12653. [Google Scholar] [CrossRef]
- Abdu, P.A. Evolution and the pathogenicity of Newcastle disease virus and its implications for diagnosis and control. In Proceedings of the Workshop on Improved Disease Diagnosis, Health, Nutrition and Risk Management Practice in Poultry, Zaria, Nigeria, 29 November–1 December 2005. [Google Scholar]
- Liu, M.; Wei, Y.Y.; Dai, Y.B.; Cheng, X.; Zhou, S.; Pan, Z.M.; Xu, L.X.; Jiao, X.A. Isolation and preliminary identification of a virulent Newcastle disease virus isolate of duck origin. Chin. J. Anim. Infect. Dis. 2010, 18, 67–71. [Google Scholar]
- Gowthaman, V.; Singh, S.D.; Barathidasan, R.; Ayanur, A.; Dhama, K. Natural outbreak of Newcastle disease in turkeys and Japanese quails housed along with chicken in a multi-species poultry farm in northern India. Adv. Anim. Vet. Sci. 2013, 1, 17–20. [Google Scholar]
- El-Morshidy, Y.; Abdo, W.; Elmahallawy, E.K.; Abd EL-Dayem, G.A.; El-Sawak, A.; El-Habashi, N.; Mosad, S.M.; Lokman, M.S.; Albrakati, A.; Abou Asa, S. Pathogenesis of Velogenic Genotype VII. 1.1 Newcastle Disease Virus Isolated from Chicken in Egypt via Different Inoculation Routes: Molecular, Histopathological, and Immunohistochemical Study. Animals 2021, 11, 3567. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.; Ozsemir, C.; Yilmaz, A.; Cizmecigil, U.Y.; Aydin, O.; Bamac, O.E.; Gurel, A.; Kutukcu, A.; Özsemir, K.G.; Tali, H.E.; et al. Identification of Newcastle disease virus subgenotype VII. 2 in wild birds in Turkey. BMC Vet. Res. 2020, 16, 277. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Hu, Q.L.; Chen, S.L.; Cheng, X.X.; Wu, N.Y.; Lin, F.Q.; Zhu, X.L.; Cheng, Y.Q. Isolation and identification of duck paramyxovirus. Chin. J. Prev. Vet. Med. 2004, 26, 118–120. [Google Scholar]
- Yu, H.; Li, W.Y.; Cheng, L.F.; Shi, S.H.; Peng, C.X.; Fu, G.H. Isolation and identification of paramyxovirus type 1 from duck. Chin. J. Prev. Vet. Med. 2005, 2, 148–150. [Google Scholar]
