Convalescent Plasma for Pregnant Women with COVID-19: A Systematic Literature Review
Abstract
:1. Introduction
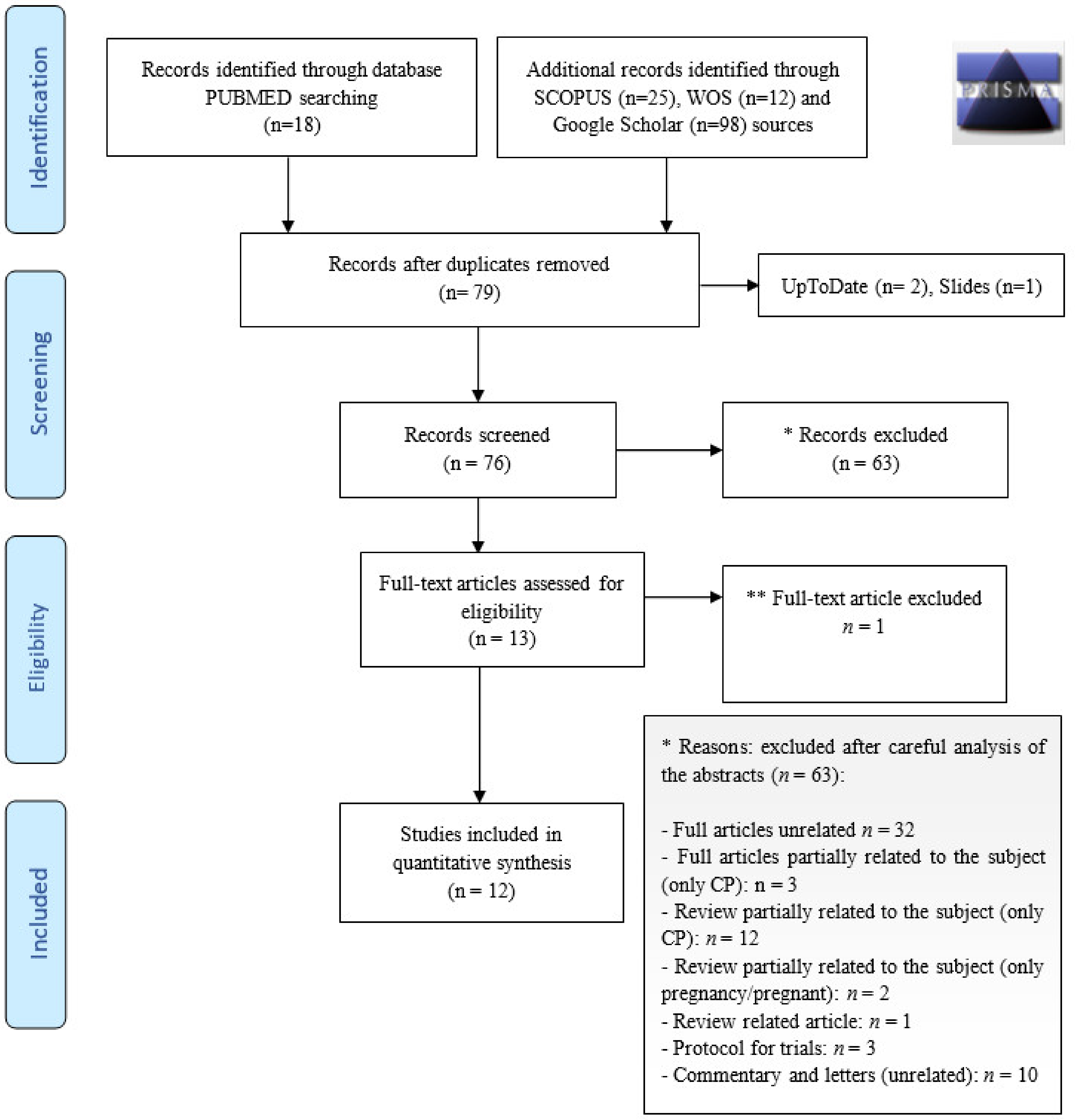
2. Sources
3. Selection of Studies
4. Ongoing Trials Involving Pregnant Patients
5. Results
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 5 August 2020).
