Pathogenicity of La Jolla Virus in Drosophila suzukii following Oral Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Husbandry
2.2. Cultivation and Extraction of LJV
2.3. Oral Infection of Flies
2.4. Oral Infection of Larvae
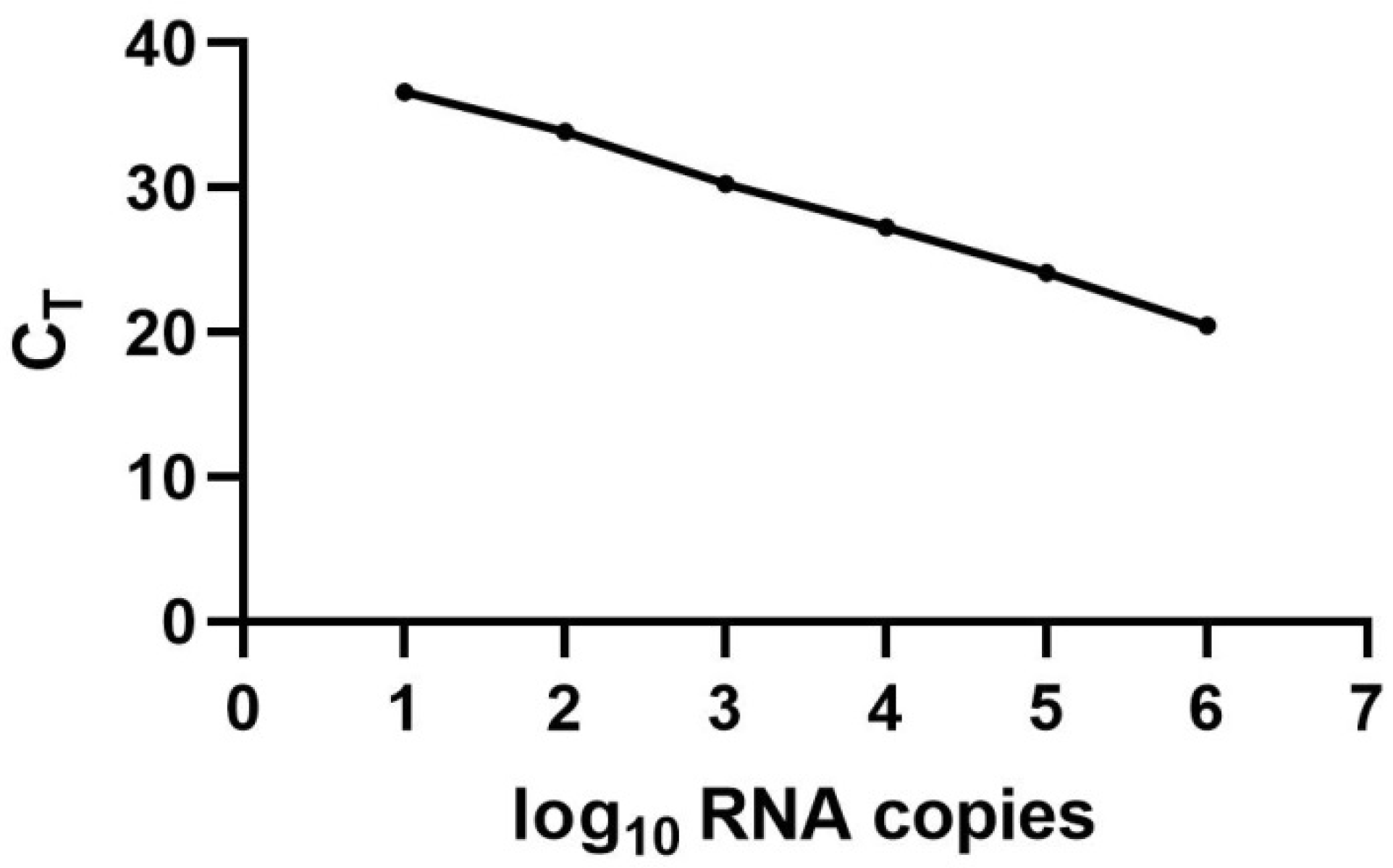
2.5. Standard Curve and Quantification of LJV
2.6. LJV Stability Test
2.7. Statistical Analysis
3. Results
3.1. Standard Curve for the Quantification of LJV Based on TaqMan qPCR
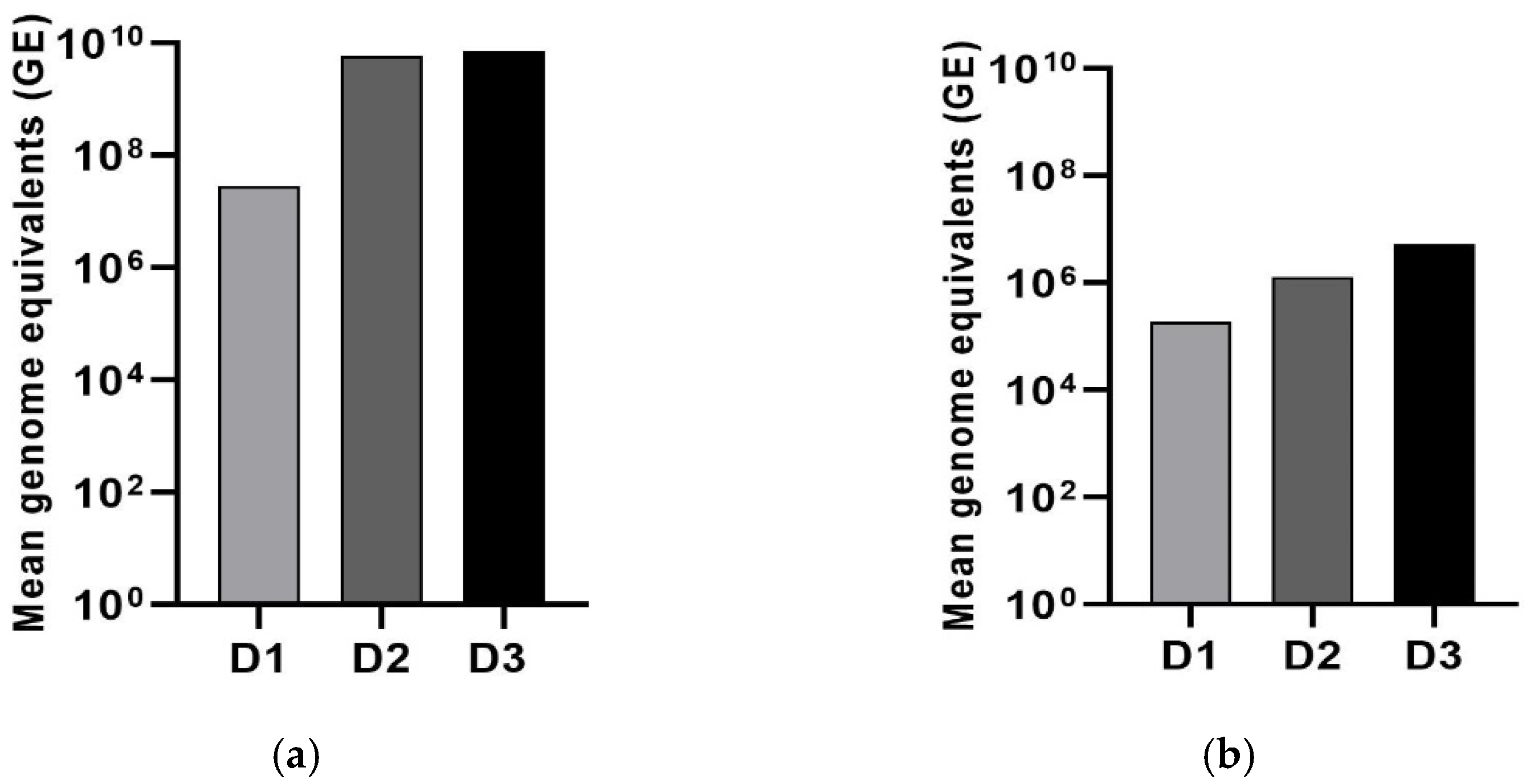
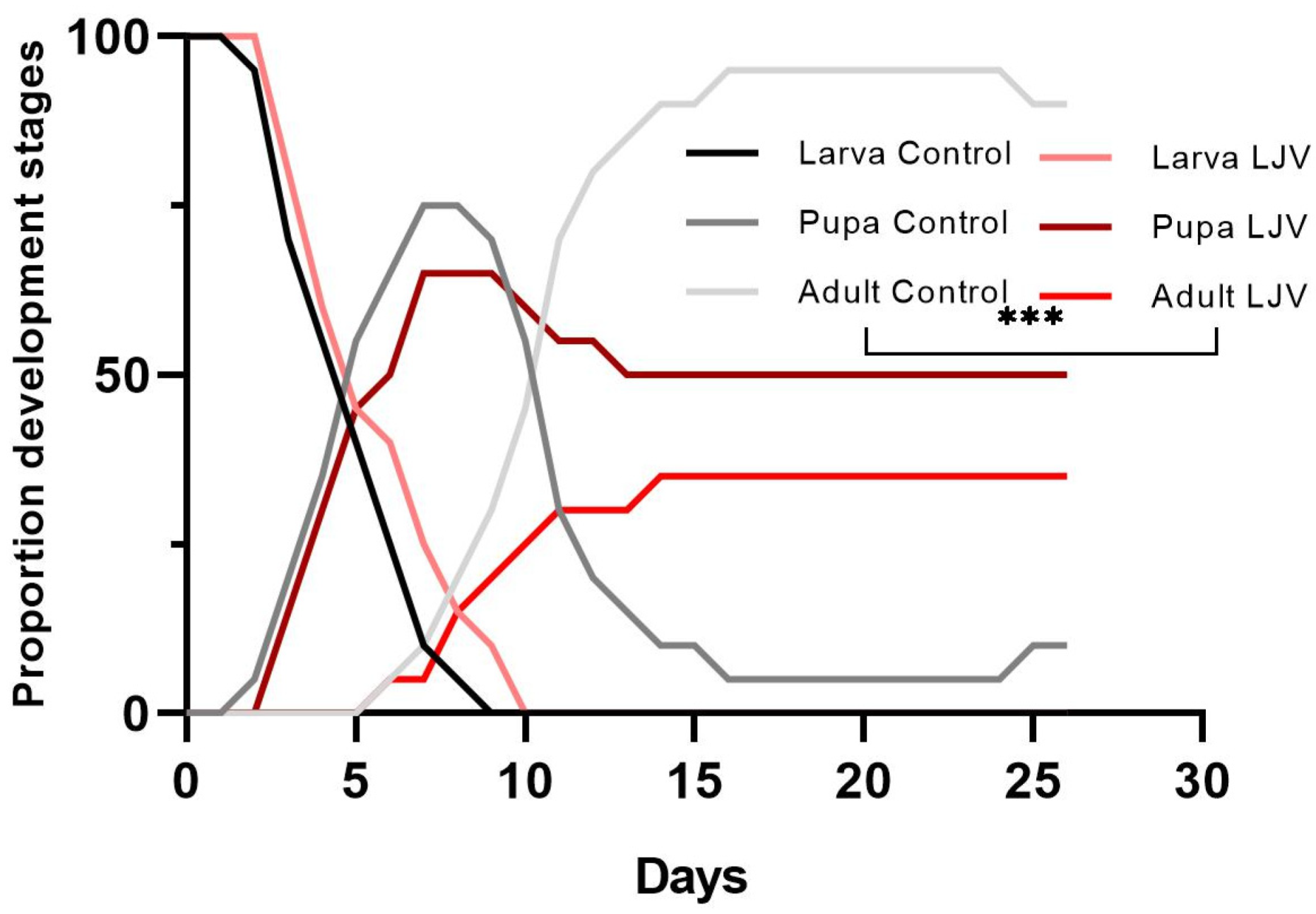
3.2. The Oral Administration of LJV Has Adverse Effects on Adult Flies and Larvae
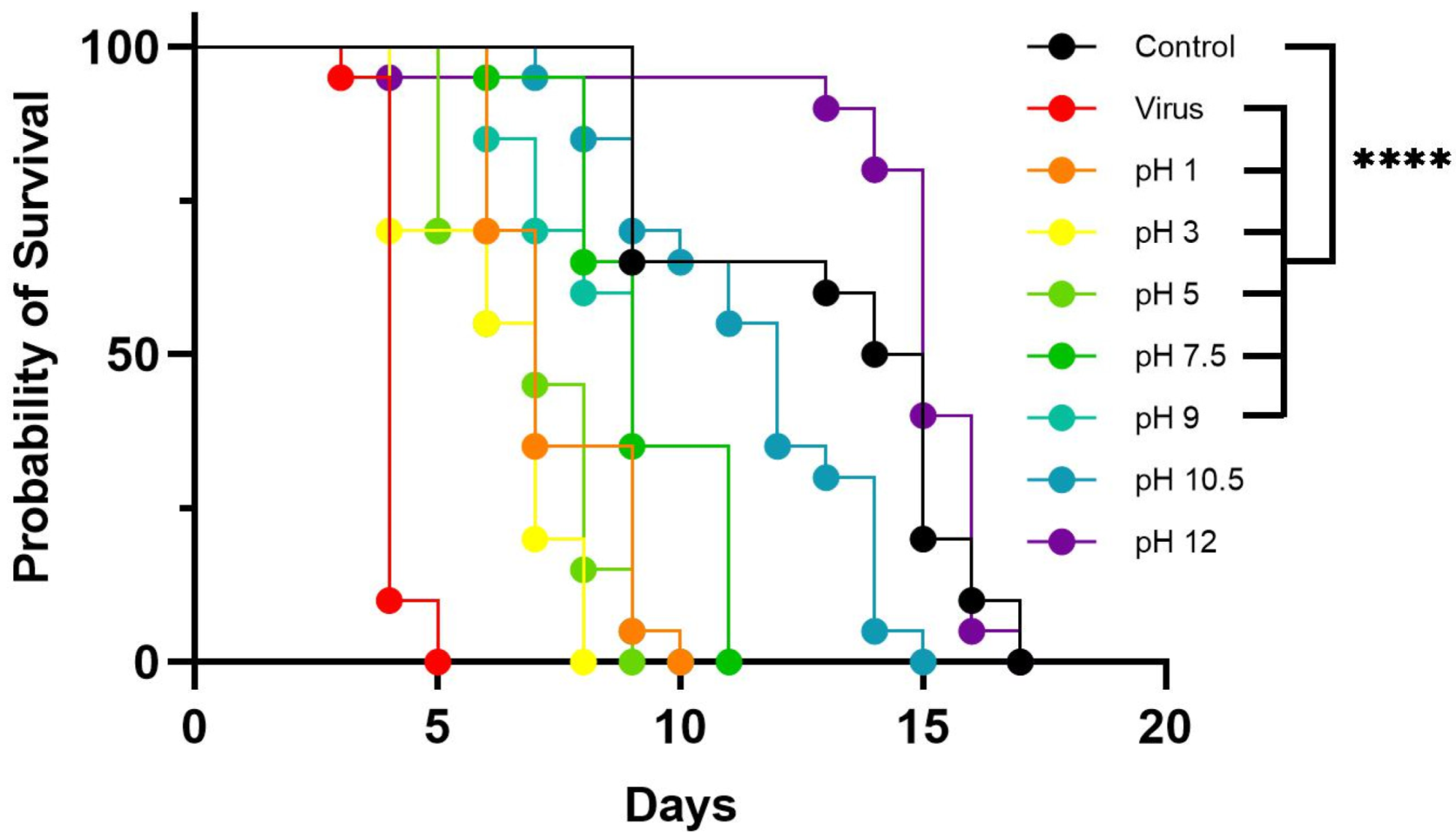
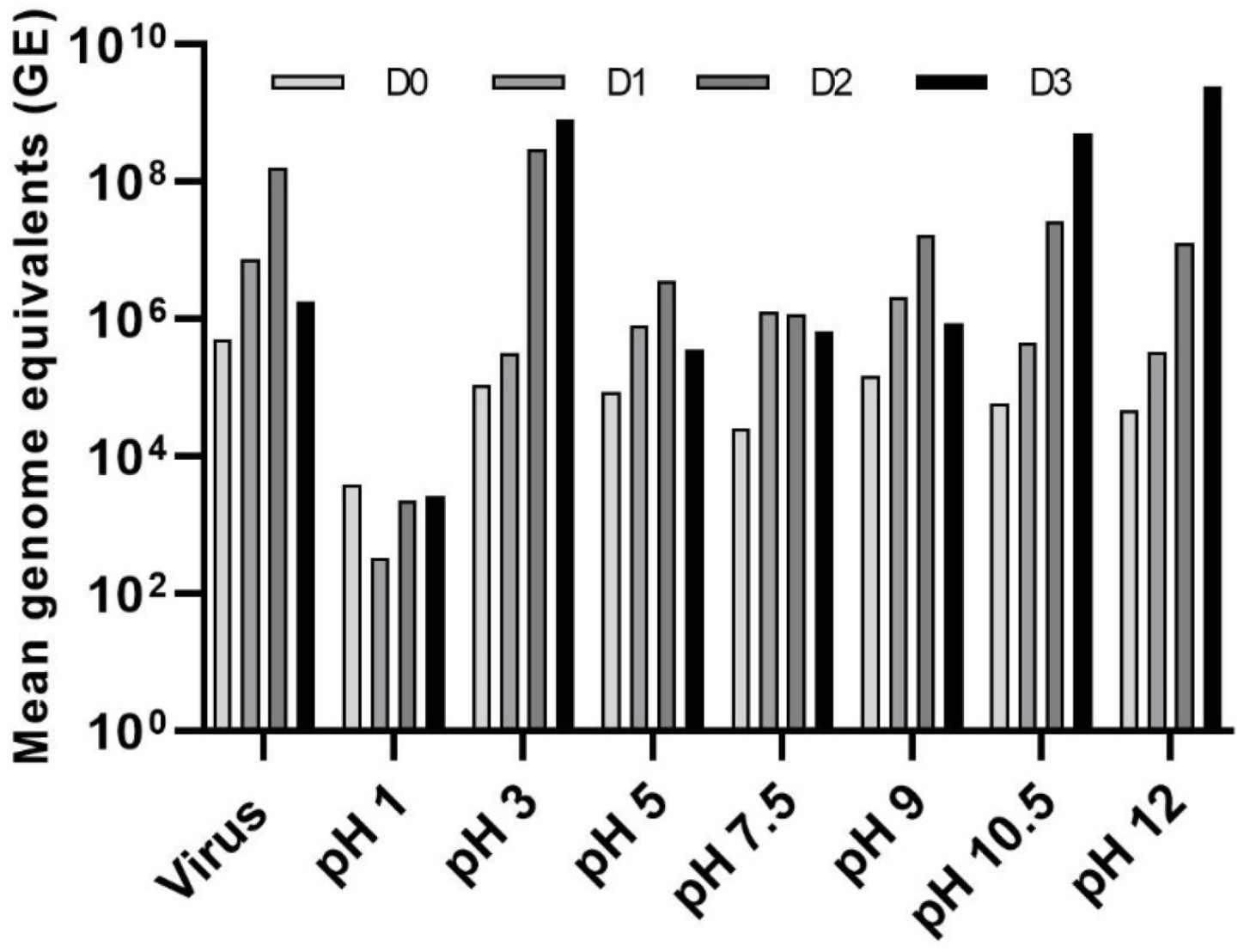
3.3. Effect of pH on LJV Pathogenicity
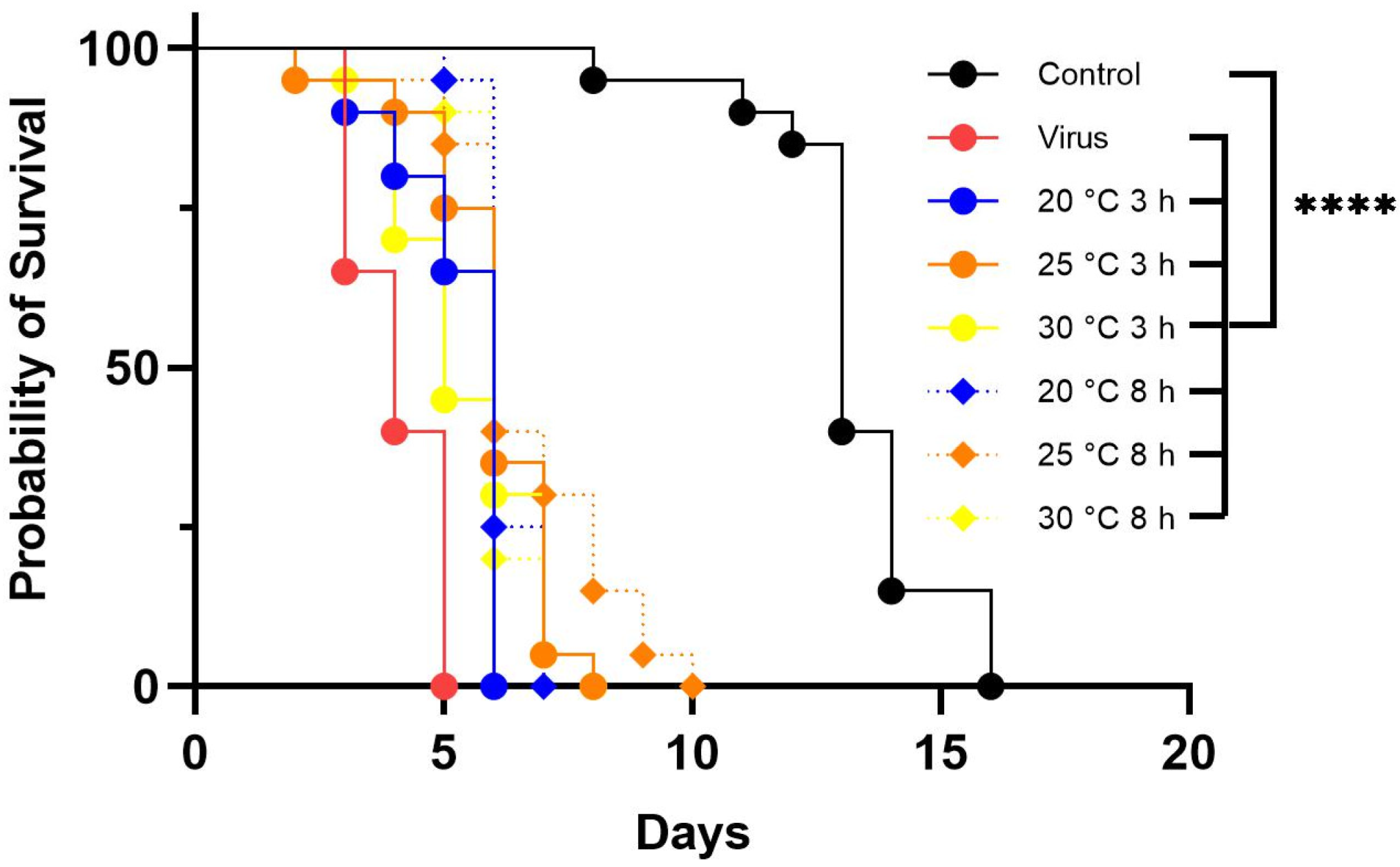
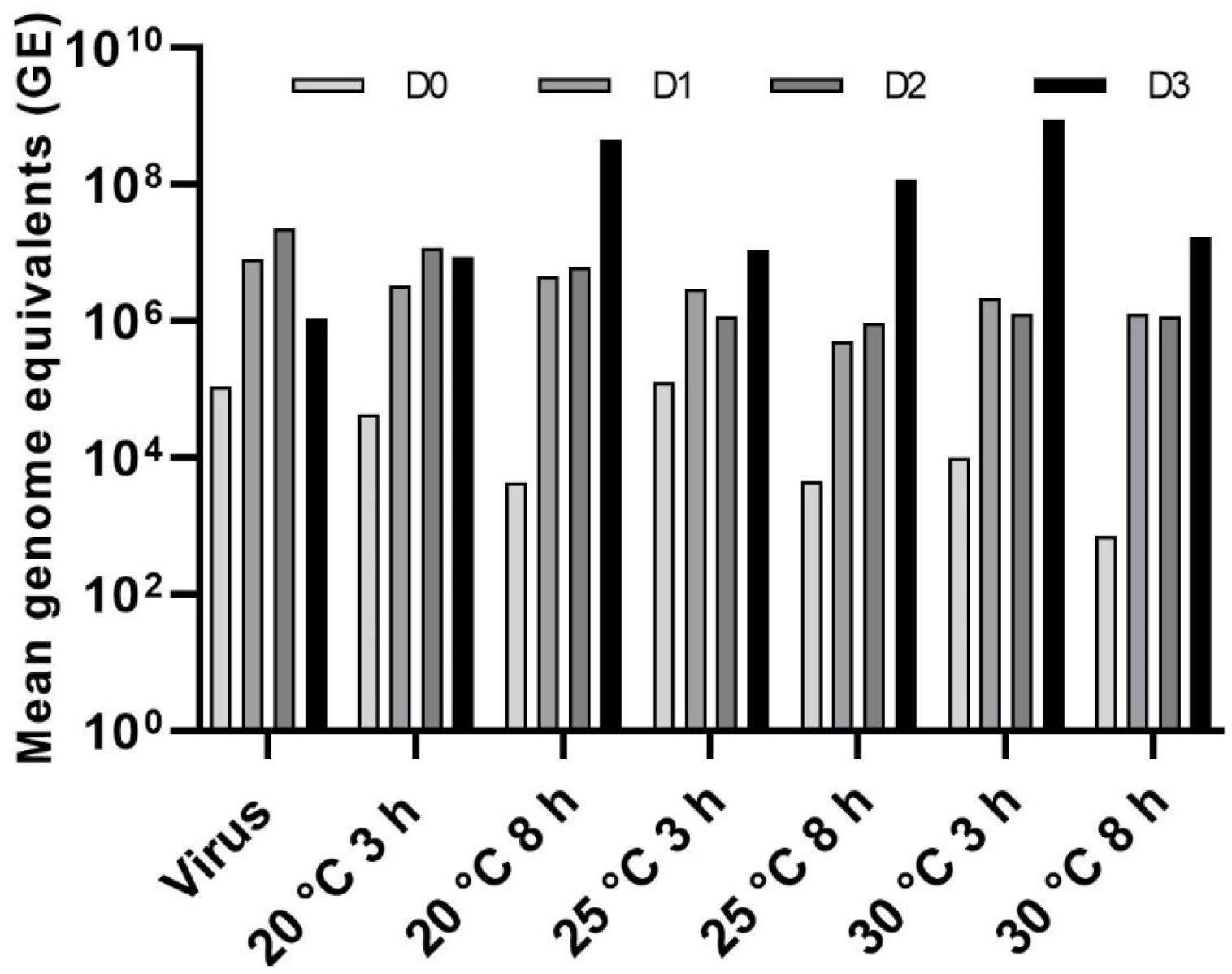
3.4. Effect of Temperature on LJV Pathogenicity
4. Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haubrock, P.J.; Turbelin, A.J.; Cuthbert, R.N.; Novoa, A.; Taylor, N.G.; Angulo, E.; Ballesteros-Mejia, L.; Bodey, T.W.; Capinha, C.; Diagne, C.; et al. Economic costs of invasive alien species across Europe. NeoBiota 2021, 67, 153–190. [Google