GABAA-Receptor Agonists Limit Pneumonitis and Death in Murine Coronavirus-Infected Mice
Abstract
1. Introduction
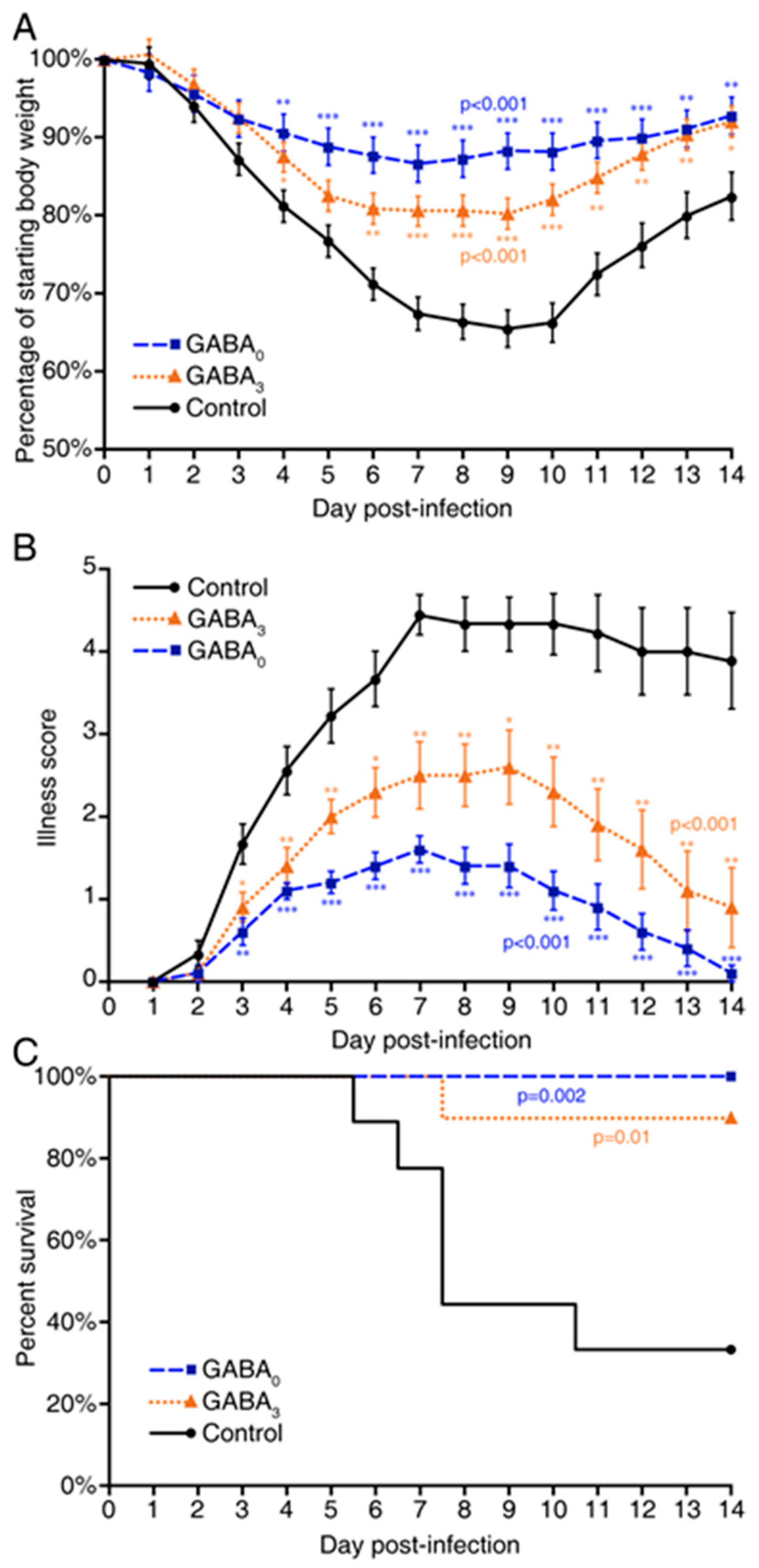
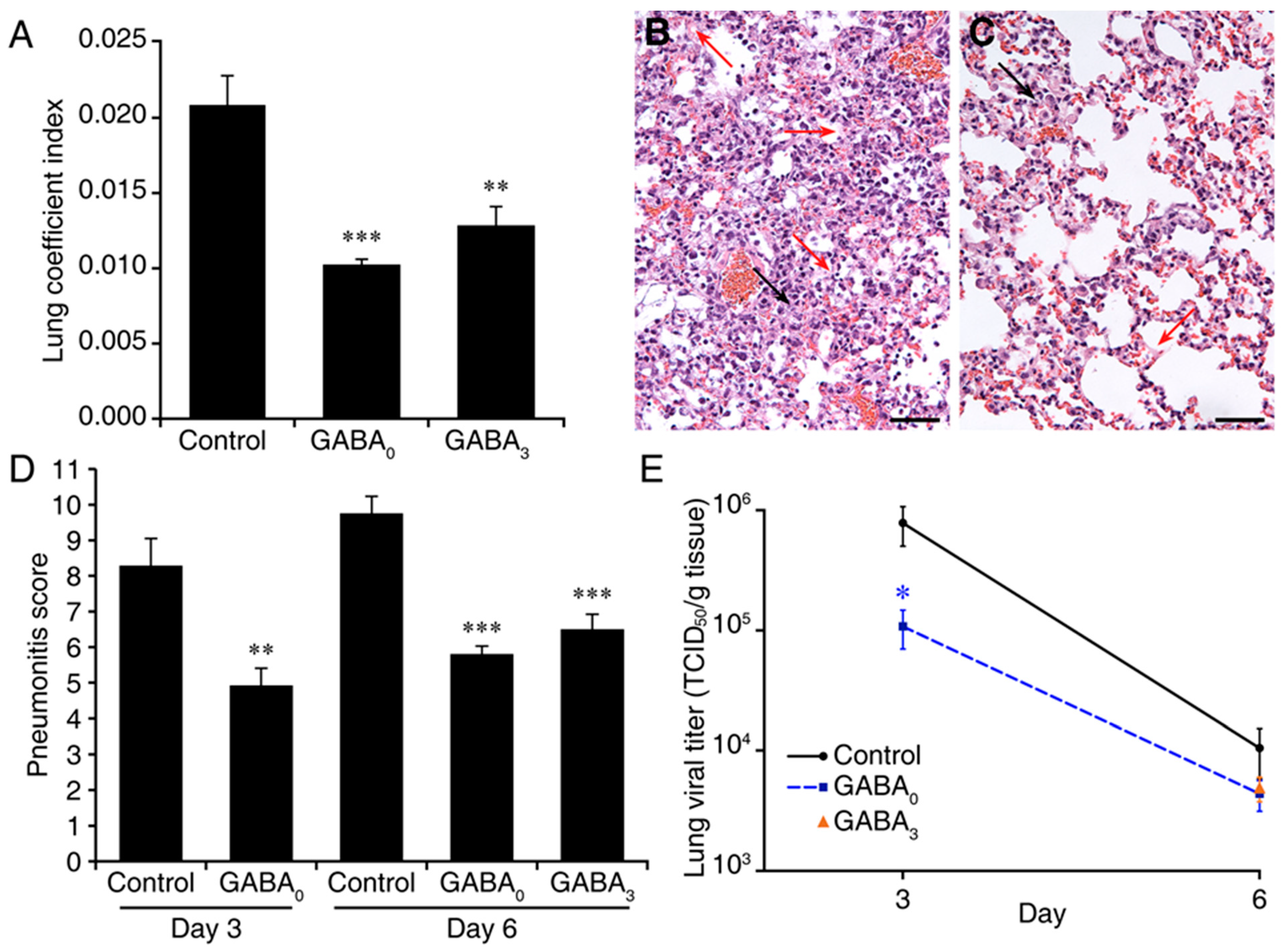
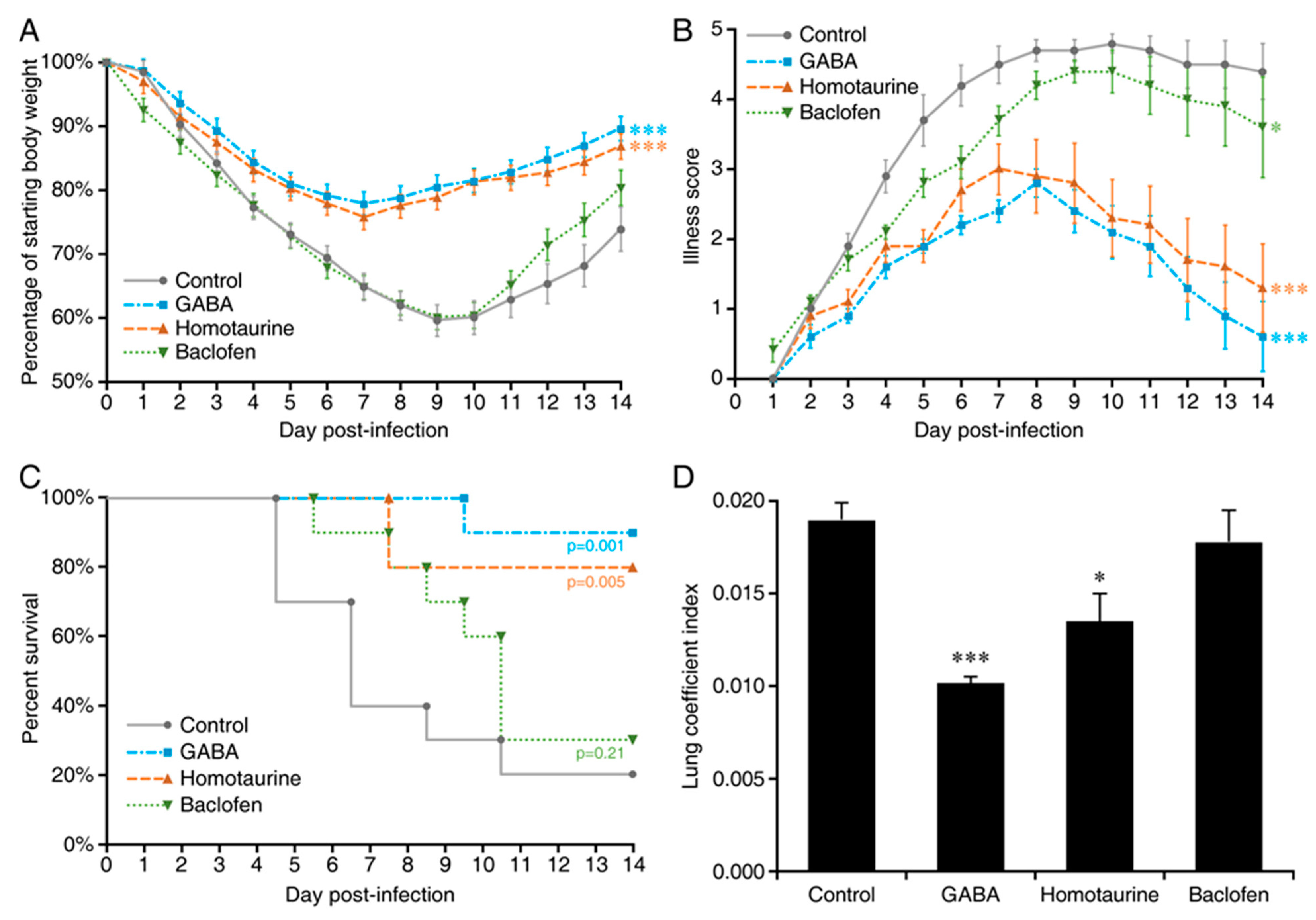
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olsen, R.W.; Sieghart, W. GABAA receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology 2009, 56, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Bettler, B.; Kaupmann, K.; Mosbacher, J.; Gassmann, M. Molecular structure and physiological functions of GABA(B) receptors. Physiol. Rev. 2004, 84, 835–867. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yong, J.; Dang, H.; Kaufman, D.L. Oral GABA treatment downregulates inflammatory responses in a mouse model of rheumatoid arthritis. Autoimmunity 2011, 44, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Axtell, R.; Mitra, A.; Miranda, M.; Lock, C.; Tsien, R.W.; Steinman, L. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2580–2585. [Google Scholar] [CrossRef] [PubMed]
- Januzi, L.; Poirier, J.W.; Maksoud, M.J.; Xiang, Y.-Y.; Veldhuizen, R.A.; Gill, S.E.; Cregan, S.P.; Zhang, H.; Dekaban, G.A.; Lu, W.-Y. Autocrine GABA signaling distinctively regulates phenotypic activation of mouse pulmonary macrophages. Cell. Immunol. 2018, 332, 7–23. [Google Scholar] [CrossRef]
- Prud’Homme, G.J.; Glinka, Y.; Hasilo, C.; Paraskevas, S.; Li, X.; Wang, Q. GABA Protects Human Islet Cells Against the Deleterious Effects of Immunosuppressive Drugs and Exerts Immunoinhibitory Effects Alone. Transplantation 2013, 96, 616–623. [Google Scholar] [CrossRef]
- Fuks, J.; Arrighi, R.B.G.; Weidner, J.M.; Mendu, S.K.; Jin, Z.; Wallin, R.P.A.; Rethi, B.; Birnir, B.; Barragan, A. GABAergic Signaling Is Linked to a Hypermigratory Phenotype in Dendritic Cells Infected by Toxoplasma gondii. PLoS Pathog. 2012, 8, e1003051. [Google Scholar] [CrossRef]
- Bhandage, A.K.; Jin, Z.; Korol, S.V.; Shen, Q.; Pei, Y.; Deng, Q.; Espes, D.; Carlsson, P.-O.; Kamali-Moghaddam, M.; Birnir, B. GABA Regulates Release of Inflammatory Cytokines from Peripheral Blood Mononuclear Cells and CD4+ T Cells and Is Immunosuppressive in Type 1 Diabetes. EBioMedicine 2018, 30, 283–294. [Google Scholar] [CrossRef]
