A Cellular Assay for Spike/ACE2 Fusion: Quantification of Fusion-Inhibitory Antibodies after COVID-19 and Vaccination
Abstract
:1. Introduction
2. Material and Methods
2.1. Cells and Reagents
2.2. Blood Samples
2.3. Molecular Biology
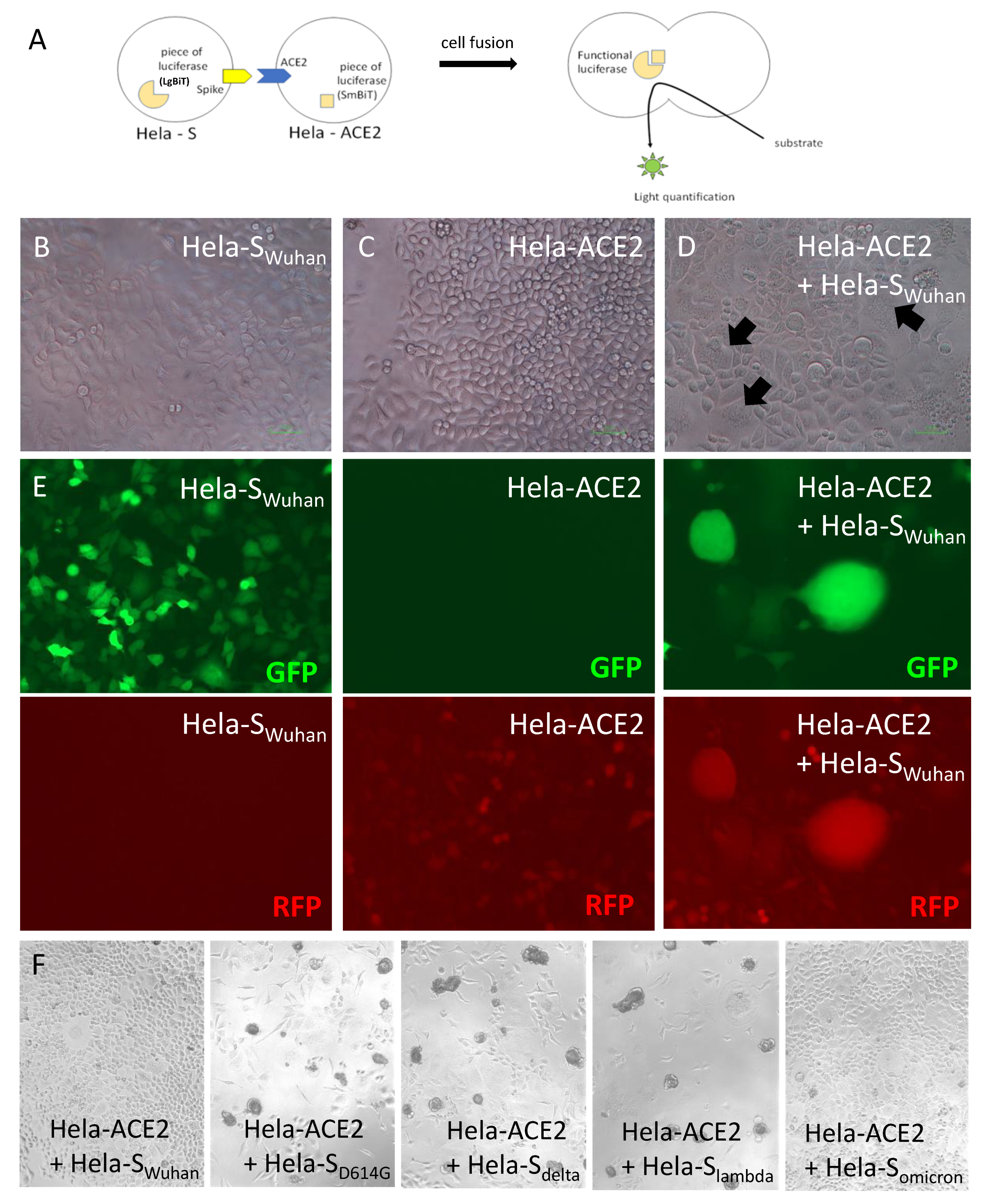
2.4. Cell Fusion Assay
2.5. Competitive ELISA for the RBD/ACE2 Interaction
2.6. Pseudotyped-Based Assay for the Detection of nAbs
3. Results
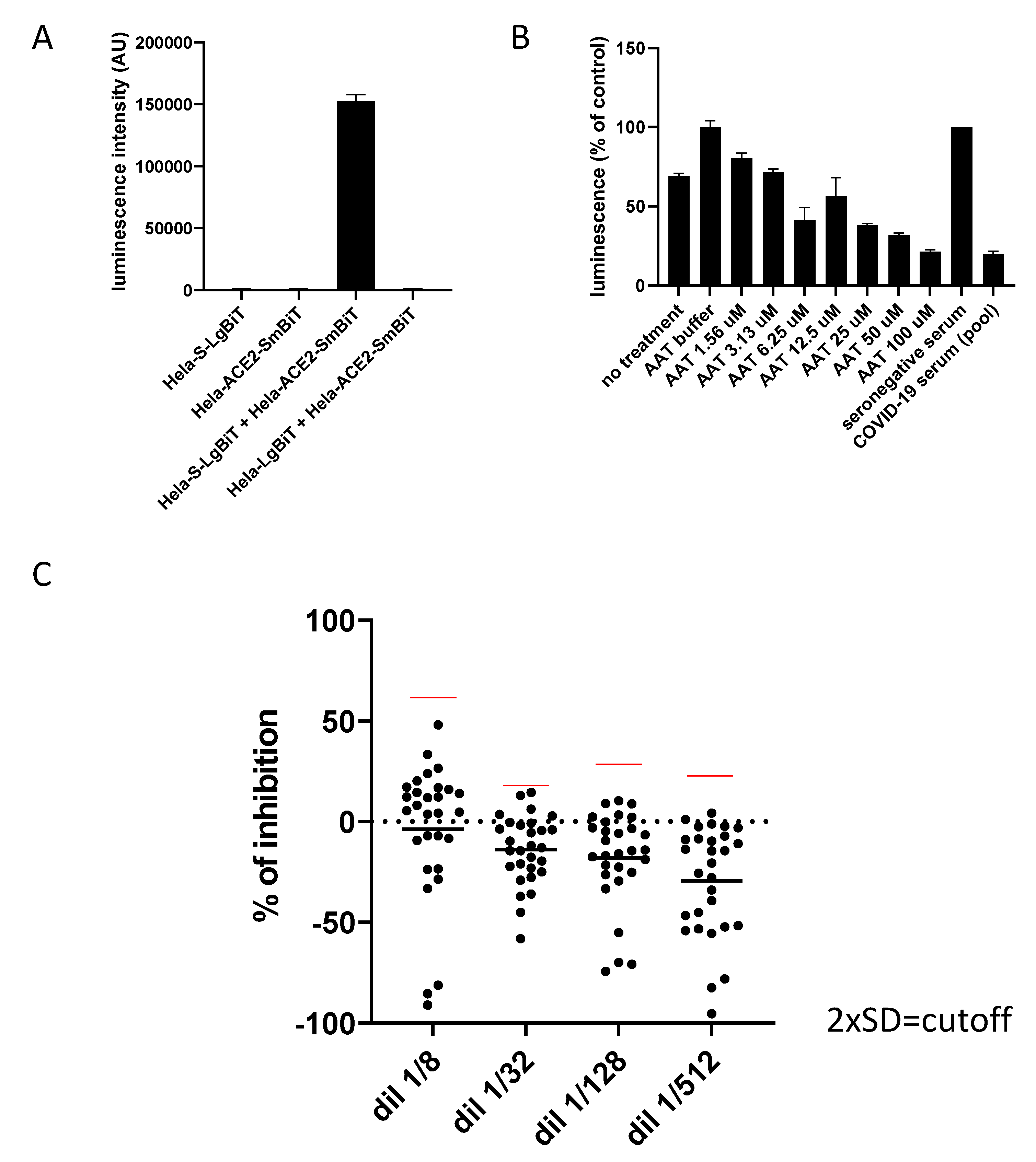
3.1. Coculture of Hela-S and Hela-ACE2 in the Presence of Various Dilutions of Seronegative Human Serum Induces Emission of a Luminescent Signal
3.2. Impact of Seropositive Sera from COVID-19 Patients on Hela-S and Hela-ACE2 Fusion
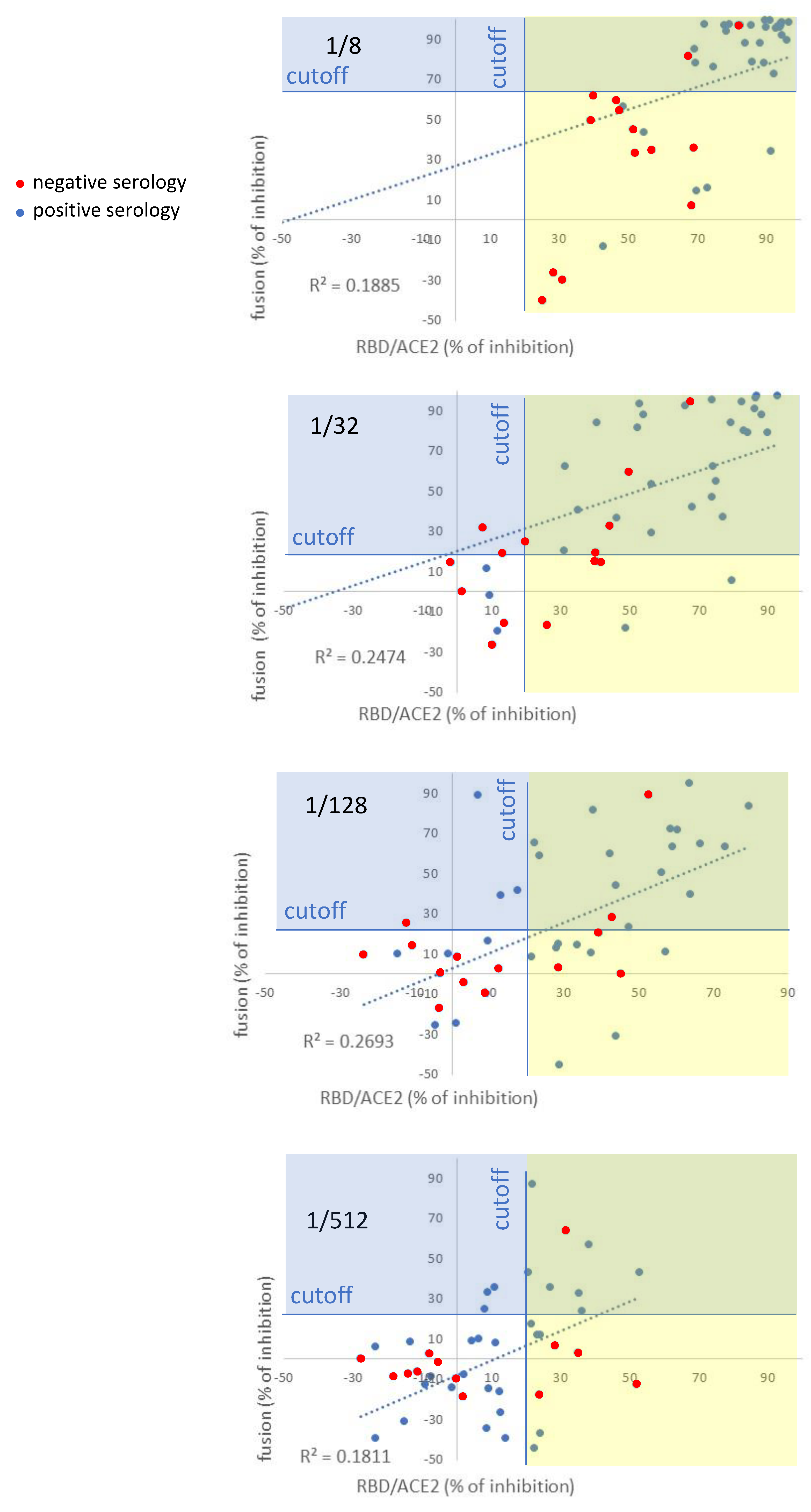
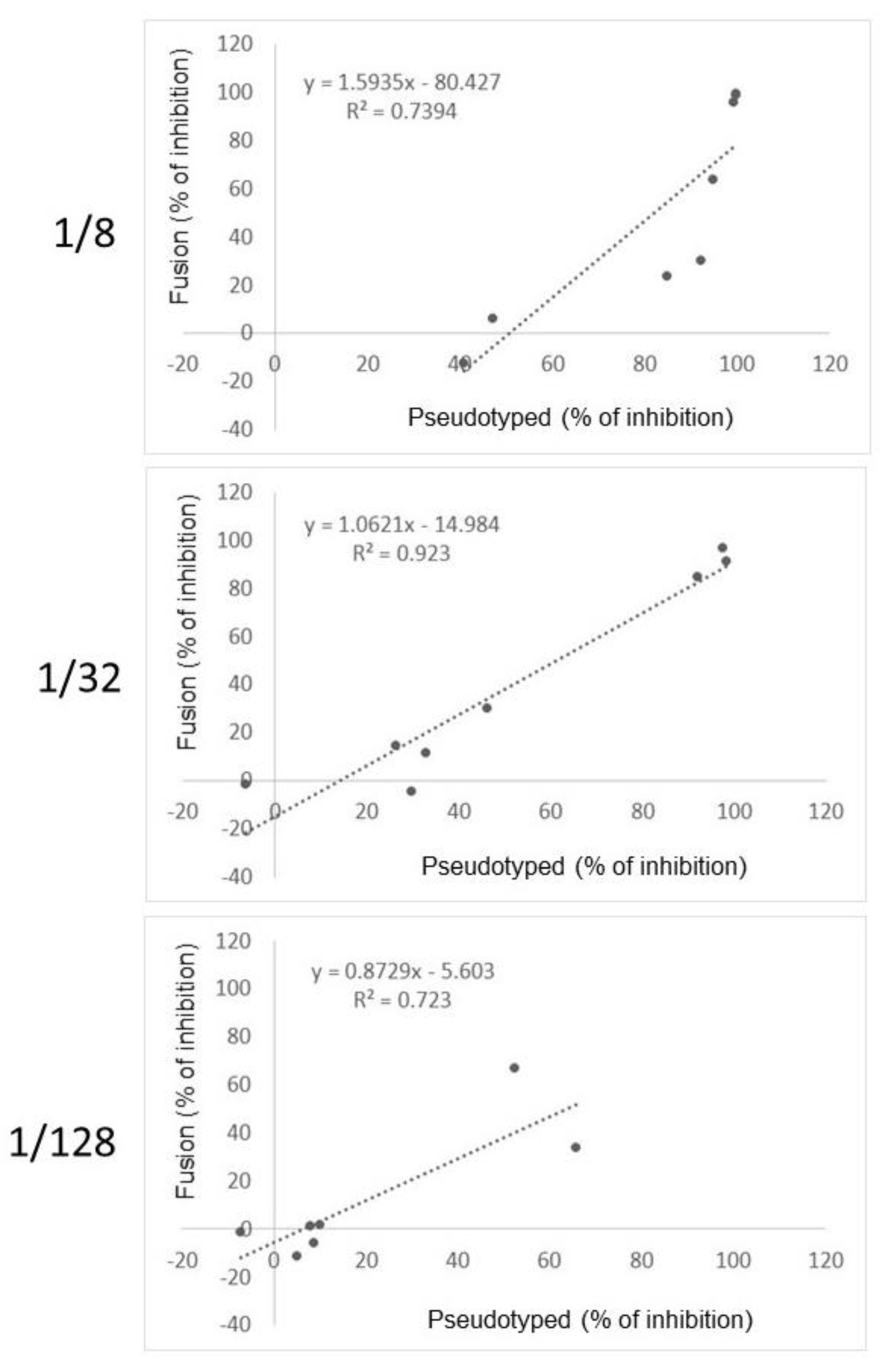
3.3. Comparison of the Fusion-Based Assay with an Assay Measuring the Inhibition of RBD/ACE2 Interaction, Serology and Pseudotyped-Based Assay
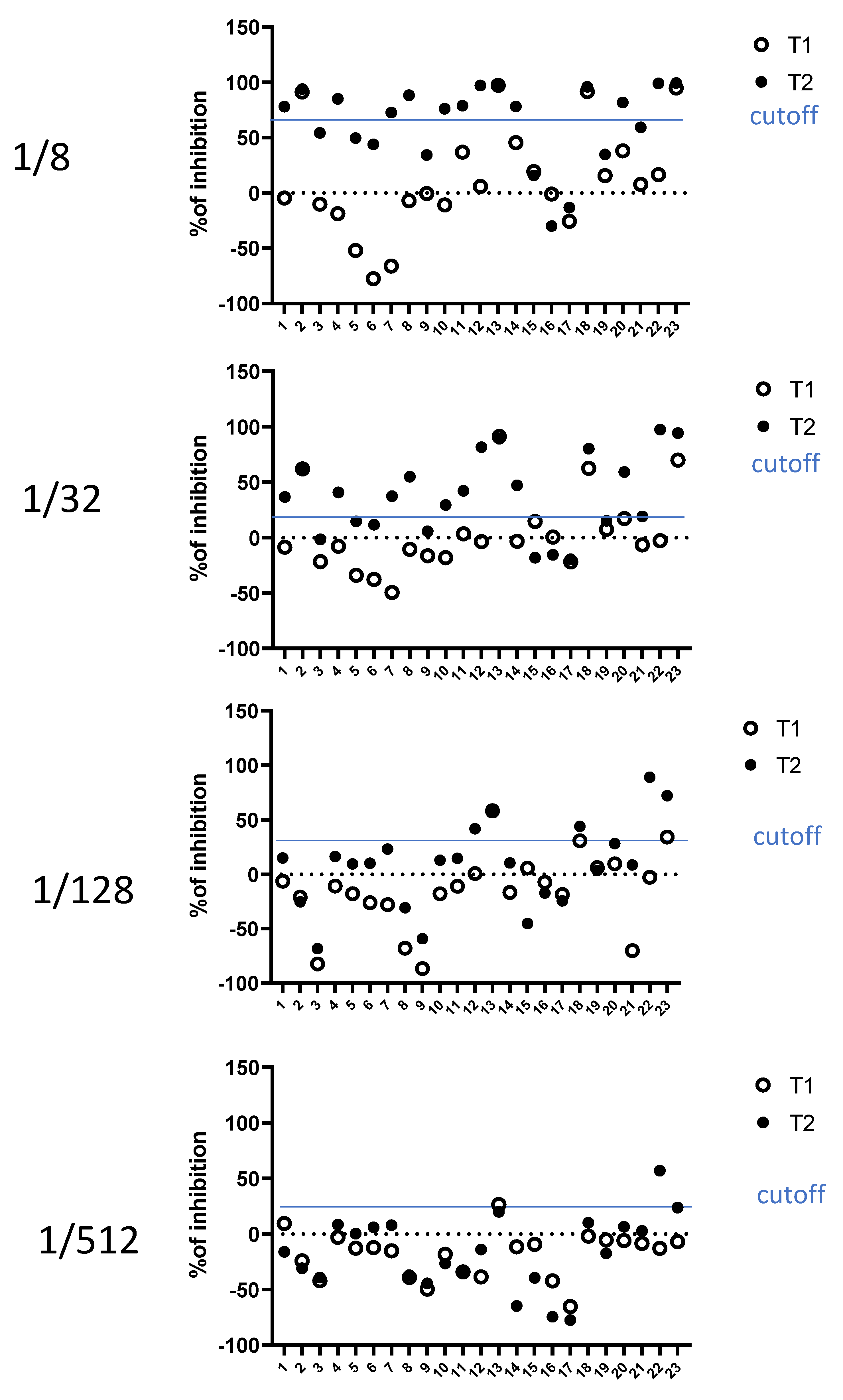
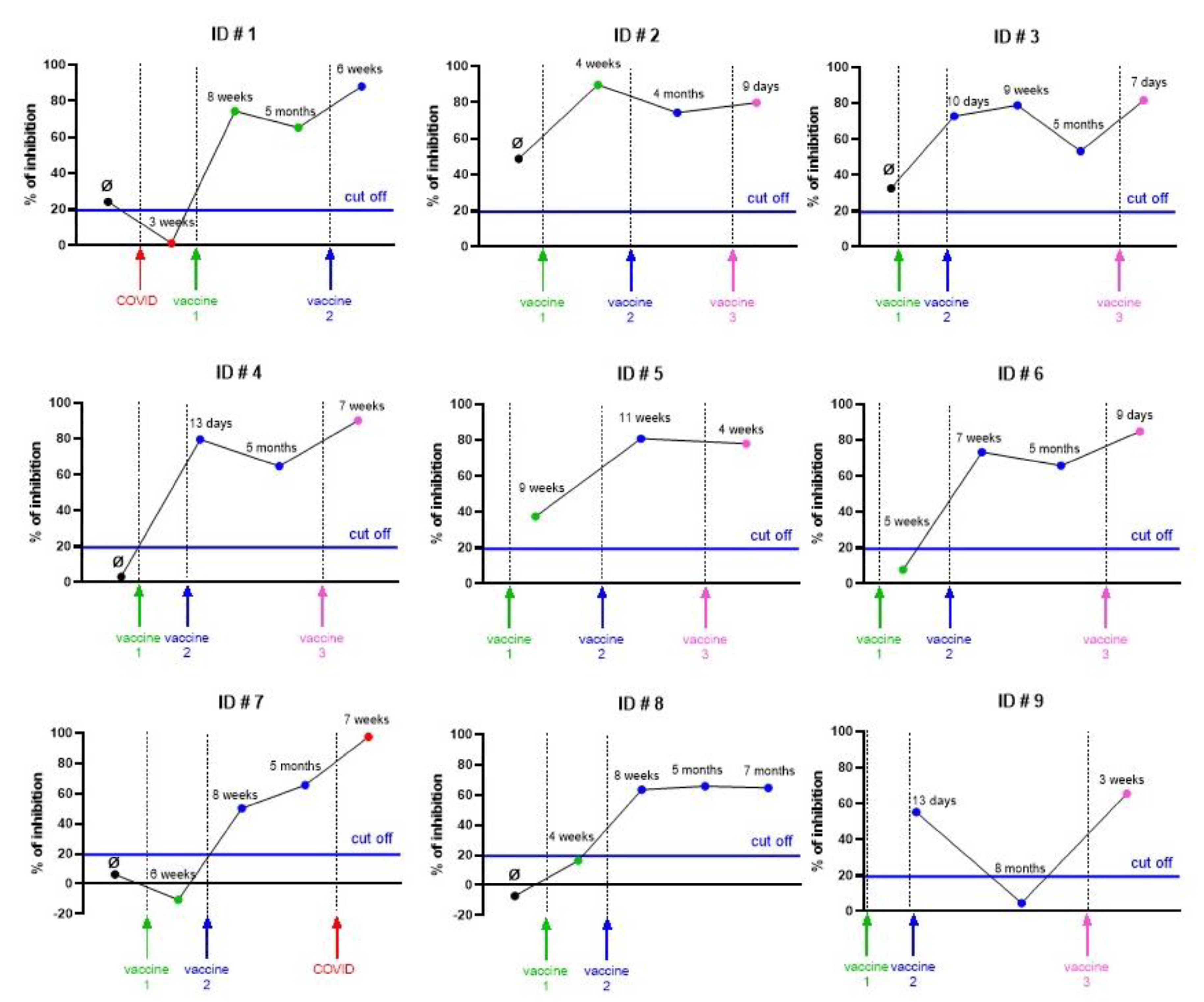
3.4. Case-Reports of the Kinetic of Fusion Inhibitions in Vaccinated Individuals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jeremiah, S.S.; Miyakawa, K.; Ryo, A. Detecting SARS-CoV-2 neutralizing immunity: Highlighting the potential of split nanoluciferase technology. J. Mol. Cell Biol. 2022, 14, mjac023. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, 6529. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Matthews, P.C.; Stoesser, N.; Maddox, T.; Lorenzi, L.; Studley, R.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat. Commun. 2021, 12, 6250. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Gavriatopoulou, M.; Terpos, E.; Ntanasis-Stathopoulos, I.; Briasoulis, A.; Gumeni, S.; Malandrakis, P.; Fotiou, D.; Migkou, M.; Theodorakakou, F.; Eleutherakis-Papaiakovou, E.; et al. Poor neutralizing antibody responses in 106 patients with WM after vaccination against SARS-CoV-2: A prospective study. Blood Adv. 2021, 5, 4398–4405. [Google Scholar] [CrossRef]
