Comparative Upper Respiratory Tract Transcriptomic Profiling Reveals a Potential Role of Early Activation of Interferon Pathway in Severe COVID-19
Abstract
1. Introduction
2. Materials and Methods
3. Quantification and Statistical Analysis
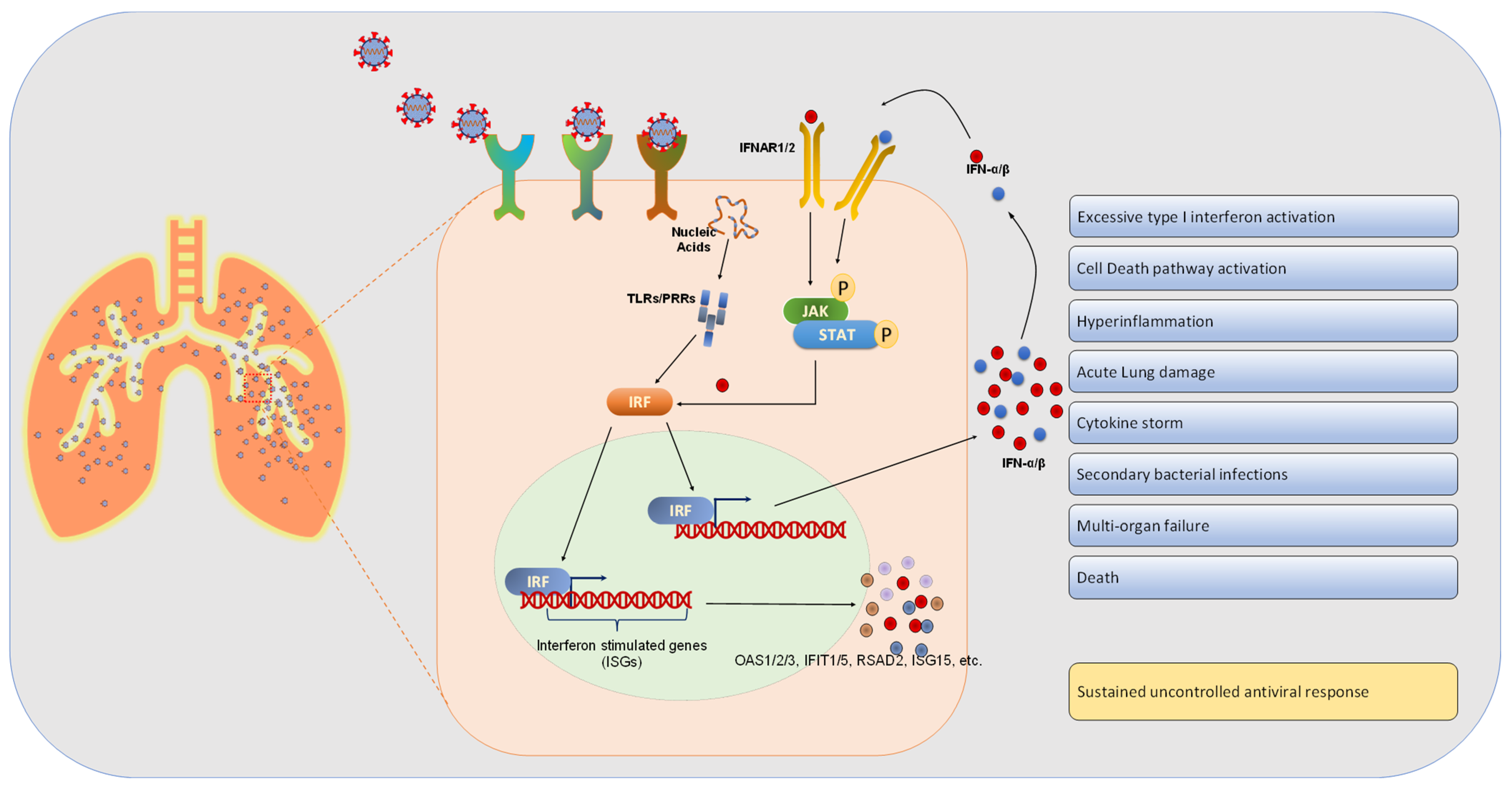
4. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Cheng, Z.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Baßler, K.; Schlickeiser, S.; Zhang, B.; Krämer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 2020, 182, 1419–1440.e23. [Google Scholar] [CrossRef] [PubMed]
- Wilk, A.J.; Rustagi, A.; Zhao, N.Q.; Roque, J.; Martínez-Colón, G.J.; McKechnie, J.L.; Ivison, G.T.; Ranganath, T.; Vergara, R.; Hollis, T.; et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020, 26, 1070–1076. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, 6515. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Mick, E.; Kamm, J.; Pisco, A.O.; Ratnasiri, K.; Babik, J.M.; Castañeda, G.; DeRisi, J.L.; Detweiler, A.M.; Hao, S.L.; Kangelaris, K.N.; et al. Upper airway gene expression reveals suppressed immune responses to SARS-CoV-2 compared with other respiratory viruses. Nat. Commun. 2020, 11, 5854. [Google Scholar] [CrossRef]
- Sanders, J.M.; Monogue, L.M.; Jodlowski, Z.T.; Cutrell, B.J. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Yang, Y.; Dai, H.; Xiong, Y.; Wang, J.Q.; Lin, L.; Chen, Z.S. Recent Updates in Experimental Research and Clinical Evaluation on Drugs for COVID-19 Treatment. Front. Pharmacol. 2021, 12, 732403. [Google Scholar] [CrossRef] [PubMed]
- Barlow, A.; Landolf, K.M.; Barlow, B.; Yeung, S.; Heavner, J.J.; Claassen, C.W.; Heavner, M.S. Review of Emerging Pharmacotherapy for the Treatment of Coronavirus Disease 2019. Pharmacotherapy 2020, 40, 416–437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kilian, C.; Turner, J.E.; Bosurgi, L.; Roedl, K.; Bartsch, P.; Gnirck, A.C.; Cortesi, F.; Schultheiß, C.; Hellmig, M.; et al. Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci. Immunol. 2021, 6, eabf6692. [Google Scholar] [CrossRef]
- Trinchieri, G.; Type I interferon: Friend or foe?, J. Type I interferon: Friend or foe? J. Exp. Med. 2010, 207, 2053–2063. [Google Scholar] [CrossRef]
- McClain, M.T.; Constantine, F.J.; Henao, R.; Liu, Y.; Tsalik, E.L.; Burke, T.W.; Steinbrink, J.M.; Petzold, E.; Nicholson, B.P.; Rolfe, R.; et al. Dysregulated transcriptional responses to SARS-CoV-2 in the periphery. Nat. Commun. 2021, 12, 1079. [Google Scholar] [CrossRef]