- Tian, J.; Chau, C.; Hales, T.G.; Kaufman, D.L. GABA(A) receptors mediate inhibition of T cell responses. J. Neuroimmunol. 1999, 96, 21–28. [Google Scholar] [CrossRef]
- Tian, J.; Dang, H.; O’Laco, K.A.; Song, M.; Tiu, B.-C.; Gilles, S.; Zakarian, C.; Kaufman, D.L. Homotaurine Treatment Enhances CD4+ and CD8+ Regulatory T Cell Responses and Synergizes with Low-Dose Anti-CD3 to Enhance Diabetes Remission in Type 1 Diabetic Mice. ImmunoHorizons 2019, 3, 498–510. [Google Scholar] [CrossRef]
- Tian, J.; Dang, H.; Wallner, M.; Olsen, R.; Kaufman, D.L. Homotaurine, a safe blood-brain barrier permeable GABAA-R-specific agonist, ameliorates disease in mouse models of multiple sclerosis. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Mendu, S.K.; Åkesson, L.; Jin, Z.; Edlund, A.; Cilio, C.; Lernmark, Å.; Birnir, B. Increased GABAA channel subunits expression in CD8+ but not in CD4+ T cells in BB rats developing diabetes compared to their congenic littermates. Mol. Immunol. 2011, 48, 399–407. [Google Scholar] [CrossRef]
- Tian, J.; Dang, H.; Nguyen, A.V.; Chen, Z.; Kaufman, D.L. Combined Therapy With GABA and Proinsulin/Alum Acts Synergistically to Restore Long-term Normoglycemia by Modulating T-Cell Autoimmunity and Promoting ß-Cell Replication in Newly Diabetic NOD Mice. Diabetes 2014, 63, 3128–3134. [Google Scholar] [CrossRef]
- Tian, J.; Lu, Y.; Zhang, H.; Chau, C.H.; Dang, H.N.; Kaufman, D.L. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J. Immunol. 2004, 173, 5298–5304. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dang, H.N.; Yong, J.; Chui, W.-S.; Dizon, M.P.G.; Yaw, C.K.Y.; Kaufman, D.L. Oral Treatment with γ-Aminobutyric Acid Improves Glucose Tolerance and Insulin Sensitivity by Inhibiting Inflammation in High Fat Diet-Fed Mice. PLoS ONE 2011, 6, e25338. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Mao, J.; Wei, B.; Pei, G. The anti-spasticity drug baclofen alleviates collagen-induced arthritis and regulates dendritic cells. J. Cell. Physiol. 2015, 230, 1438–1447. [Google Scholar] [CrossRef]