- Terpos, E.; Gavriatopoulou, M.; Fotiou, D.; Giatra, C.; Asimakopoulos, I.; Dimou, M.; Sklirou, A.D.; Ntanasis-Stathopoulos, I.; Darmani, I.; Briasoulis, A.; et al. Poor Neutralizing Antibody Responses in 132 Patients with CLL, NHL and HL after Vaccination against SARS-CoV-2: A Prospective Study. Cancers 2021, 13, 4480. [Google Scholar] [CrossRef]
- Chia, W.N.; Zhu, F.; Ong, S.W.X.; Young, B.E.; Fong, S.W.; Le Bert, N.; Tan, C.W.; Tiu, C.; Zhang, J.; Tan, S.Y.; et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: A longitudinal study. Lancet Microbe. 2021, 2, e240–e249. [Google Scholar] [CrossRef]
- Goto, A.; Go, H.; Miyakawa, K.; Yamaoka, Y.; Ohtake, N.; Kubo, S.; Jeremiah, S.S.; Mihara, T.; Senuki, K.; Miyazaki, T.; et al. Sustained Neutralizing Antibodies 6 Months Following Infection in 376 Japanese COVID-19 Survivors. Front. Microbiol. 2021, 12, 661187. [Google Scholar] [CrossRef]
- Epaulard, O.; Buisson, M.; Nemoz, B.; Marechal, M.L.; Terzi, N.; Payen, J.F.; Froidure, M.; Blanc, M.; Mounayar, A.L.; Quenard, F.; et al. Persistence at one year of neutralizing antibodies after SARS-CoV-2 infection: Influence of initial severity and steroid use. J. Infect. 2022, 84, 418–467. [Google Scholar] [CrossRef]
- Haveri, A.; Ekstrom, N.; Solastie, A.; Virta, C.; Osterlund, P.; Isosaari, E.; Nohynek, H.; Palmu, A.A.; Melin, M. Persistence of neutralizing antibodies a year after SARS-CoV-2 infection in humans. Eur. J. Immunol. 