- Tsalik, E.L.; Henao, R.; Nichols, M.; Burke, T.; Ko, E.R.; McClain, M.T.; Hudson, L.L.; Mazur, A.; Freeman, D.H.; Veldman, T.; et al. Host gene expression classifiers diagnose acute respiratory illness etiology. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Maggi, E.; Canonica, G.W.; Moretta, L. COVID-19: Unanswered questions on immune response and pathogenesis. J. Allergy Clin. Immunol. 2020, 146, 18–22. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Hornung, V.; Hartmann, R.; Ablasser, A.; Hopfner, K.-P. OAS proteins and cGAS: Unifying concepts in sensing and responding to cytosolic nucleic acids. Nat. Rev. Immunol. 2014, 14, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.L.; Conn, G.L. RNA regulation of the antiviral protein 2'-5'-oligoadenylate synthetase. Wiley Interdiscip. Rev. RNA 2019, 10, e1534. [Google Scholar] [CrossRef] [PubMed]
- Danziger, O.; Patel, R.S.; DeGrace, E.; Rosen, M.R.; Rosenberg, B.R. Inducible CRISPR activation screen for interferon-stimulated genes identifies OAS1 as a SARS-CoV-2 restriction factor. PLoS Pathog. 2022, 18, e1010464. [Google Scholar] [CrossRef] [PubMed]
- Zaas, A.K.; Chen, M.; Varkey, J.; Veldman, T.; Hero, A.O., 3rd; Lucas, J.; Huang, Y.; Turner, R.; Gilbert, A.; Lambkin-Williams, R.; et al. Gene expression signatures diagnose influenza and other symptomatic respiratory viral infections in humans. Cell Host Microbe 2009, 6, 207–217. [Google Scholar] [CrossRef]
- Spiegel, M.; Pichlmair, A.; Martínez-Sobrido, L.; Cros, J.; García-Sastre, A.; Haller, O.; Weber, F. Inhibition of Beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 2005, 79, 2079–2165. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Lo Tartaro, D.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y.; Cao, L.; Wang, D.; Guo, M.; Jiang, A.; Guo, D.; Hu, W.; Yang, J.; Tang, Z.; et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 761–770. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef]
- Israelow, B.; Song, E.; Mao, T.; Lu, P.; Meir, A.; Liu, F.; Alfajaro, M.M.; Wei, J.; Dong, H.; Homer, R.J.; et al. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J. Exp. Med. 2020, 217, e20201241. [Google Scholar] [CrossRef]

| Patient ID | Age | Gender | Smoking | Outcome | Comorbidity | Race | Clinical Symptoms at Presentation | Lowest O2 Saturation in ED | |
|---|---|---|---|---|---|---|---|---|---|
| Negative (N)-Group | Patient 1 | 55 | F | Former | Released | SLE with nephritis, CKD, CVA, seizure disorder, wheelchair bound | Non-Hispanic; Black | Dry cough, malaise, fatigue, chest pressure, mild dyspnea | NA |
| Patient 2 | 37 | F | Never | Released | Asthma | Non-Hispanic; White | Shortness of breath, coughing | NA | |
| Patient 3 | 71 | F | Never | Deceased | PBC cirrhosis s/p liver transplant | Non-Hispanic; White | Febrile neutropenia (concern for GVHD) | NA | |
| Patient 4 | 79 | M | Former | Released | Ischemic CMY, HTN, HLD, DM, CKD | Non-Hispanic; White | Cough, shortness of breath | NA | |
| Patient 5 | 78 | F | Former | Released | Dementia | Non-Hispanic; White | Fever, facial swelling | NA | |