- Cheng, L.F.; Fu, G.H.; Huang, Y.; Shi, S.H.; Peng, C.X. Isolation and identification and sequence analysis of F protein gene of paramyxovirus type 1 strain FM01 from semi-muscovy duck. Chin. J. Prev. Vet. Med. 2006, 28, 499–502. [Google Scholar]
- Xu, Q.; Sun, J.; Gao, M.; Zhao, S.; Liu, H.; Zhang, T.; Han, Z.; Kong, X.; Liu, S. Genetic, antigenic, and pathogenic characteristics of Newcastle disease viruses isolated from geese in China. J. Vet. Diagn Invest. 2017, 29, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Elbestawy, A.R.; Ellakany, H.F.; Abd El-Hamid, H.S.; Zidan, R.E.; Gado, A.R.; Mahmoud, E.; Abd El-Hack, M.E.; Saadeldin, I.M.; Alowaimer, A.N.; Ba-Awadh, H.A.; et al. Muscovy ducks infected with velogenic Newcastle disease virus (genotype VIId) act as carriers to infect in-contact chickens. Poult. Sci. 2019, 98, 4441–4448. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.E.; El-Kady, M.F.; EL-Sawah, A.A.; Luttermann, C.; Parvin, R.; Shany, S.; Beer, M.; Harder, T. Respiratory disease due to mixed viral infections in poultry flocks in Egypt between 2017 and 2018: Upsurge of highly pathogenic avian influenza virus subtype H5N8 since 2018. Transbound. Emerg. Dis. 2021, 68, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Darwish, S.F.; El-Sabagh, I.M.; El-Sanousi, A.A.; Shalaby, M.A. Isolation and molecular characterization of Newcastle disease virus genotypes II and VIId in Egypt between 2011 and 2012. Virus Genes 2013, 47, 311–316. [Google Scholar] [CrossRef]
- Shakal, M.; Maher, M.; Metwally, A.S.; AbdelSabour, M.A.; Madbbouly, Y.M.; Safwat, G. Molecular Identification of a Velogenic Newcastle Disease Virus Strain Isolated from Egypt. J. World Poult. Res. 2020, 10, 195–202. [Google Scholar] [CrossRef]