Scholar] [CrossRef]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research Towards a Sustainable Integrated Pest Management Program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M. A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag. Sci. 2011, 67, 1352–1357. [Google Scholar] [CrossRef]
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectology 2012, 65, 149–160. [Google Scholar]
- Bolda, M.P.; Goodhue, R.E.; Zalom, F.G. Spotted Wing Drosophila: Potential Economic Impact of a Newly Established Pest. Univ. Calif. Giannini Found. Agric. Econ. 2010, 13, 5–8. [Google Scholar]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; van Steenwyk, R.A.; Yorgey, B.M. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef]
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The making of a pest: The evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. Biol. Sci. 2014, 281, 20132840. [Google Scholar] [CrossRef] [PubMed]
- Wiman, N.G.; Walton, V.M.; Dalton, D.T.; Anfora, G.; Burrack, H.J.; Chiu, J.C.; Daane, K.M.; Grassi, A.; Miller, B.; Tochen, S.; et al. Integrating temperature-dependent life table data into a matrix projection model for Drosophila suzukii population estimation. PLoS ONE 2014, 9, e106909. [Google Scholar] [CrossRef]
- Tochen, S.; Woltz, J.M.; Dalton, D.T.; Lee, J.C.; Wiman, N.G.; Walton, V.M. Humidity affects populations of Drosophila suzukii (Diptera: Drosophilidae) in blueberry. J. Appl. Entomol. 2016, 140, 47–57. [Google Scholar] [CrossRef]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Paul DeBach, D.R. Biological Control by Natural Enemies, 2nd ed.; Cambridge University Press: Cambridge, UK, 1991; ISBN 0521391911. [Google Scholar]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Bundesamt für Verbraucherschutzmittel und Lebensmittelsicherheit. Zugelassene Pflanzenschutzmittel: Auswahl für den ökologischen Landbau nach der Durhführungverordnung (EU) 2021/1165; Bundesamt für Verbraucherschutzmittel und Lebensmittelsicherheit: Braunschweig, Germany, 2021.
- Schetelig, M.F.; Lee, K.-Z.; Otto, S.; Talmann, L.; Stökl, J.; Degenkolb, T.; Vilcinskas, A.; Halitschke, R. Environmentally sustainable pest control options for Drosophila suzukii. J. Appl. Entomol. 2018, 142, 3–17. [Google Scholar] [CrossRef]