- Duthey, B.; Hübner, A.; Diehl, S.; Boehncke, S.; Pfeffer, J.; Boehncke, W.-H. Anti-inflammatory effects of the GABAB receptor agonist baclofen in allergic contact dermatitis. Exp. Dermatol. 2010, 19, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Beales, P.E.; Hawa, M.; Williams, A.J.K.; Albertini, M.C.; Giorgini, A.; Pozzilli, P. Baclofen, a gamma-aminobutyric acid-b receptor agonist, delays diabetes onset in the non-obese diabetic mouse. Acta Diabetol. 1995, 32, 53–56. [Google Scholar] [CrossRef]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef]
- Lucas, C.; Team, Y.I.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nat. Cell Biol. 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Sariol, A.; Perlman, S. Lessons for COVID-19 Immunity from Other Coronavirus Infections. Immunity 2020, 53, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, Y.; Wang, C.; Gao, J. Propofol reduces acute lung injury by up-regulating gamma-aminobutyric acid type a receptors. Exp. Mol. Pathol. 2019, 110, 104295. [Google Scholar] [CrossRef] [PubMed]
- Fortis, S.; Spieth, P.M.; Lu, W.-Y.; Parotto, M.; Haitsma, J.J.; Slutsky, A.S.; Zhong, N.; Mazer, C.D.; Zhang, H. Effects of anesthetic regimes on inflammatory responses in a rat model of acute lung injury. Intensiv. Care Med. 2012, 38, 1548–1555. [Google Scholar] [CrossRef]
- Chintagari, N.R.; Liu, L. GABA receptor ameliorates ventilator-induced lung injury in rats by improving alveolar fluid clearance. Crit. Care 2012, 16, R55. [Google Scholar] [CrossRef]
- Jin, S.; Merchant, M.L.; Ritzenthaler, J.D.; McLeish, K.R.; Lederer, E.D.; Torres-Gonzalez, E.; Fraig, M.; Barati, M.T.; Lentsch, A.B.; Roman, J.; et al. Baclofen, a GABABR Agonist, Ameliorates Immune-Complex Mediated Acute Lung Injury by Modulating Pro-Inflammatory Mediators. PLoS ONE 2015, 10, e0121637. [Google Scholar] [CrossRef] [PubMed]
- Voigtsberger, S.; Lachmann, R.A.; Leutert, A.C.; Schlaepfer, M.; Booy, C.; Reyes, L.; Urner, M.; Schild, J.; Schimmer, R.C.; Beck-Schimmer, B. Sevoflurane Ameliorates Gas Exchange and Attenuates Lung Damage in Experimental Lipopolysaccharide-induced Lung Injury. Anesthesiology 2009, 111, 1238–1248. [Google Scholar] [CrossRef]