- Dewidar, A.A.; El-Sawah, A.A.; Shany, S.A.; Dahshan, A.H.M.; Ali, A. Genetic characterization of genotype VII. 1.1 Newcastle Disease viruses from commercial and backyard broiler chickens in Egypt. Ger. J. Vet. Res. 2021, 1, 11–17. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Wu, Y.; Wu, Y.; Sun, C.; Zheng, D.; Xu, T.; Li, J. Molecular characterization and phylogenetic analysis of new Newcastle disease virus isolates from the mainland of China. Res. Vet. Sci. 2008, 85, 612–616. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Shao, M.Y.; Yu, X.H.; Zhao, J.; Zhang, G.Z. Molecular characterization of chicken-derived genotype VIId Newcastle disease virus isolates in China during 2005–2012 reveals a new length in hemagglutinin-neuraminidase. Infect. Genet. Evol. 2014, 21, 359–366. [Google Scholar] [CrossRef]
- Kang, Y.; Xiang, B.; Yuan, R.; Zhao, X.; Feng, M.; Gao, P.; Li, Y.; Li, Y.; Ning, Z.; Ren, T. Phylogenetic and pathotypic characterization of Newcastle disease viruses circulating in South China and transmission in different birds. Front. Microbiol. 2016, 7, 119. [Google Scholar] [CrossRef]
- Haddas, R.; Meir, R.; Perk, S.; Horowitz, I.; Lapin, E.; Rosenbluth, E.; Lublin, A. Newcastle disease virus in little owls (Athene noctua) and African penguins (Spheniscus demersus) in an Israeli Zoo. Transbound. Emerg. Dis. 2014, 61, e79–e82. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.; Löndt, B.; Dimitrov, K.M.; Lewis, N.; van Boheemen, S.; Fouchier, R.; Brown, I. An epizootiological report of the re-emergence and spread of a lineage of virulent Newcastle disease virus into Eastern Europe. Transbound. Emerg. Dis. 2017, 64, 1001–1007. [Google Scholar] [CrossRef]
- Omony, J.B.; Wanyana, A.; Mugimba, K.K.; Kirunda, H.; Nakavuma, J.L.; Otim-Onapa, M.; Byarugaba, D.K. Epitope peptide-based predication and other functional regions of antigenic F and HN proteins of waterfowl and poultry avian avulavirus serotype-1 isolates from Uganda. Front. Vet. Sci. 2021, 8, 551. [Google Scholar] [CrossRef] [PubMed]