- Berling, M.; Blachere-Lopez, C.; Soubabere, O.; Lery, X.; Bonhomme, A.; Sauphanor, B.; Lopez-Ferber, M. Cydia pomonella granulovirus genotypes overcome virus resistance in the codling moth and improve virus efficiency by selection against resistant hosts. Appl. Environ. Microbiol. 2009, 75, 925–930. [Google Scholar] [CrossRef]
- Lee, K.-Z.; Vilcinskas, A. Analysis of virus susceptibility in the invasive insect pest Drosophila suzukii. J. Invertebr. Pathol. 2017, 148, 138–141. [Google Scholar] [CrossRef]
- Carrau, T.; Hiebert, N.; Vilcinskas, A.; Lee, K.-Z. Identification and characterization of natural viruses associated with the invasive insect pest Drosophila suzukii. J. Invertebr. Pathol. 2018, 154, 74–78. [Google Scholar] [CrossRef]
- Carrau, T.; Lamp, B.; Reuscher, C.M.; Vilcinskas, A.; Lee, K.-Z. Organization of the Structural Protein Region of La Jolla Virus Isolated from the Invasive Pest Insect Drosophila suzukii. Viruses 2021, 13, 740. [Google Scholar] [CrossRef]
- Webster, C.L.; Waldron, F.M.; Robertson, S.; Crowson, D.; Ferrari, G.; Quintana, J.F.; Brouqui, J.-M.; Bayne, E.H.; Longdon, B.; Buck, A.H.; et al. The Discovery, Distribution, and Evolution of Viruses Associated with Drosophila melanogaster. PLoS Biol. 2015, 13, e1002210. [Google Scholar] [CrossRef] [PubMed]