| Patient 6 | 36 | F | Never | Released | Migraines, anxiety | Hispanic; White | Cough, chest tightness | NA | |
| Positive Not Admitted (A)-Group | Patient 7 | 51 | F | Never | Released | None | Non-Hispanic; White | Chest pain, palpitations | 99 |
| Patient 8 | 43 | F | Never | Released | Pregnant, None | Non-Hispanic; Black | Shortness of breath, chills, malaise/fatigue, cough, wheezing | 98 | |
| Patient 9 | 64 | M | Former | Released | HLD, HTN | Non-Hispanic; White | Fever, nasal congestion, sore throat | 94 | |
| Patient 10 | 28 | M | Never | Released | GAD, Depression, Obesity | Hispanic | Chest pain, coughing, wheezing, heartburn | 99 | |
| Patient 11 | 36 | F | Never | Released | None | Non-Hispanic; Asian | Fever, body aches, chills, loss of taste/smell, cough, shortness of breath, | 97 | |
| Patient 12 | 32 | M | Never | Released | None | Non-Hispanic | Subjective fever and chills | 100 | |
| Positive with Severe Symptoms (S)-Group | Patient 13 | 69 | M | Never | Deceased | GAD | Unknown | Chills, fevers, cough, shortness of breath | 86 on NRB 15L |
| Patient 14 | 73 | F | Never | Deceased | Quadriplegia, DM, large sacral wound present | Non-Hispanic; White | Fever, rhinorrhea, AMS | 97 | |
| Patient 15 | 59 | M | Never | Deceased | HTN | Non-Hispanic; Black | Shortness of breath, cough, fevers, abdominal pain | 92 on 3L nasal cannula | |
| Patient 16 | 47 | M | Never | Deceased | None | Non-Hispanic; Black | Fevers, myalgias, fatigue, headache, sore throat, shortness of breath | 95 | |
| Patient 17 | 87 | M | Never | Deceased | HTN, Dementia | Non-Hispanic; Black | Weakness, nausea, vomiting | 94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, S.A.; Shibata, T.; Leong, M.; Plummer, J.; Vail, E.; Khan, Z. Comparative Upper Respiratory Tract Transcriptomic Profiling Reveals a Potential Role of Early Activation of Interferon Pathway in Severe COVID-19. Viruses 2022, 14, 2182. https://doi.org/10.3390/v14102182
Bhat SA, Shibata T, Leong M, Plummer J, Vail E, Khan Z. Comparative Upper Respiratory Tract Transcriptomic Profiling Reveals a Potential Role of Early Activation of Interferon Pathway in Severe COVID-19. Viruses. 2022; 14(10):2182. https://doi.org/10.3390/v14102182
Chicago/Turabian StyleBhat, Shabir A., Tomohiro Shibata, Matthew Leong, Jasmine Plummer, Eric Vail, and Zakir Khan. 2022. "Comparative Upper Respiratory Tract Transcriptomic Profiling Reveals a Potential Role of Early Activation of Interferon Pathway in Severe COVID-19" Viruses 14, no. 10: 2182. https://doi.org/10.3390/v14102182
APA StyleBhat, S. A., Shibata, T., Leong, M., Plummer, J., Vail, E., & Khan, Z. (2022). Comparative Upper Respiratory Tract Transcriptomic Profiling Reveals a Potential Role of Early Activation of Interferon Pathway in Severe COVID-19. Viruses, 14(10), 2182. https://doi.org/10.3390/v14102182