- Orabi, A.; Hussein, A.; Saleh, A.A.; El-Magd, M.A.; Munir, M. Evolutionary insights into the fusion protein of Newcastle disease virus isolated from vaccinated chickens in 2016 in Egypt. Arch. Virol. 2017, 162, 3069–3079. [Google Scholar] [CrossRef]
- Chong, Y.L.; Padhi, A.; Hudson, P.J.; Poss, M. The effect of vaccination on the evolution and population dynamics of avian paramyxovirus 1. PLoS Pathog. 2010, 6, e1000872. [Google Scholar] [CrossRef]
- Ke, G.M.; Yu, S.W.; Ho, C.H.; Chu, P.Y.; Ke, L.Y.; Lin, K.H.; Tsai, Y.C.; Liu, H.J.; Lin, M.Y. Characterization of newly emerging Newcastle disease viruses isolated during 2002-2008 in Taiwan. Virus. Res. 2010, 147, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.M.; Tan, L.T.; Xu, H.Y.; Ma, B.C.; Wang, Y.L.; Yuan, X.Y.; Liu, W.J. Pathotypical characterization and molecular epidemiology of Newcastle disease virus isolates from different hosts in China from 1996 to 2005. J. Clin. Microbiol. 2008, 46, 601–611. [Google Scholar] [CrossRef] [PubMed]
- McGinnes, L.W.; Sergel, T.; Chen, H.; Hamo, L.; Schwertz, S.; Li, D.; Morrison, T.G. Mutational analysis of the membrane proximal heptad repeat of the Newcastle disease virus fusion protein. Virology 2001, 289, 343–352. [Google Scholar] [CrossRef]
- McGinnes, L.W.; Pantua, H.; Reitter, J.; Morrison, T.G. Newcastle disease virus: Propagation, quantification, and storage. Curr. Protoc. Microbiol. 2006, 1, 15F.2.11–15F.2.18. [Google Scholar] [CrossRef]
- Okpe, G.C.; Ezema, W.S.; Shoyinka, S.V.O.; Okoye, J.O.A. Vitamin A dietary supplementation reduces the mortality of velogenic Newcastle disease significantly in cockerels. Int. J. Exp. Pathol. 2015, 96, 326–331. [Google Scholar] [CrossRef]
- Selim, K.M.; Selim, A.; Arafa, A.; Hussein, H.A.; Elsanousi, A.A. Molecular characterization of full fusion protein (F) of Newcastle disease virus genotype VIId isolated from Egypt during 2012–2016. Vet. World 2018, 11, 930–938. [Google Scholar] [CrossRef] [PubMed]
