Age and Infectious Dose Significantly Affect Disease Progression after RHDV2 Infection in Naïve Domestic Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Trials
2.2. Virus Inoculum
2.3. Monitoring
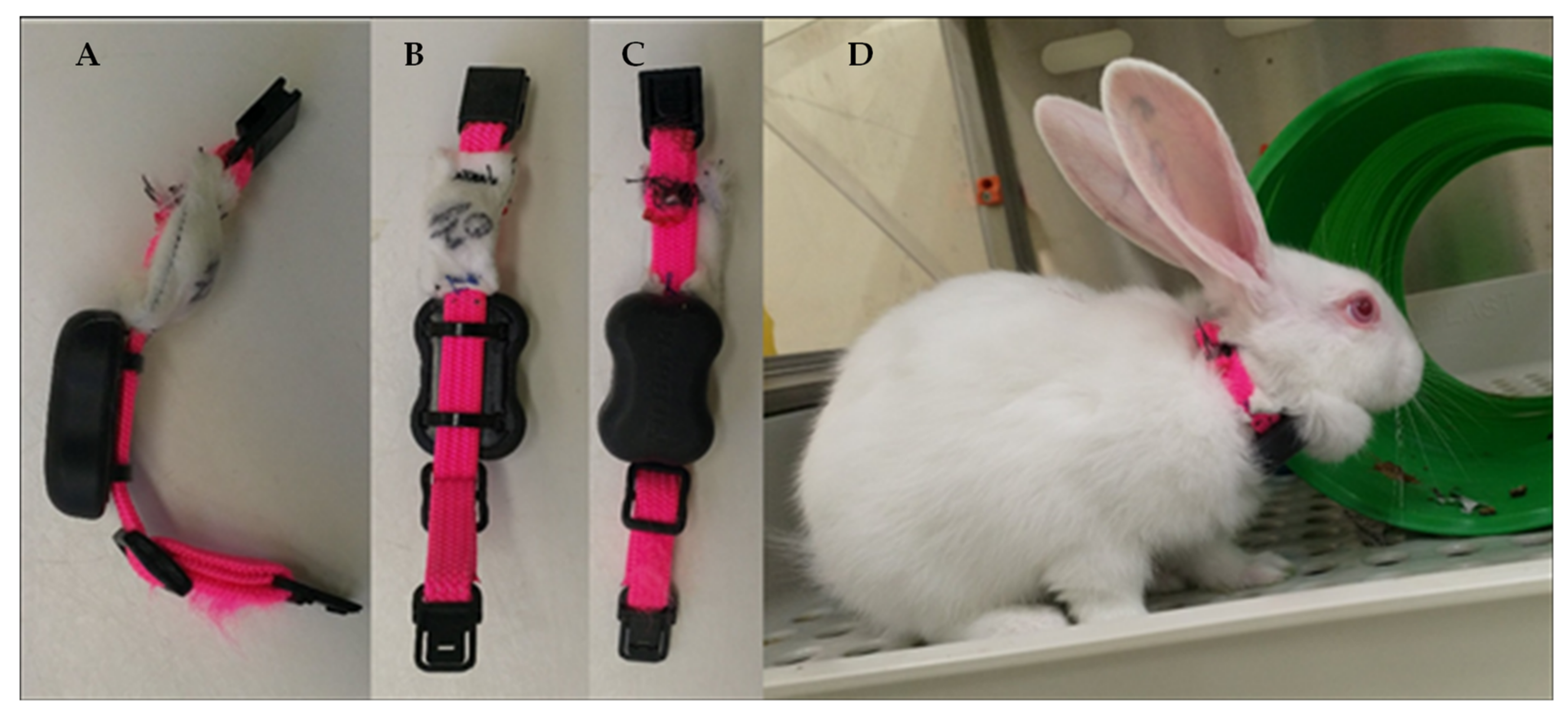
2.4. Sample Collection
2.5. Laboratory Analyses
2.6. Data Analyses
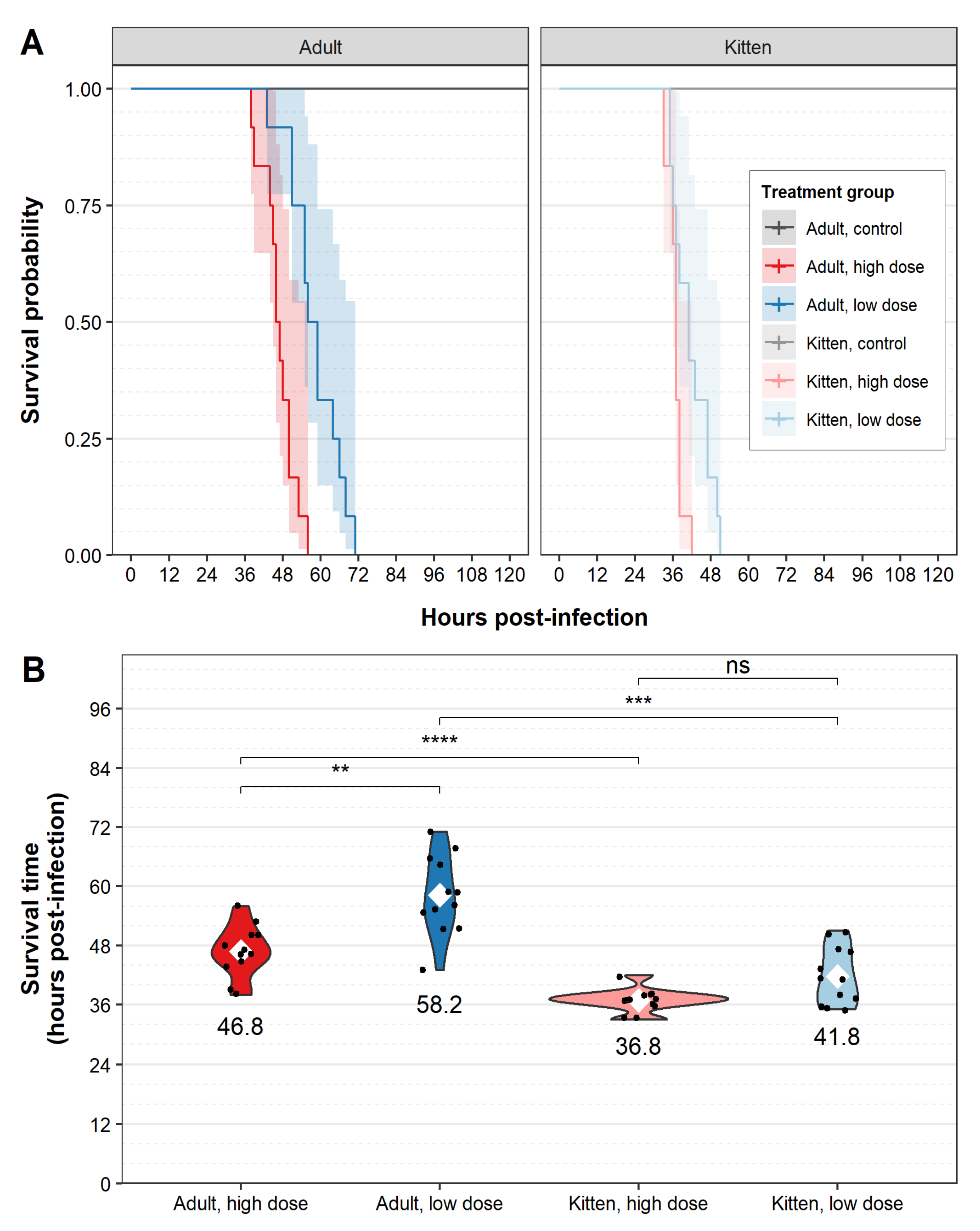
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrantes, J.; van der Loo, W.; Le Pendu, J.; Esteves, P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): A review. Vet. Res. 2012, 43, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.J.; Xue, H.P.; Pu, B.Q.; Qian, N.H. A new viral disease in rabbits. J. Vet. Diagn. Invest. 1984, 16, 253–255. [Google Scholar]
- Spickler, A. Rabbit Hemorrhagic Disease. 2016. Available online: https://www.cfsph.iastate.edu/Factsheets/pdfs/rabbit_hemorrhagic_disease.pdf (accessed on 5 March 2021).