- Faller, S.; Strosing, K.M.; Ryter, S.W.; Buerkle, H.; Loop, T.; Schmidt, R.; Hoetzel, A. The Volatile Anesthetic Isoflurane Prevents Ventilator-Induced Lung Injury via Phosphoinositide 3-Kinase/Akt Signaling in Mice. Anesthesia Analg. 2012, 114, 747–756. [Google Scholar] [CrossRef]
- Taniguchi, T.; Yamamoto, K.; Ohmoto, N.; Ohta, K.; Kobayashi, T. Effects of propofol on hemodynamic and inflammatory responses to endotoxemia in rats. Crit. Care Med. 2000, 28, 1101–1106. [Google Scholar] [CrossRef]
- Lin, X.; Ju, Y.-N.; Gao, W.; Li, D.-M.; Guo, C.-C. Desflurane Attenuates Ventilator-Induced Lung Injury in Rats with Acute Respiratory Distress Syndrome. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Mahmoud, K.; Ammar, A. Immunomodulatory Effects of Anesthetics during Thoracic Surgery. Anesthesiol. Res. Pr. 2011, 2011, 1–6. [Google Scholar] [CrossRef][Green Version]
- De Conno, E.; Steurer, M.P.; Wittlinger, M.; Zalunardo, M.P.; Weder, W.; Schneiter, D.; Schimmer, R.C.; Klaghofer, R.; Neff, T.A.; Schmid, E.R.; et al. Anesthetic-induced Improvement of the Inflammatory Response to One-lung Ventilation. Anesthesiology 2009, 110, 1316–1326. [Google Scholar] [CrossRef]
- Schilling, T.; Kozian, A.; Kretzschmar, M.; Huth, C.; Welte, T.; Bühling, F.; Hedenstierna, G.; Hachenberg, T. Effects of propofol and desflurane anaesthesia on the alveolar inflammatory response to one-lung ventilation. Br. J. Anaesth. 2007, 99, 368–375. [Google Scholar] [CrossRef]
- Kochiyama, T.; Li, X.; Nakayama, H.; Kage, M.; Yamane, Y.; Takamori, K.; Iwabuchi, K.; Inada, E. Effect of Propofol on the Production of Inflammatory Cytokines by Human Polarized Macrophages. Mediat. Inflamm. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Forkuo, G.S.; Nieman, A.N.; Kodali, R.; Zahn, N.M.; Li, G.; Roni, S.R.; Stephen, M.R.; Harris, T.W.; Jahan, R.; Guthrie, M.L.; et al. A Novel Orally Available Asthma Drug Candidate That Reduces Smooth Muscle Constriction and Inflammation by Targeting GABAA Receptors in the Lung. Mol. Pharm. 2018, 15, 1766–1777. [Google Scholar] [CrossRef]