- Dashraath, P.; Wong, J.L.J.; Lim, M.X.K.; Lim, L.M.; Li, S.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Pierce-Williams, R.A.; Burd, J.; Felder, L.; Khoury, R.; Bernstein, P.S.; Avila, K.; Penfield, C.A.; Roman, A.; DeBolt, C.A.; Stone, J.L.; et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: A United States cohort study. Am. J. Obstet. Gynecol. MFM 2020, 2, 100134. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, K.; LaSala, A. Risk factors associated with the increasing prevalence of pneumonia during pregnancy. Am. J. Obstet. Gynecol. 1990, 163, 981–985. [Google Scholar] [CrossRef]
- Stumpfe, F.M.; Titzmann, A.; Schneider, M.O.; Stelzl, P.; Kehl, S.; Fasching, P.A.; Beckmann, M.W.; Ensser, A. SARS-CoV-2 Infection in Pregnancy—A Review of the Current Literature and Possible Impact on Maternal and Neonatal Outcome. Geburtshilfe Frauenheilkd. 2020, 80, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madinger, N.E.; Greenspoon, J.S.; Ellrodt, A.G. Pneumonia during pregnancy: Has modern technology improved maternal and fetal outcome? Am. J. Obstet. Gynecol. 1989, 161, 657–662. [Google Scholar] [CrossRef]
- Benedetti, T.J.; Valle, R.; Ledger, W.J. Antepartum pneumonia in pregnancy. Am. J. Obstet. Gynecol. 1982, 144, 413–417. [Google Scholar] [CrossRef]
- Novoa, R.H.; Quintana, W.; Llancarí, P.; Urbina-Quispe, K.; Guevara-Ríos, E.; Ventura, W. Maternal clinical characteristics and perinatal outcomes among pregnant women with coronavirus disease 2019. A systematic review. Travel Med. Infect. Dis. 2021, 39, 101919. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef]
- Papapanou, M.; Papaioannou, M.; Petta, A.; Routsi, E.; Farmaki, M.; Vlahos, N.; Siristatidis, C. Maternal and neonatal char-acteristics and outcomes of covid-19 in pregnancy: An overview of systematic reviews. Int. J. Environ. Res. Public Health 2021, 18, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Salem, D.; Katranji, F.; Bakdash, T. COVID-19 infection in pregnant women: Review of maternal and fetal outcomes. Int. J. Gynecol. Obstet. 2021, 152, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Pandita, A.; Panza, R. Maternal and perinatal outcomes in pregnant women infected by SARS-CoV-2: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 194–204. [Google Scholar] [CrossRef]
- National Institutes of Health. COVID-19 Treatment Guidelines Panel Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2020. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 20 February 2021).
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Ahmed, S.; Hill, K. Acute respiratory distress syndrome in a pregnant patient with COVID-19 improved after delivery: A case report and brief review. Respir. Med. Case Rep. 2020, 31, 101171. [Google Scholar] [CrossRef]
- Soleimani, Z.; Soleimani, A. ADRS due to COVID-19 in midterm pregnancy: Successful management with plasma transfusion and corticosteroids. J. Matern. Neonatal Med. 2020, 1–4. [Google Scholar] [CrossRef]
- Grisolia, G.; Franchini, M.; Glingani, C.; Inglese, F.; Garuti, M.; Beccaria, M.; Capuzzo, M.; Pinto, A.; Pavan, G.; Righetto, L.; et al. Convalescent plasma for coronavirus disease 2019 in pregnancy: A case report and review. Am. J. Obstet. Gynecol. MFM 2020, 2, 100174. [Google Scholar] [CrossRef]
- Jafari, R.; Jonaidi-Jafari, N.; Dehghanpoor, F.; Saburi, A. Convalescent plasma therapy in a pregnant COVID-19 patient with a dramatic clinical and imaging response: A case report. World J. Radiol. 2020, 12, 137–141. [Google Scholar] [CrossRef]
- Lam, H.; Lacasse, A. Combination of remdesivir, corticosteroid, and convalescent plasma for severe COVID-19 in periviable pregnancy. Infect. Dis. Clin. Pract. 2020, 28, e21–e23. [Google Scholar] [CrossRef]