- Medd, N.C.; Fellous, S.; Waldron, F.M.; Xuéreb, A.; Nakai, M.; Cross, J.V.; Obbard, D.J. The Virome of Drosophila suzukii, an Invasive Pest of Soft Fruit. Virus Evol. 2018, 4, vey009. [Google Scholar] [CrossRef]
- Jakobs, R.; Gariepy, T.D.; Sinclair, B.J. Adult plasticity of cold tolerance in a continental-temperate population of Drosophila suzukii. J. Insect Physiol. 2015, 79, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lehane, M.J. Peritrophic Matrix Structure and Function. Annu. Rev. Entomol. 1997, 42, 525–550. [Google Scholar] [CrossRef]
- Villegas-Ospina, S.; Merritt, D.J.; Johnson, K.N. Physical and Chemical Barriers in the Larval Midgut Confer Developmental Resistance to Virus Infection in Drosophila. Viruses 2021, 13, 894. [Google Scholar] [CrossRef] [PubMed]
- Kuraishi, T.; Binggeli, O.; Opota, O.; Buchon, N.; Lemaitre, B. Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2011, 108, 15966–15971. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, A.L.; Johnson, K.N. Infectivity of Drosophila C virus following oral delivery in Drosophila larvae. J. Gen. Virol. 2015, 96, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, Á.G.; Naylor, H.; Esteves, S.S.; Pais, I.S.; Martins, N.E.; Teixeira, L. The Toll-dorsal pathway is required for resistance to viral oral infection in Drosophila. PLoS Pathog. 2014, 10, e1004507. [Google Scholar] [CrossRef]
- Habayeb, M.S.; Cantera, R.; Casanova, G.; Ekström, J.-O.; Albright, S.; Hultmark, D. The Drosophila Nora virus is an enteric virus, transmitted via feces. J. Invertebr. Pathol. 2009, 101, 29–33. [Google Scholar] [CrossRef]
- Meeus, I.; de Miranda, J.R.; de Graaf, D.C.; Wäckers, F.; Smagghe, G. Effect of oral infection with Kashmir bee virus and Israeli acute paralysis virus on bumblebee (Bombus terrestris) reproductive success. J. Invertebr. Pathol. 2014, 121, 64–69. [Google Scholar] [CrossRef]
- Škubník, K.; Sukeník, L.; Buchta, D.; Füzik, T.; Procházková, M.; Moravcová, J.; Šmerdová, L.; Přidal, A.; Vácha, R.; Plevka, P. Capsid opening enables genome release of iflaviruses. Sci. Adv. 2021, 7, eabd7130. [Google Scholar] [CrossRef]