- Wheeler, D.W.; Thompson, A.J.; Corletto, F.; Reckless, J.; Loke, J.C.T.; Lapaque, N.; Grant, A.J.; Mastroeni, P.; Grainger, D.J.; Padgett, C.L.; et al. Anaesthetic Impairment of Immune Function Is Mediated via GABAA Receptors. PLoS ONE 2011, 6, e17152. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.-Y.; Chen, X.; Li, J.; Wang, S.; Faclier, G.; MacDonald, J.F.; Hogg, J.C.; Orser, B.A.; Lu, W.-Y. Isoflurane Regulates Atypical Type-A γ-Aminobutyric Acid Receptors in Alveolar Type II Epithelial Cells. Anesthesiology 2013, 118, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Boost, K.A.; Leipold, T.; Scheiermann, P.; Hoegl, S.; Sadik, C.D.; Hofstetter, C.; Zwissler, B. Sevoflurane and isoflurane decrease TNF-α-induced gene expression in human monocytic THP-1 cells: Potential role of intracellular IκBα regulation. Int. J. Mol. Med. 2009, 23, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Lu, W.-J.; Wang, S.-H.; Fong, T.-H.; Chou, D.-S.; Chang, C.-C.; Chang, N.-C.; Chiang, Y.-C.; Huang, S.-Y.; Sheu, J.-R. Characteristics of endogenous γ-aminobutyric acid (GABA) in human platelets: Functional studies of a novel collagen glycoprotein VI inhibitor. J. Mol. Med. 2014, 92, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Azevedo-Quintanilha, I.G.; Palhinha, L.; Teixeira, L.; Barreto, E.A.; Pão, C.R.R.; Righy, C.; Franco, S.; Souza, T.M.L.; Kurtz, P.; et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 2020, 136, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, N.; Baig, E.; Ma, X.; Zhang, J.; He, W.; Rowe, A.; Habal, M.; Liu, M.; Shalev, I.; Downey, G.P.; et al. MurineHepatitis Virus Strain 1 Produces a Clinically Relevant Model of Severe Acute Respiratory Syndrome in A/J Mice. J. Virol. 2006, 80, 10382–10394. [Google Scholar] [CrossRef] [PubMed]
- Khanolkar, A.; Hartwig, S.M.; Haag, B.A.; Meyerholz, D.K.; Epping, L.L.; Haring, J.S.; Varga, S.M.; Harty, J.T. Protective and Pathologic Roles of the Immune Response to Mouse Hepatitis Virus Type 1: Implications for Severe Acute Respiratory Syndrome. J. Virol. 2009, 83, 9258–9272. [Google Scholar] [CrossRef] [PubMed]
- Khanolkar, A.; Hartwig, S.M.; Haag, B.A.; Meyerholz, D.K.; Harty, J.T.; Varga, S.M. Toll-Like Receptor 4 Deficiency Increases Disease and Mortality after Mouse Hepatitis Virus Type 1 Infection of Susceptible C3H Mice. J. Virol. 2009, 83, 8946–8956. [Google Scholar] [CrossRef]