- Easterlin, M.C.; de Beritto, T.; Yeh, A.M.; Wertheimer, F.B.; Ramanathan, R. Extremely Preterm Infant Born to a Mother With Severe COVID-19 Pneumonia. J. Investig. Med. High Impact Case Rep. 2020, 8, 232470962094662. [Google Scholar] [CrossRef]
- Yaqoub, S.; Ahmad, S.; Mansouri, Z.; Pallivalapila, A.; El Kassem, W.; Maslamani, M.; Abu Jubara, M.; Minisha, F.; Tarannum, A.; Babarinsa, I.; et al. Management of life-threatening acute respiratory syndrome and severe pneumonia secondary to COVID-19 in pregnancy: A case report and literature review. Clin. Case Rep. 2021, 9, 137–143. [Google Scholar] [CrossRef]
- Donzelli, M.; Ippolito, M.; Catalisano, G.; Renda, B.; Tarantino, F.; Diquattro, O.; Cortegiani, A. Prone positioning and convalescent plasma therapy in a critically ill pregnant woman with COVID-19. Clin. Case Rep. 2020, 8, 3352–3358. [Google Scholar] [CrossRef]
- Magallanes-Garza, G.I.; Valdez-Alatorre, C.; Dávila-González, D.; Martínez-Reséndez, M.F.; Sánchez-Salazar, S.S.; Castilleja-Leal, F.; Cardona-Huerta, S. Rapid improvement of a critically ill obstetric patient with SARS-CoV-2 infection after administration of convalescent plasma. Int. J. Gynecol. Obstet. 2021, 152, 439–441. [Google Scholar] [CrossRef]
- Pelayo, J.; Pugliese, G.; Salacup, G.; Quintero, E.; Khalifeh, A.; Jaspan, D.; Sharma, B. Severe COVID-19 in third trimester pregnancy: Multidisciplinary approach. Case Rep. Crit. Care 2020, 2020, 8889487. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, S.; Tan, T.; Huang, W.; Dong, Y.; Chen, L.; Chen, Q.; Zhang, L.; Zhong, Q.; Zhang, X.; et al. Treatment with convalescent plasma for critically Ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest 2020, 158, e9–e13. [Google Scholar] [CrossRef]
- Anderson, J.; Schauer, J.; Bryant, S.; Graves, C.R. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: A case report. Case Rep. Women’s Health 2020, 27, e00221. [Google Scholar] [CrossRef]
- Jacobson, J.; Antony, K.; Beninati, M.; Alward, W.; Hoppe, K.K. Use of dexamethasone, remdesivir, convalescent plasma and prone positioning in the treatment of severe COVID-19 infection in pregnancy: A case report. Case Rep. Women’s Health 2021, 29, e00273. [Google Scholar] [CrossRef]
- Eckhardt, C.M.; Cummings, M.J.; Rajagopalan, K.N.; Borden, S.; Bitan, Z.C.; Wolf, A.; Kantor, A.; Briese, T.; Meyer, B.J.; Ja-cobson, S.D.; et al. Evalua ting the efficacy and safety of human anti-SARS-CoV-2 convalescent plasma in severely ill adults with COVID-19: A structured summary of a study protocol for a randomized controlled trial. Trials 2020, 21, 499. [Google Scholar] [CrossRef]
- Chowdhury, F.R.; Hoque, A.; Chowdhury, F.U.H.; Amin, M.R.; Rahim, A.; Rahman, M.M.; Yasmin, R.; Amin, M.R.; Miah, M.T.; Kalam, M.A.; et al. Convalescent plasma transfusion therapy in severe COVID-19 patients—A safety, efficacy and dose response study: A structured summary of a study protocol of a phase II randomized controlled trial. Trials 2020, 21, 883. [Google Scholar] [CrossRef]
- Ali, S.; Luxmi, S.; Anjum, F.; Muhaymin, S.M.; Uddin, S.M.; Ali, A.; Ali, M.R.; Tauheed, S.; Khan, M.; Bajwa, M.; et al. Hy-perimmune anti-COVID-19 IVIG (C-IVIG) therapy for passive immunization of severe and critically Ill COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 1–3. [Google Scholar] [CrossRef]
- Boban, M. Novel coronavirus disease (COVID-19) update on epidemiology, pathogenicity, clinical course and treatments. Int. J. Clin. Pract. 2021, 75, e13868. [Google Scholar] [CrossRef]
- Lyngbakken, M.N.; Berdal, J.-E.; Eskesen, A.; Kvale, D.; Olsen, I.C.; Rueegg, C.S.; Rangberg, A.; Jonassen, C.M.; Omland, T.; Røsjø, H.; et al. A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics. Nat. Commun. 2020, 11, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. Available online: https://www.medrxiv.org/content/10.1101/2020.10.15.20209817v1 (accessed on 28 January 2021).