- Dalton, K.P.; Nicieza, I.; Balseiro, A.; Muguerza, M.A.; Rosell, J.M.; Casais, R.; Alvarez, A.L.; Parra, F. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg. Infect. Dis. 2012, 18, 2009–2012. [Google Scholar] [CrossRef] [PubMed]
- Le Gall-Recule, G.; Zwingelstein, F.; Boucher, S.; Le Normand, B.; Plassiart, G.; Portejoie, Y.; Decors, A.; Bertagnoli, S.; Guerin, J.L.; Marchandeau, S. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet. Rec. 2011, 168, 137–138. [Google Scholar] [CrossRef] [Green Version]
- Puggioni, G.; Cavadini, P.; Maestrale, C.; Scivoli, R.; Botti, G.; Ligios, C.; Le Gall-Recule, G.; Lavazza, A.; Capucci, L. The new French 2010 rabbit hemorrhagic disease virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus). Vet. Res. 2013, 44, 96. [Google Scholar] [CrossRef] [Green Version]
- Camarda, A.; Pugliese, N.; Cavadini, P.; Circella, E.; Capucci, L.; Caroli, A.; Legretto, M.; Mallia, E.; Lavazza, A. Detection of the new emerging rabbit haemorrhagic disease type 2 virus (RHDV2) in Sicily from rabbit (Oryctolagus cuniculus) and Italian hare (Lepus corsicanus). Res. Vet. Sci. 2014, 97, 642–645. [Google Scholar] [CrossRef]
- Velarde, R.; Cavadini, P.; Neimanis, A.; Cabezon, O.; Chiari, M.; Gaffuri, A.; Lavin, S.; Grilli, G.; Gavier-Widen, D.; Lavazza, A.; et al. Spillover events of infection of Brown hares (Lepus europaeus) with rabbit haemorrhagic disease type 2 virus (RHDV2) caused sporadic cases of an European Brown Hare syndrome-like disease in Italy and Spain. Transbound. Emerg. Dis. 2017, 64, 1750–1761. [Google Scholar] [CrossRef]
- Neimanis, A.S.; Ahola, H.; Larsson Pettersson, U.; Lopes, A.M.; Abrantes, J.; Zohari, S.; Esteves, P.J.; Gavier-Widen, D. Overcoming species barriers: An outbreak of Lagovirus europaeus GI.2/RHDV2 in an isolated population of mountain hares (Lepus timidus). BMC Vet. Res. 2018, 14, 367. [Google Scholar] [CrossRef] [Green Version]
- World Organisation for Animal Health. World Animal Health Information Database (WAHIS Interface)—Version 1. Available online: https://wahis.oie.int/#/home (accessed on 5 March 2021).
- World Organisation for Animal Health. Follow-Up Report 9 Rabbit Haemorrhagic Disease, United States of America. 2020. Available online: https://wahis.oie.int/#/report-info?reportId=14804 (accessed on 5 March 2021).
- Mahar, J.E.; Hall, R.N.; Peacock, D.; Kovaliski, J.; Piper, M.; Mourant, R.; Huang, N.; Campbell, S.; Gu, X.; Read, A.; et al. Rabbit hemorrhagic disease virus 2 (RHDV2; GI.2) is replacing endemic strains of RHDV in the Australian landscape within 18 months of its arrival. J. Virol. 2018, 92, e01374-17. [Google Scholar] [CrossRef] [Green Version]
- Bull, R.A.; Tanaka, M.M.; White, P. Norovirus recombination. J. Gen. Virol. 2007, 88, 3347–3359. [Google Scholar] [CrossRef]
- Le Gall-Recule, G.; Lavazza, A.; Marchandeau, S.; Bertagnoli, S.; Zwingelstein, F.; Cavadini, P.; Martinelli, N.; Lombardi, G.; Guerin, J.L.; Lemaitre, E.; et al. Emergence of a new lagovirus related to rabbit haemorrhagic disease virus. Vet. Res. 2013, 44, 81. [Google Scholar] [CrossRef] [Green Version]
- Almeida, T.; Lopes, A.M.; Magalhaes, M.J.; Neves, F.; Pinheiro, A.; Goncalves, D.; Leitao, M.; Esteves, P.J.; Abrantes, J. Tracking the evolution of the G1/RHDVb recombinant strains introduced from the Iberian Peninsula to the Azores islands, Portugal. Infect. Genet. Evol. 2015, 34, 307–313. [Google Scholar] [CrossRef]
- Lopes, A.M.; Dalton, K.P.; Magalhaes, M.J.; Parra, F.; Esteves, P.J.; Holmes, E.C.; Abrantes, J. Full genomic analysis of new variant rabbit hemorrhagic disease virus revealed multiple recombination events. J. Gen. Virol. 2015, 96, 1309–1319. [Google Scholar] [CrossRef]