- Khanolkar, A.; Fulton, R.B.; Epping, L.L.; Pham, N.-L.; Tifrea, D.; Varga, S.M.; Harty, J.T. T Cell Epitope Specificity and Pathogenesis of Mouse Hepatitis Virus-1–Induced Disease in Susceptible and Resistant Hosts. J. Immunol. 2010, 185, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.S.; Lee, H.-M.; Jin, H.S.; Neupane, C.; Kim, S.; Lee, S.-H.; Min, J.-J.; Sasai, M.; Jeong, J.-H.; et al. GABAergic signaling linked to autophagy enhances host protection against intracellular bacterial infections. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Kolliputi, N.; Gou, D.; Weng, T.; Liu, L. A Novel Function of Ionotropic γ-Aminobutyric Acid Receptors Involving Alveolar Fluid Homeostasis. J. Biol. Chem. 2006, 281, 36012–36020. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Fang, L.; Xia, S.; Ke, W.; Wang, J.; Wu, X.; Fang, P.; Xiao, S. Porcine deltacoronavirus (PDCoV) modulates calcium influx to favor viral replication. Virology 2020, 539, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Kraeft, S.-K.; Chen, D.S.; Lib, H.-P.; Chen, L.B.; Lai, M.M. Mouse Hepatitis Virus Infection Induces an Early, Transient Calcium Influx in Mouse Astrocytoma Cells. Exp. Cell Res. 1997, 237, 55–62. [Google Scholar] [CrossRef]
- Tian, J.; Middleton, B.; Lee, V.S.; Park, H.W.; Zhang, Z.; Kim, B.; Lowe, C.; Nguyen, N.; Liu, H.; Beyer, R.S.; et al. GABAB-Receptor Agonist-Based Immunotherapy for Type 1 Diabetes in NOD Mice. Biomedicines 2021, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Otomo, E.; Araki, G.; Mori, A.; Kurihara, M. Clinical evaluation of GABA in the treatment of cerebrovascular disorders. Multi-center double-blind study in comparison with pyrithioxine and placebo. Arzneimittelforschung 1981, 31, 1511–1523. [Google Scholar]
- Loeb, C.; Benassi, E.; Bo, G.-P.; Cocito, L.; Maffini, M.; Scotto, P. Preliminary evaluation of the effect of GABA and phosphatidylserine in epileptic patients. Epilepsy Res. 1987, 1, 209–212. [Google Scholar] [CrossRef]
- Tower, D.B.; Roberts, E. (Eds.) Inhibition in the Nervous System and GABA; Pergamon Press: New York, NY, USA, 1960. [Google Scholar]