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA 2020, 323, 1582. [Google Scholar] [CrossRef] [PubMed]
- Luke, T.C.; Kilbane, E.M.; Jackson, J.L.; Hoffman, S.L. Meta-analysis: Convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment? Ann. Intern. Med. 2006, 145, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Marano, G.; Vaglio, S.; Pupella, S.; Facco, G.; Catalano, L.; Liumbruno, G.M.; Grazzini, G. Convalescent plasma: New evidence for an old therapeutic tool? Blood Transfus. 2016, 14, 152–157. [Google Scholar] [PubMed] [Green Version]
- Mair-Jenkins, J.; Saavedra-Campos, M.; Baillie, J.K.; Cleary, P.; Khaw, F.-M.; Lim, W.S.; Makki, S.; Rooney, K.D.; Nguyen-Van-Tam, J.S.; Beck, C.R.; et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2015, 211, 80–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perotti, C.; Baldanti, F.; Bruno, R.; Del Fante, C.; Seminari, E.; Casari, S.; Percivalle, E.; Glingani, C.; Musella, V.; Belliato, M.; et al. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica 2020, 105, 2834–2840. [Google Scholar] [CrossRef]
- Piechotta, V.; Chai, K.L.; Valk, S.J.; Doree, C.; Monsef, I.; Wood, E.M.; Lamikanra, A.; Kimber, C.; McQuilten, Z.; So-Osman, C.; et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Klassen, S.A.; Senefeld, J.W.; Johnson, P.W.; Carter, R.E.; Wiggins, C.C.; Shoham, S.; Grossman, B.J.; Henderson, J.P.; Musser, J.; Salazar, E.; et al. Evidence favoring the efficacy of convalescent plasma for COVID-19 therapy. medRxiv 2020. [Google Scholar] [CrossRef]
- Focosi, D.; Anderson, A.O.; Tang, J.W.; Tuccori, M. Convalescent plasma therapy for COVID-19: State of the art. Clin. Microbiol. Rev. 2020, 33, 1–17. [Google Scholar] [CrossRef]
- Amaral, W.; Moraes, C.; Rodrigues, A.; Noll, M.; Arruda, J.; Mendonça, C. Maternal coronavirus infections and neonates born to mothers with SARS-CoV-2: A systematic review. Healthcare 2020, 8, 511. [Google Scholar] [CrossRef]
- Hcini, N.; Maamri, F.; Picone, O.; Carod, J.-F.; Lambert, V.; Mathieu, M.; Carles, G.; Pomar, L. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: A single-center prospective comparative study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 257, 11–18. [Google Scholar] [CrossRef]
- Di Guardo, F.; Di Grazia, F.M.; Di Gregorio, L.M.; Zambrotta, E.; Carrara, G.; Gulino, F.A.; Tuscano, A.; Palumbo, M. Poor maternal–neonatal outcomes in pregnant patients with confirmed SARS-Cov-2 infection: Analysis of 145 cases. Arch. Gynecol. Obstet. 2021, 303, 1483–1488. [Google Scholar] [CrossRef]
- Wiese, M.D.; Berry, M.J.; Hissaria, P.; Darby, J.R.; Morrison, J.L. COVID-19: Can we treat the mother without harming her baby? J. Dev. Orig. Health Dis. 2021, 1–11. [Google Scholar] [CrossRef]
- Hessami, K.; Homayoon, N.; Hashemi, A.; Vafaei, H.; Kasraeian, M.; Asadi, N. COVID-19 and maternal, fetal and neonatal mortality: A systematic review. J. Matern. Fetal Neonatal Med. 2020, 1–6. [Google Scholar] [CrossRef]