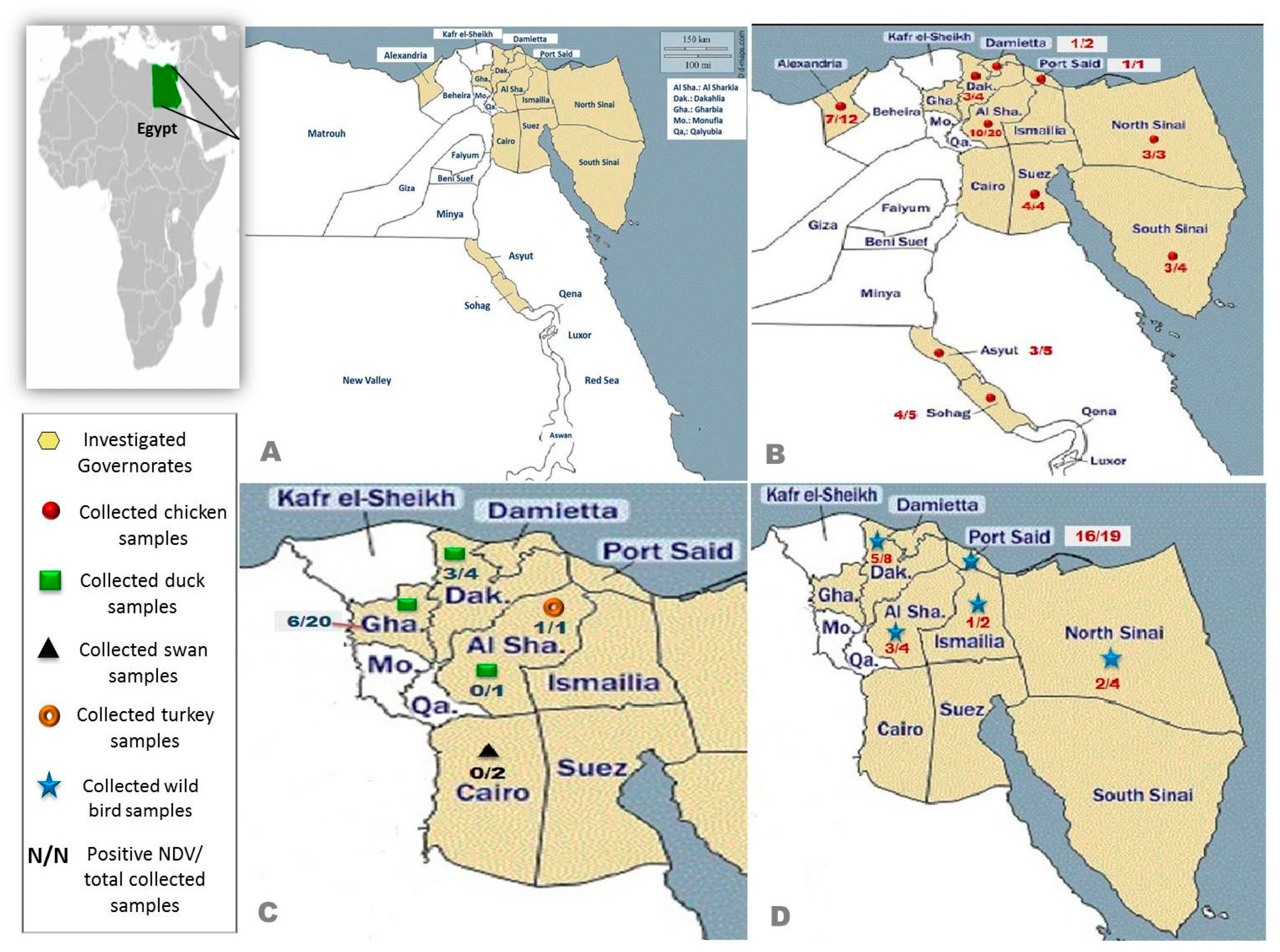
| Species | Breed | No. of Flocks | Age/Day | Type of Poultry Flock | Location |
|---|---|---|---|---|---|
| Chickens | Cobb | 25 | 23–45 | Broiler | Sharkia, Dakahlia, Sohag, Alexandria, Assuit, North and South Sinai |
| Sasso | 3 | 40–310 | Broiler/Layer | Dakahlia, Sohag, Damietta | |
| Avian 48 | 3 | 30–33 | Broiler | Suez, South Sinai | |
| Hay-line | 2 | 200 | Layer | Dakahlia | |
| Ross | 4 | 28–35 | Broiler | Alexandria, North Sinai | |
| Indian river | 3 | 32–48 | Broiler | Suez, North Sinai | |
| Bovanes | 5 | 210–252 | Layer | Sharkia, Alexandria | |
| Hubbard | 2 | 27–29 | Broiler | Damietta, Port-Said | |
| Lehman | 2 | 200–330 | Layer | South Sinai, Alexandria | |
| Isapapcoack | 2 | 100 | Layer | Sharkia | |
| Baladi | 9 | 20–40 | Native | Sharkia, Sohag, Assuit | |
| Ducks | Muscovy | 17 | 25–360 | Broiler/Breeder | Dakahlia, Gharbia |
| Mallard | 5 | 187–622 | Broiler/Breeder | Dakahlia, Gharbia | |
| Pekin | 16 | 30–700 | Broiler | Sharkia, Dakahlia, Gharbia | |
| Turkeys | Converter | 1 | 47 | Sharkia | |
| Pelican | Pelecanus crispus | 2 | 4320–5400 | Cairo |
| Species | Common Name | Number of Birds | Collection Date (Month/Year) | Location |
|---|---|---|---|---|
| Corvus cornix | Hooded crow | 8 | 4, 8, 10/2019 | Sharkia, Port-Said, Dakahlia |
| Larus canus | Common gull | 1 | 3/2019 | Port-Said |
| Numenius minutus | Little curlew | 1 | 3/2019 | Port-Said |
| Coturnix ypsilophora | Brown quail | 3 | 4/2019 | North Sinai |
| Streptopelia turtur | European turtle dove | 3 | 4, 10/2019 | Port-Said, Sharkia |
| Nycticorax nycticorax | Black-crowned night heron | 2 | 10/2019 | Port-Said |
| Gallinula chloropus | Common moorhen | 2 | 10/2019 | Port-Said |
| Bubulcus ibis | Cattle egret | 8 | 8, 10/2019 | Port-Said, Ismailia, Dakahlia |
| Anas platyrhynchos | Mallard duck | 1 | 7/2019 | North Sinai |
| Ardenna pacifica | Wedge-tailed shearwater | 1 | 3/2019 | Port-Said |