2021, 51, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, K.; Kubo, S.; Stanleyraj Jeremiah, S.; Go, H.; Yamaoka, Y.; Ohtake, N.; Kato, H.; Ikeda, S.; Mihara, T.; Matsuba, I.; et al. Persistence of Robust Humoral Immune Response in Coronavirus Disease 2019 Convalescent Individuals over 12 Months after Infection. Open Forum Infect. Dis. 2022, 9, ofab626. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R.; Fleming, T.R.; Longini, I.M.; Peto, R.; Briand, S.; Heymann, D.L.; Beral, V.; Snape, M.D.; Rees, H.; Ropero, A.M.; et al. SARS-CoV-2 Variants and Vaccines. N. Engl. J. Med. 2021, 385, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.A.; Lee, J.H.; Brescia, P.; Kumar, D.; Nong, T.; Shih, R.; Woodle, E.S.; Maltzman, J.S.; Gebel, H.M. Development and Validation of a Multiplex, Bead-based Assay to Detect Antibodies Directed against SARS-CoV-2 Proteins. Transplantation 2021, 105, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Ohtake, N.; Miyakawa, K.; Jeremiah, S.S.; Yamaoka, Y.; Murohashi, K.; Hagiwara, E.; Mihara, T.; Goto, A.; Yamazaki, E.; et al. Development of an Automated Chemiluminescence Assay System for Quantitative Measurement of Multiple Anti-SARS-CoV-2 Antibodies. Front. Microbiol. 2020, 11, 628281. [Google Scholar] [CrossRef]
- Perera, R.; Ko, R.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.M.; Brackman, C.J.; To, E.M.W.; Yen, H.L.; Leung, K.; Cheng, S.M.S.; et al. Evaluation of a SARS-CoV-2 Surrogate Virus Neutralization Test for Detection of Antibody in Human, Canine, Cat, and Hamster Sera. J. Clin. Microbiol. 2021, 59, e02504-20. [Google Scholar] [CrossRef]
- Putcharoen, O.; Wacharapluesadee, S.; Chia, W.N.; Paitoonpong, L.; Tan, C.W.; Suwanpimolkul, G.; Jantarabenjakul, W.; Ruchisrisarod, C.; Wanthong, P.; Sophonphan, J.; et al. Early detection of neutralizing antibodies against SARS-CoV-2 in COVID-19 patients in Thailand. PLoS ONE 2021, 16, e0246864. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Taylor, S.C.; Hurst, B.; Charlton, C.L.; Bailey, A.; Kanji, J.N.; McCarthy, M.K.; Morrison, T.E.; Huey, L.; Annen, K.; DomBourian, M.G.; et al. A New SARS-CoV-2 Dual-Purpose Serology Test: Highly Accurate Infection Tracing and Neutralizing Antibody Response Detection. J. Clin. Microbiol. 2021, 59, e02438-20. [Google Scholar] [CrossRef]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; et al. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT). Emerg. Microbes. Infect. 2020, 9, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, E.J.; Manguiat, K.; Robinson, A.; Chen, J.C.; Dimitrova, K.; Philipson, C.; Lamoureux, L.; McLachlan, E.; Schiffman, Z.; Drebot, M.A.; et al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Diagn. Microbiol. Infect. Dis. 2021, 99, 115294. [Google Scholar] [CrossRef] [PubMed]
- Alsoussi, W.B.; Turner, J.S.; Case, J.B.; Zhao, H.; Schmitz, A.J.; Zhou, J.Q.; Chen, R.E.; Lei, T.; Rizk, A.A.; McIntire, K.M.; et al. A Potently Neutralizing Antibody Protects Mice against SARS-CoV-2 Infection. J. Immunol. 2020, 205, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 2020, 369, 650–655. [Google Scholar] [CrossRef]
- Duan, J.; Yan, X.; Guo, X.; Cao, W.; Han, W.; Qi, C.; Feng, J.; Yang, D.; Gao, G.; Jin, G. A human SARS-CoV neutralizing antibody against epitope on S2 protein. Biochem. Biophys. Res. Commun. 2005, 333, 186–193. [Google Scholar] [CrossRef]
- Muruato, A.E.; Fontes-Garfias, C.R.; Ren, P.; Garcia-Blanco, M.A.; Menachery, V.D.; Xie, X.; Shi, P.Y. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat. Commun. 2020, 11, 4059. [Google Scholar] [CrossRef]