- Di Martino, D.; Chiaffarino, F.; Patanè, L.; Prefumo, F.; Vergani, P.; Ornaghi, S.; Savasi, V.; Spinillo, A.; Cromi, A.; D’Ambrosi, F.; et al. Assessing risk factors for severe forms of COVID-19 in a pregnant population: A clinical series from Lombardy, Italy. Int. J. Gynecol. Obstet. 2021, 152, 275–277. [Google Scholar] [CrossRef]
- Libster, R.; Marc, G.P.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef]
- Joyner, M.J.; Bruno, K.A.; Klassen, S.A.; Kunze, K.L.; Johnson, P.W.; Lesser, E.R.; Wiggins, C.C.; Senefeld, J.W.; Klompas, A.M.; Hodge, D.O.; et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin. Proc. 2020, 95, 1888–1897. [Google Scholar] [CrossRef]
| Author, Year [Ref] | Design | Country | Age, y | Gestational Age | Severity of Disease | Comorbidity | Procedures | CP Treatment | Other Medications | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units Transfused | NAbT | Days from Hospitalization | AR | Maternal | Fetal/Neonatal | |||||||||
| Grisolia, 2020 [17] | CR | Italy | 29 | 24 w and 2 d | Mild ARDS | Class I obesity | VD | 2 | 160 | +1, +4 | None | Ceftriaxone, azithromycin, hydroxychloroquine, methylprednisolone, LMWH | Maternal well-being | Full-term, well neonate with VD |
| Zhang, 2020 [25] | CR | China | 31 | 35 w and 2 d | Severe ARDS | - | CD (35 w), IMV, ECMO | 1 | NR | +17 | None | Lopinavir/ritonavir, ribavirin, imipenem, vancomycin | Maternal survival | Neonatal death due to intrauterine asphyxia |
| Anderson, 2020 [26] | CR | USA | 35 | 22 w and 2 d | Severe ARDS | Type 2 DM, asthma, class III obesity | Forego delivery (25 w) | 1 | NR | +1 | None | Remdesivir, ceftriaxone, azithromycin, hydroxychloroquine, hydrocortisone, LMWH | Maternal well-being | Normal ongoing pregnancy |
| Donzelli, 2020 [22] | CR | Italy | 34 | 27 w and 4 d | Severe ARDS | - | IMV, PP, tracheostomy, CD (30 w) | 2 | NR | +2, +3 | None | Clarithromycin, ceftriaxone, betamethasone, LMWH | Maternal well-being | Normal ongoing pregnancy |
| Jacobson, 2021 [27] | CR | USA | 42 | 26 w | Severe ARDS | - | CD (29 w), IMV, PP, ECMO, tracheostomy | 1 | NR | +2 | None | Remdesivir, dexamethasone, azithromycin, ceftriaxone | Discharged with home oxygen | Neonatal adrenal insufficiency, then good condition |
| Magallanes-Garza, 2020 [23] | CR | Mexico | 33 | 27 w and 4 d | Severe ARDS | - | VD (39 w), IMV | 2 | NR | +4, +5 | None | Lopinavir/ritonavir, LMWH, azithromycin, ceftaroline, methylprednisolone | Maternal well-being | Neonatal GR |
| Pelayo, 2020 [24] | CR | USA | 35 | 36 w and 2 d | Severe ARDS, PE | Asthma, class III obesity, ileal carcinoma, HCV | IMV, CD (36 w) | 1 | NR | NR | NR | Methylprednisolone, remdesivir, heparin, vancomycin, ceftriaxone | Discharged to acute inpatient rehabilitation unit | Neonate intubation due to hypoxia, then positive outcome |
| Jafari, 2020 [18] | CR | Iran | 26 | 36 w and 1 d | Moderate ARDS | - | CD (36 w) | NR | NR | NR | NR | Favipiravir, meropenem, azithromycin, hydroxychloroquine | Maternal well-being | Neonate well |