- Li, J.; Zhang, Z.; Liu, X.; Wang, Y.; Mao, F.; Mao, J.; Lu, X.; Jiang, D.; Wan, Y.; Lv, J.-Y.; et al. Study of GABA in Healthy Volunteers: Pharmacokinetics and Pharmacodynamics. Front. Pharmacol. 2015, 6, 260. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Gauthier, S.; Ferris, S.H.; Saumier, D.; Haine, D.; Garceau, D.; Duong, A.; Suhy, J.; Oh, J.; Lau, W.C.; et al. Tramiprosate in mild-to-moderate Alzheimer’s disease—A randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study). Arch. Med Sci. 2011, 1, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Aisen, P.S.; Ferris, S.H.; Saumier, D.; Duong, A.; Haine, D.; Garceau, D.; Suhy, J.; Oh, J.; Lau, W.; et al. Effect of tramiprosate in patients with mild-to-moderate alzheimer’s disease: Exploratory analyses of the MRI sub-group of the alphase study. J. Nutr. Health Aging 2009, 13, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, M. Future strategies of management of Alzheimer’s Disease. The role of homotaurine. Hell. J. Nucl Med. 2019, 22, 82–94. [Google Scholar]
- Leibowitz, J.; Kaufman, G.; Liu, P. Coronaviruses: Propagation, Quantification, Storage, and Construction of Recombinant Mouse Hepatitis Virus. Curr. Protoc. Microbiol. 2011, 21. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Russo, R.C.; Thurston, R.V. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1977, 11, 714–719. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Middleton, B.; Kaufman, D.L. GABAA-Receptor Agonists Limit Pneumonitis and Death in Murine Coronavirus-Infected Mice. Viruses 2021, 13, 966. https://doi.org/10.3390/v13060966
Tian J, Middleton B, Kaufman DL. GABAA-Receptor Agonists Limit Pneumonitis and Death in Murine Coronavirus-Infected Mice. Viruses. 2021; 13(6):966. https://doi.org/10.3390/v13060966
Chicago/Turabian StyleTian, Jide, Blake Middleton, and Daniel L. Kaufman. 2021. "GABAA-Receptor Agonists Limit Pneumonitis and Death in Murine Coronavirus-Infected Mice" Viruses 13, no. 6: 966. https://doi.org/10.3390/v13060966
APA StyleTian, J., Middleton, B., & Kaufman, D. L. (2021). GABAA-Receptor Agonists Limit Pneumonitis and Death in Murine Coronavirus-Infected Mice. Viruses, 13(6), 966. https://doi.org/10.3390/v13060966