- Ye, C.; Chiem, K.; Park, J.G.; Silvas, J.A.; Morales Vasquez, D.; Sourimant, J.; Lin, M.J.; Greninger, A.L.; Plemper, R.K.; Torrelles, J.B.; et al. Analysis of SARS-CoV-2 infection dynamic in vivo using reporter-expressing viruses. Proc. Natl. Acad. Sci. USA 2021, 118, e2111593118. [Google Scholar] [CrossRef]
- Schmidt, F.; Weisblum, Y.; Muecksch, F.; Hoffmann, H.H.; Michailidis, E.; Lorenzi, J.C.C.; Mendoza, P.; Rutkowska, M.; Bednarski, E.; Gaebler, C.; et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020, 217, e20201181. [Google Scholar] [CrossRef]
- Rockx, B.; Kuiken, T.; Herfst, S.; Bestebroer, T.; Lamers, M.M.; Oude Munnink, B.B.; de Meulder, D.; van Amerongen, G.; van den Brand, J.; Okba, N.M.A.; et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 2020, 368, 1012–1015. [Google Scholar] [CrossRef]
- Liu, X.; Drelich, A.; Li, W.; Chen, C.; Sun, Z.; Shi, M.; Adams, C.; Mellors, J.W.; Tseng, C.T.; Dimitrov, D.S. Enhanced elicitation of potent neutralizing antibodies by the SARS-CoV-2 spike receptor binding domain Fc fusion protein in mice. Vaccine 2020, 38, 7205–7212. [Google Scholar] [CrossRef]
- Sha, Y.; Wu, Y.; Cao, Z.; Xu, X.; Wu, W.; Jiang, D.; Mao, X.; Liu, H.; Zhu, Y.; Gong, R.; et al. A convenient cell fusion assay for the study of SARS-CoV entry and inhibition. IUBMB Life 2006, 58, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kiso, M.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Takeda, M.; Kinoshita, N.; Ohmagari, N.; Gohda, J.; Semba, K.; et al. The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner. Viruses 2020, 12, 629. [Google Scholar] [CrossRef] [PubMed]
- Theuerkauf, S.A.; Michels, A.; Riechert, V.; Maier, T.J.; Flory, E.; Cichutek, K.; Buchholz, C.J. Quantitative assays reveal cell fusion at minimal levels of SARS-CoV-2 spike protein and fusion from without. iScience 2021, 24, 102170. [Google Scholar] [CrossRef] [PubMed]