| Spatula clypeata | Northern Shoveler | 2 | 3/2019 | Port-Said |
| Ardea cinerea | Grey heron | 2 | 3/2019 | Port-Said |
| Porphyrio madagascariensis | Purple gallinule (African swamphen) | 2 | 3, 4/2019 | Port-Said |
| Anthus rubescens | American pipit | 1 | 4/2019 | Port-Said |
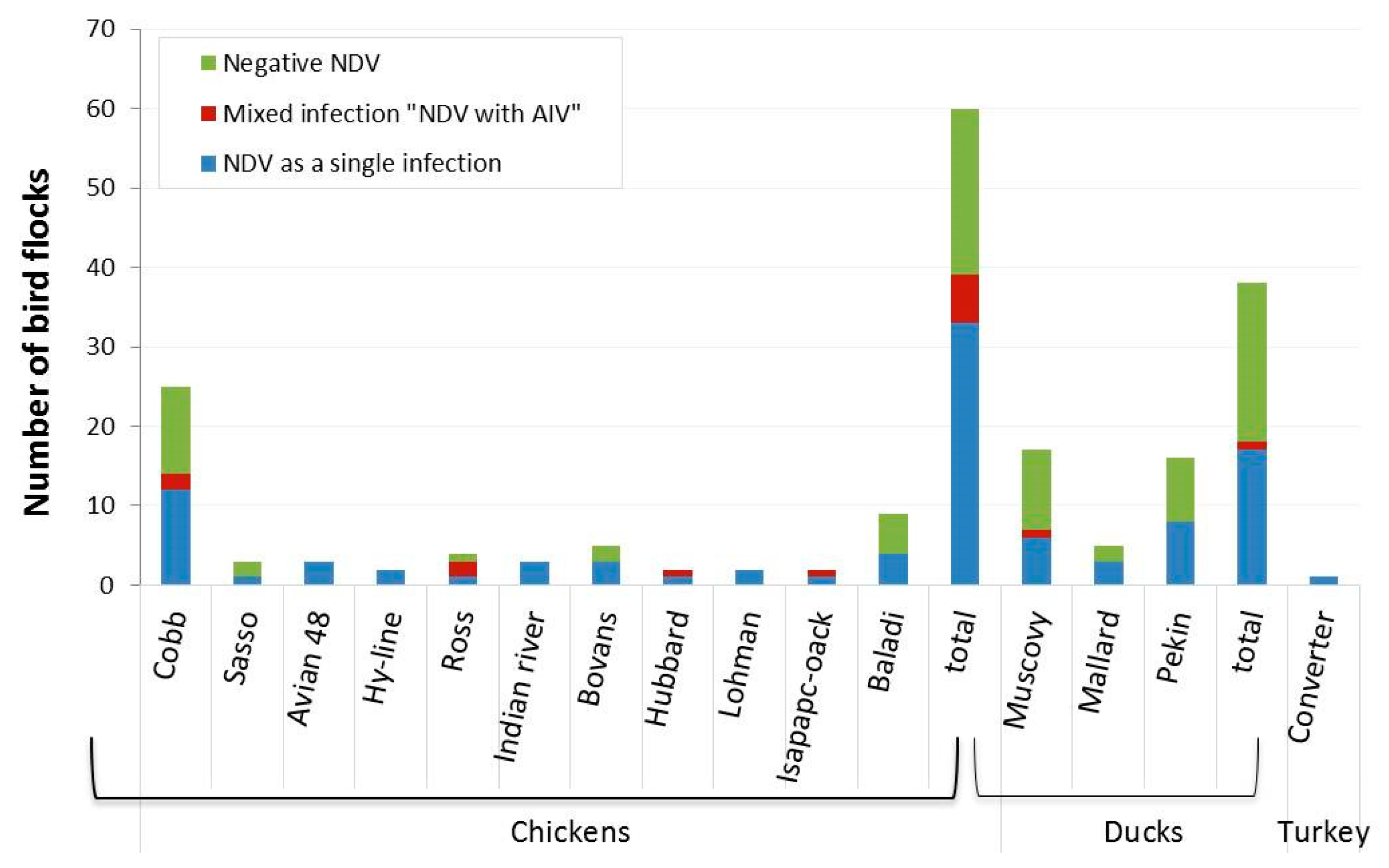
| Breeds | No. of Flocks | Detected Virus(es) | |||
|---|---|---|---|---|---|
| No of Positive NDV (%) | 95% CI | No of Positive AIV (%) | 95% CI | ||
| Chickens | |||||
| Cobb | 25 | 14 (56) | 34.9–75.6 | 4 (16) | 4.54–36.08 |
| Sasso | 3 | 1 (33.3) | 0.84–90.57 | 0 | 0 |
| Avian 48 | 3 | 3 (100) | 29.24–1.00 | 0 | 0 |
| Hy-line | 2 | 2 (100) | 15.81–1.00 | 0 | 0 |
| Ross | 4 | 3 (75) | 19.41–99.37 | 2 (50) | 6.76–93.24 |
| Indian river | 3 | 3 (100) | 29.24–1.00 | 0 | 0 |
| Bovans | 5 | 3 (60) | 14.66–94.73 | 1 (20) | 0.51–71.64 |
| Hubbard | 2 | 2 (100) | 15.81–1.00 | 1 (50) | 1.26–98.74 |
| Lohman | 2 | 2 (100) | 15.81–1.00 | 0 | 0 |
| Isapapcoack | 2 | 2 (100) | 15.81–1.00 | 1 (50) | 1.26–98.74 |
| Baladi | 9 | 4 (44.4) | 13.70–78.80 | 0 | 0 |
| Total | 60 | 39 (65) | 51.60–76.87 | 9 (15) | 7.10–26.57 |
| Ducks | |||||
| Muscovy | 17 | 7 (41.2) | 18.44–67.08 | 3 (17.6) | 3.80–43.43 |
| Mallard | 5 | 3 (60) | 14.66–94.73 | 0 | 0 |
| Pekin | 16 | 8 (50) | 24.65–75.35 | 1 (6.3) | 0.16–30.23 |
| Total | 38 | 18 (47.4) | 30.97–64.19 | 4 (10.5) | 2.94–24.80 |
| Turkeys | |||||
| Converter | 1 | 1 (100) | 2.50–1.00 | 0 | 0 |
| Pelicans | |||||
| Pelican crispus | 2 | 0 | 0 | 0 | 0 |
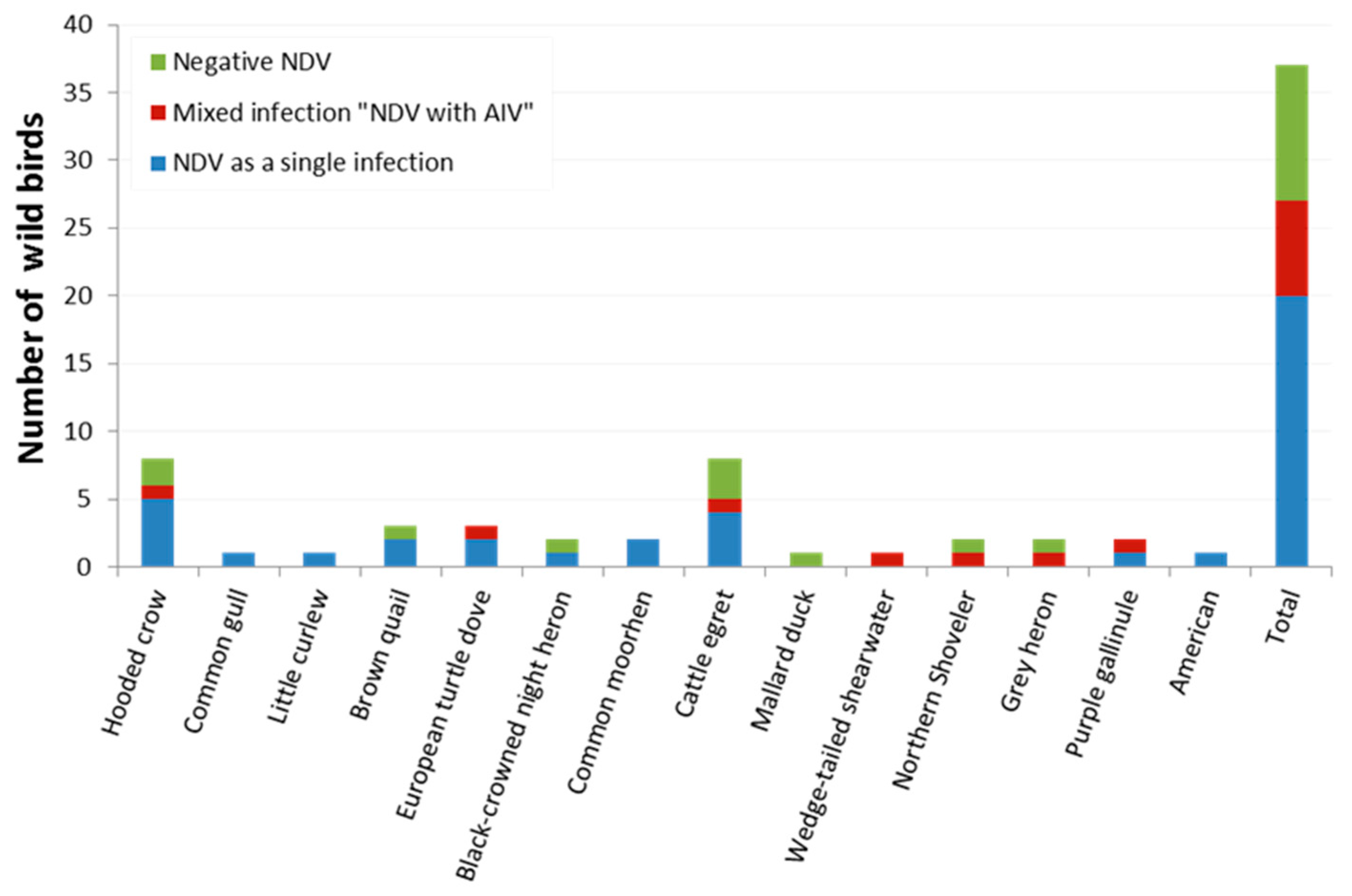
| Species | Common Name | No. of Birds | Detected Virus(es) | |||
|---|---|---|---|---|---|---|
| No. of Positive NDV (%) | 95% CI | No. of Positive AIV (%) | 95% CI | |||
| Corvus cornix | Hooded crow | 8 | 6 (75) | 34.91–96.61 | 1 (12.5) | 0.32–52.65 |
| Larus canus | Common gull | 1 | 1 (100) | 2.50–1.00 | 0 | 0 |