| Easterlin, 2020 [20] | CR | USA | 22 | 23 w and 6 d | Severe ARDS | Tuberous sclerosis, nephrectomy, leiomyosarcoma | CD (25 w), PP, tracheostomy | NR | NR | NR | NR | Azithromycin, hydroxychloroquine, remdesivir, tocilizumab, LMWH | Pre-eclampsia, post-delivery critically ill condition | Critically ill preterm neonate with severe respiratory failure |
| Soleimani, 2020 [16] | CR | Iran | 30 | 21 w and 2 d | Severe ARDS | Class II obesity | - | 2 | NR | +10, +11 | None | Lopinavir/ritonavir, LMWH, azithromycin, methylprednisolone | Maternal well-being | Normal ongoing pregnancy |
| Lam, 2020 [19] | CR | USA | 30 | 23 w and 1 d | Severe ARDS | Type 2 DM, hypertension, pre-eclampsia | CD (25 w) | NR | NR | +1 | None | Remdesivir, dexamethasone, azithromycin, ceftriaxone | Pre-eclampsia, discharged on day +28 | Neonate intubated due to hypoxia, stable condition |
| Yaqoub, 2020 [21] | CR | Qatar | 33 | 32 w | Severe ARDS | Asthma, gestational diabetes | CD (32 w), IMV, ECMO | 2 | NR | +5 | NR | Lopinavir/ritonavir, tocilizumab, hydroxychloroquine, azithromycin, ceftriaxone | Clinical improvement, discharged on day +40 | Neonate intubated due to hypoxia, then positive outcome |
| Author | Selection | Ascertainment | Causality | Reporting |
|---|---|---|---|---|
| Grisolia, 2020 [17] | ★ | ★ | ★★ | ★ |
| Zhang, 2020 [25] | ★ | ★★ | ★ | ★ |
| Anderson, 2020 [26] | ★ | ★ | ★★ | ★ |
| Donzelli, 2020 [22] | ★ | ★★ | ★★ | ★ |
| Jacobson, 2021 [27] | ★ | ★★ | ★ | ★ |
| Magallanes-Garza, 2020 [23] | ★ | ★★ | ★★ | ★ |
| Pelayo, 2020 [24] | ★ | ★ | ★ | ★ |
| Jafari, 2020 [18] | ★ | ★ | ★ | ★ |
| Easterlin, 2020 [20] | ★ | ★ | ★ | ★ |
| Soleimani,2020 [16] | ★ | ★ | ★★ | ★ |
| Lam, 2020 [19] | ★ | ★ | ★ | ★ |
| Yaqoub, 2020 [21] | ★ | ★★ | ★ | ★ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchini, M.; Prefumo, F.; Grisolia, G.; Bergamini, V.; Glingani, C.; Pisello, M.; Presti, F.; Zaffanello, M. Convalescent Plasma for Pregnant Women with COVID-19: A Systematic Literature Review. Viruses 2021, 13, 1194. https://doi.org/10.3390/v13071194
Franchini M, Prefumo F, Grisolia G, Bergamini V, Glingani C, Pisello M, Presti F, Zaffanello M. Convalescent Plasma for Pregnant Women with COVID-19: A Systematic Literature Review. Viruses. 2021; 13(7):1194. https://doi.org/10.3390/v13071194
Chicago/Turabian StyleFranchini, Massimo, Federico Prefumo, Gianpaolo Grisolia, Valentino Bergamini, Claudia Glingani, Marlene Pisello, Francesca Presti, and Marco Zaffanello. 2021. "Convalescent Plasma for Pregnant Women with COVID-19: A Systematic Literature Review" Viruses 13, no. 7: 1194. https://doi.org/10.3390/v13071194
APA StyleFranchini, M., Prefumo, F., Grisolia, G., Bergamini, V., Glingani, C., Pisello, M., Presti, F., & Zaffanello, M. (2021). Convalescent Plasma for Pregnant Women with COVID-19: A Systematic Literature Review. Viruses, 13(7), 1194. https://doi.org/10.3390/v13071194