- Mancek-Keber, M.; Hafner-Bratkovic, I.; Lainscek, D.; Bencina, M.; Govednik, T.; Orehek, S.; Plaper, T.; Jazbec, V.; Bergant, V.; Grass, V.; et al. Disruption of disulfides within RBD of SARS-CoV-2 spike protein prevents fusion and represents a target for viral entry inhibition by registered drugs. FASEB J. 2021, 35, e21651. [Google Scholar] [CrossRef]
- Plaper, T.; Aupic, J.; Dekleva, P.; Lapenta, F.; Keber, M.M.; Jerala, R.; Bencina, M. Coiled-coil heterodimers with increased stability for cellular regulation and sensing SARS-CoV-2 spike protein-mediated cell fusion. Sci. Rep. 2021, 11, 9136. [Google Scholar] [CrossRef]
- Wettstein, L.; Weil, T.; Conzelmann, C.; Muller, J.A.; Gross, R.; Hirschenberger, M.; Seidel, A.; Klute, S.; Zech, F.; Prelli Bozzo, C.; et al. Alpha-1 antitrypsin inhibits TMPRSS2 protease activity and SARS-CoV-2 infection. Nat. Commun. 2021, 12, 1726. [Google Scholar] [CrossRef]
- Rajah, M.M.; Bernier, A.; Buchrieser, J.; Schwartz, O. The Mechanism and Consequences of SARS-CoV-2 Spike-Mediated Fusion and Syncytia Formation. J. Mol. Biol. 2022, 434, 167280. [Google Scholar] [CrossRef]
- Hornich, B.F.; Grosskopf, A.K.; Schlagowski, S.; Tenbusch, M.; Kleine-Weber, H.; Neipel, F.; Stahl-Hennig, C.; Hahn, A.S. SARS-CoV-2 and SARS-CoV Spike-Mediated Cell-Cell Fusion Differ in Their Requirements for Receptor Expression and Proteolytic Activation. J. Virol. 2021, 95, e00002-21. [Google Scholar] [CrossRef]
- Yamamoto, M.; Gohda, J.; Kobayashi, A.; Tomita, K.; Hirayama, Y.; Koshikawa, N.; Seiki, M.; Semba, K.; Akiyama, T.; Kawaguchi, Y.; et al. Metalloproteinase-Dependent and TMPRSS2-Independent Cell Surface Entry Pathway of SARS-CoV-2 Requires the Furin Cleavage Site and the S2 Domain of Spike Protein. mBio 2022, 13, e0051922. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Rajah, M.M.; Hubert, M.; Bishop, E.; Saunders, N.; Robinot, R.; Grzelak, L.; Planas, D.; Dufloo, J.; Gellenoncourt, S.; Bongers, A.; et al. SARS-CoV-2 Alpha, Beta, and Delta variants display enhanced Spike-mediated syncytia formation. EMBO J. 2021, 40, e108944. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetskiy, L.; Wang, X.; Pascal, K.E.; Tomkins-Tinch, C.; Nyalile, T.P.; Wang, Y.; Baum, A.; Diehl, W.E.; Dauphin, A.; Carbone, C.; et al. Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. Cell 2020, 183, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Thao, T.T.N.; Hoffmann, D.; Taddeo, A.; Ebert, N.; Labroussaa, F.; Pohlmann, A.; King, J.; Steiner, S.; Kelly, J.N.; et al. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature 2021, 592, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Ozono, S.; Zhang, Y.; Ode, H.; Sano, K.; Tan, T.S.; Imai, K.; Miyoshi, K.; Kishigami, S.; Ueno, T.; Iwatani, Y.; et al. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat. Commun. 2021, 12, 848. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, Z.; Wang, C.; Ren, H.; Gao, L.; Peng, H.; Niu, Z.; Ren, H.; Huang, H.; Sun, Q. Bimodular effects of D614G mutation on the spike glycoprotein of SARS-CoV-2 enhance protein processing, membrane fusion, and viral infectivity. Signal. Transduct. Target Ther. 2020, 5, 268. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Irie, T.; Suzuki, R.; Maemura, T.; Nasser, H.; Uriu, K.; Kosugi, Y.; Shirakawa, K.; Sadamasu, K.; Kimura, I.; et al. Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature 2022, 602, 300–306. [Google Scholar] [CrossRef] [PubMed]

| Serum Dilution | Average | 2 Standard Deviations from the Average |