| Numenius minutus | Little curlew | 1 | 1(100) | 2.50–1.00 | 0 | 0 |
| Coturnix ypsilophora | Brown quail | 3 | 2 (66.7) | 9.43–99.16 | 0 | 0 |
| Streptopelia turtur | European turtle dove | 3 | 3 (100) | 29.24–1.00 | 1 (33.33) | 0.84–90.57 |
| Nycticorax nycticorax | Black-crowned night heron | 2 | 1 (50) | 1.26–98.74 | 0 | 0 |
| Gallinula chloropus | Common moorhen | 2 | 2 (100) | 15.81–1.00 | 0 | 0 |
| Bubulcus ibis | Cattle egret | 8 | 5 (62.5) | 24.49–91.48 | 1 (12.5) | 0.32–52.65 |
| Anas platyrhynchos | Mallard duck | 1 | 0 | 0 | 0 | 0 |
| Ardenna pacifica | Wedge-tailed shearwater | 1 | 1 (100) | 2.50–1.00 | 1 (100) | 2.50–1.00 |
| Spatula clypeata | Northern shoveler | 2 | 1 (50) | 1.26–98.74 | 1 (50) * | 1.26–98.74 |
| Ardea cinerea | Grey heron | 2 | 1 (50) | 1.26–98.74 | 1 (50) * | 1.26–98.74 |
| Porphyrio madagascariensis | Purple gallinule (African swamphen) | 2 | 2 (100) | 15.81–1.00 | 1 (50) | 1.26–98.74 |
| Anthus rubescens | American pipit | 1 | 1 (100) | 2.50–1.00 | 0 | 0 |
| Total | 37 | 27 (72.97) | 55.88–86.21 | 7 (18.9) | 7.96–35.16 | |
| Name of Isolates | Breed | Locality | Descriptive Data | Sequencing | Accession Number | ||||
|---|---|---|---|---|---|---|---|---|---|
| Date | Age/Days | Flock Density | Mortality Rate/ 3 Days | Clinical Finding | |||||
| NDV/Chicken/Egypt/NOR/ZU-NM76/2019 | Ross (Domestic) | North Sinai | 03/2019 | 33 | 14,000 | 600 | Respiratory signs, greenish diarrhoea, septicaemia | Approx. 99% genome coverage (103 nucleotides gap), Full F gene | OP219678 |
| NDV/Chicken/Egypt/ALEX/ZU-NM97/2019 | Ross (Domestic) | Alexandria | 05/2019 | 30 | 5000 | 62 | Respiratory signs, greenish diarrhoea, haemorrhages in caecal tonsils and proventriculus | Approx. 90% genome coverage, Full F gene | OP219679 |
| NDV/Chicken/Egypt/ALEX/ZU-NM99/2019 | Ross (Domestic) | Alexandria | 05/2019 | 25 | 6000 | 85 | Respiratory signs, greenish diarrhoea, haemorrhages in caecal tonsils and proventriculus | Approx. 99% genome coverage (150 nucleotides gap), Full F gene | OP219680 |