- Kalynych, S.; Füzik, T.; Přidal, A.; de Miranda, J.; Plevka, P. Cryo-EM study of slow bee paralysis virus at low pH reveals iflavirus genome release mechanism. Proc. Natl. Acad. Sci. USA 2017, 114, 598–603. [Google Scholar] [CrossRef]
- Snijder, J.; Uetrecht, C.; Rose, R.J.; Sanchez-Eugenia, R.; Marti, G.A.; Agirre, J.; Guérin, D.M.; Wuite, G.J.; Heck, A.J.; Roos, W.H. Probing the biophysical interplay between a viral genome and its capsid. Nat. Chem. 2013, 6, 502–509. [Google Scholar] [CrossRef]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef]
- Farnsworth, D.; Hamby, K.A.; Bolda, M.; Goodhue, R.E.; Williams, J.C.; Zalom, F.G. Economic analysis of revenue losses and control costs associated with the spotted wing drosophila, Drosophila suzukii (Matsumura), in the California raspberry industry. Pest Manag. Sci. 2017, 73, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Ribière, M.; Olivier, V.; Blanchard, P. Chronic bee paralysis: A disease and a virus like no other? J. Invertebr. Pathol. 2010, 103, S120–S131. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Kurata, K. Infection under aseptic conditions with virus of infectious flacherie in silkworm Bombyx Mori (Linnaeus). Insect Pathol. 1964, 1964, 130. [Google Scholar]