|---|---|---|
| 1/8 | −3.7 | 62.6% |
| 1/32 | −14.1 | 19.4% |
| 1/128 | −18.1 | 27.8% |
| 1/512 | −29.5 | 24.1% |
| Serum Dilution | Fusion vs. RBD/ACE2 | Serology vs. RBD/ACE2 | Fusion vs. Serology | Fusion vs. Pseudotyped |
|---|---|---|---|---|
| 1/8 | 0.19 | 0.36 | 0.20 | 0.74 |
| 1/32 | 0.25 | 0.40 | 0.28 | 0.92 |
| 1/128 | 0.27 | 0.38 | 0.20 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul, F.; Ribaux, P.; Caillon, A.; Malézieux-Picard, A.; Prendki, V.; Vernaz, N.; Zhukovsky, N.; Delhaes, F.; Krause, K.-H.; Preynat-Seauve, O. A Cellular Assay for Spike/ACE2 Fusion: Quantification of Fusion-Inhibitory Antibodies after COVID-19 and Vaccination. Viruses 2022, 14, 2118. https://doi.org/10.3390/v14102118
Abdul F, Ribaux P, Caillon A, Malézieux-Picard A, Prendki V, Vernaz N, Zhukovsky N, Delhaes F, Krause K-H, Preynat-Seauve O. A Cellular Assay for Spike/ACE2 Fusion: Quantification of Fusion-Inhibitory Antibodies after COVID-19 and Vaccination. Viruses. 2022; 14(10):2118. https://doi.org/10.3390/v14102118
Chicago/Turabian StyleAbdul, Fabien, Pascale Ribaux, Aurélie Caillon, Astrid Malézieux-Picard, Virginie Prendki, Nathalie Vernaz, Nikolay Zhukovsky, Flavien Delhaes, Karl-Heinz Krause, and Olivier Preynat-Seauve. 2022. "A Cellular Assay for Spike/ACE2 Fusion: Quantification of Fusion-Inhibitory Antibodies after COVID-19 and Vaccination" Viruses 14, no. 10: 2118. https://doi.org/10.3390/v14102118
APA StyleAbdul, F., Ribaux, P., Caillon, A., Malézieux-Picard, A., Prendki, V., Vernaz, N., Zhukovsky, N., Delhaes, F., Krause, K.-H., & Preynat-Seauve, O. (2022). A Cellular Assay for Spike/ACE2 Fusion: Quantification of Fusion-Inhibitory Antibodies after COVID-19 and Vaccination. Viruses, 14(10), 2118. https://doi.org/10.3390/v14102118