| NDV/Duck/Egypt/DAK/ZU-NM09/2019 | Mallard (Domestic) | Dakahlia | 03/2019 | 300 | 3300 | 185 | Nervous signs, greenish diarrhoea, congestion in parenchymatous organs | Approx. 99% genome coverage (153 nucleotides gap), Full F gene | OP219681 |
| NDV/Black-crowned night heron/Egypt/POR/ZU-NM85/2019 | Migratory (Wild Bird) | Port-Said | 09/2019 | N/A | N/A | N/A | Apparently healthy without PM lesions | Approx. 90% genome coverage, Full F gene | OP219682 |
| NDV/Duck/Egypt/DAK/ZU-NM54/2019 | Muscovy (Domestic) | Dakahlia | 03/2019 | 60 | 4000 | 113 | Greenish diarrhoea, septicaemia | Approx. 70% genome coverage (3918 nucleotide gap), Partial F gene | OP219683 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eid, A.A.M.; Hussein, A.; Hassanin, O.; Elbakrey, R.M.; Daines, R.; Sadeyen, J.-R.; Abdien, H.M.F.; Chrzastek, K.; Iqbal, M. Newcastle Disease Genotype VII Prevalence in Poultry and Wild Birds in Egypt. Viruses 2022, 14, 2244. https://doi.org/10.3390/v14102244
Eid AAM, Hussein A, Hassanin O, Elbakrey RM, Daines R, Sadeyen J-R, Abdien HMF, Chrzastek K, Iqbal M. Newcastle Disease Genotype VII Prevalence in Poultry and Wild Birds in Egypt. Viruses. 2022; 14(10):2244. https://doi.org/10.3390/v14102244
Chicago/Turabian StyleEid, Amal A. M., Ashraf Hussein, Ola Hassanin, Reham M. Elbakrey, Rebecca Daines, Jean-Remy Sadeyen, Hanan M. F. Abdien, Klaudia Chrzastek, and Munir Iqbal. 2022. "Newcastle Disease Genotype VII Prevalence in Poultry and Wild Birds in Egypt" Viruses 14, no. 10: 2244. https://doi.org/10.3390/v14102244
APA StyleEid, A. A. M., Hussein, A., Hassanin, O., Elbakrey, R. M., Daines, R., Sadeyen, J.-R., Abdien, H. M. F., Chrzastek, K., & Iqbal, M. (2022). Newcastle Disease Genotype VII Prevalence in Poultry and Wild Birds in Egypt. Viruses, 14(10), 2244. https://doi.org/10.3390/v14102244