| Description | Sequence | Product Length (bp) |
|---|---|---|
| LJ specific probe * | 5′-ACTCGGCGTTATCGTTACAACCGCACATATC-3′ | |
| LJV forward primer | 5′-CAACACGTTGTGCTGCCTGA-3′ | 128 |
| LJV reverse primer | 5′-TCCATCCAAACTCCACCTCC-3′ | 128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linscheid, Y.; Kessel, T.; Vilcinskas, A.; Lee, K.-Z. Pathogenicity of La Jolla Virus in Drosophila suzukii following Oral Administration. Viruses 2022, 14, 2158. https://doi.org/10.3390/v14102158
Linscheid Y, Kessel T, Vilcinskas A, Lee K-Z. Pathogenicity of La Jolla Virus in Drosophila suzukii following Oral Administration. Viruses. 2022; 14(10):2158. https://doi.org/10.3390/v14102158
Chicago/Turabian StyleLinscheid, Yvonne, Tobias Kessel, Andreas Vilcinskas, and Kwang-Zin Lee. 2022. "Pathogenicity of La Jolla Virus in Drosophila suzukii following Oral Administration" Viruses 14, no. 10: 2158. https://doi.org/10.3390/v14102158
APA StyleLinscheid, Y., Kessel, T., Vilcinskas, A., & Lee, K.-Z. (2022). Pathogenicity of La Jolla Virus in Drosophila suzukii following Oral Administration. Viruses, 14(10), 2158. https://doi.org/10.3390/v14102158