- Hall, R.N.; Mahar, J.E.; Read, A.J.; Mourant, R.; Piper, M.; Huang, N.; Strive, T. A strain-specific multiplex RT-PCR for Australian rabbit haemorrhagic disease viruses uncovers a new recombinant virus variant in rabbits and hares. Transbound. Emerg. Dis. 2018, 65, e444–e456. [Google Scholar] [CrossRef]
- Le Gall-Recule, G.; Lemaitre, E.; Bertagnoli, S.; Hubert, C.; Top, S.; Decors, A.; Marchandeau, S.; Guitton, J.S. Large-scale lagovirus disease outbreaks in European brown hares (Lepus europaeus) in France caused by RHDV2 strains spatially shared with rabbits (Oryctolagus cuniculus). Vet. Res. 2017, 48, 70. [Google Scholar] [CrossRef] [Green Version]
- Silverio, D.; Lopes, A.M.; Melo-Ferreira, J.; Magalhaes, M.J.; Monterroso, P.; Serronha, A.; Maio, E.; Alves, P.C.; Esteves, P.J.; Abrantes, J. Insights into the evolution of the new variant rabbit haemorrhagic disease virus (GI.2) and the identification of novel recombinant strains. Transbound. Emerg. Dis. 2018, 65, 983–992. [Google Scholar] [CrossRef]
- Baily, J.L.; Dagleish, M.P.; Graham, M.; Maley, M.; Rocchi, M.S. RHDV variant 2 presence detected in Scotland. Vet. Rec. 2014, 174, 411. [Google Scholar] [CrossRef]
- Delibes-Mateos, M.; Ferreira, C.; Carro, F.; Escudero, M.A.; Gortazar, C. Ecosystem effects of variant rabbit hemorrhagic disease virus, Iberian Peninsula. Emerg. Infect. Dis. 2014, 20, 2166–2168. [Google Scholar] [CrossRef] [Green Version]
- Simpson, V.; Everest, D.; Westcott, D. RHDV variant 2 and Capillaria hepatica infection in rabbits. Vet. Rec. 2014, 174, 486. [Google Scholar] [CrossRef]
- Monterroso, P.; Garrote, G.; Serronha, A.; Santos, E.; Delibes-Mateos, M.; Abrantes, J.; Perez de Ayala, R.; Silvestre, F.; Carvalho, J.; Vasco, I.; et al. Disease-mediated bottom-up regulation: An emergent virus affects a keystone prey, and alters the dynamics of trophic webs. Sci. Rep. 2016, 6, 36072. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.L.; Duarte, E.L.; Monteiro, M.; Botelho, A.; Albuquerque, T.; Fevereiro, M.; Henriques, A.M.; Barros, S.S.; Duarte, M.D. Challenges in the rabbit haemorrhagic disease 2 (RHDV2) molecular diagnosis of vaccinated rabbits. Vet. Microbiol. 2017, 198, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Casado, J.; Carpio, A.J.; Tortosa, F.S. Recent negative trends of wild rabbit populations in southern Spain after the arrival of the new variant of the rabbit hemorrhagic disease virus RHDV2. Mammalian Biol. 2016, 81, 361–364. [Google Scholar] [CrossRef]
- Mutze, G.; De Preu, N.; Mooney, T.; Koerner, D.; McKenzie, D.; Sinclair, R.; Kovaliskli, J.; Peacock, D. Substantial numerical decline in South Australian rabbit populations following the detection of rabbit haemorrhagic disease virus 2. Vet. Rec. 2018, 182, 574. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, D.S.L.; Cox, T.; Strive, T.; Forsyth, D.M.; Stuart, I.; Hall, R.N.; Elsworth, P.; Campbell, S. Emerging RHDV2 suppresses the impact of endemic and novel strains of RHDV on wild rabbit populations. J. Appl. Ecol. 2020, 57, 630–641. [Google Scholar] [CrossRef]
- Le Minor, O.; Beilvert, F.; Le Moullec, T.; Djadour, D.; Martineau, J. Evaluation de l’efficacité d’un nouveau vaccin contre le virus variant de la maladie hémorragique virale du lapin (VHD). In 15èmes Journées de la Recherche Cunicole; Laboratoire FILAVIE: Roussay, France, 2013. [Google Scholar]
- Capucci, L.; Cavadini, P.; Schiavitto, M.; Lombardi, G.; Lavazza, A. Increased pathogenicity in rabbit haemorrhagic disease virus type 2 (RHDV2). Vet. Rec. 2017, 180, 426. [Google Scholar] [CrossRef] [Green Version]
- Calvete, C.; Mendoza, M.; Alcaraz, A.; Sarto, M.P.; Jimenez-de-Baguess, M.P.; Calvo, A.J.; Monroy, F.; Calvo, J.H. Rabbit haemorrhagic disease: Cross-protection and comparative pathogenicity of GI.2/RHDV2/b and GI.1b/RHDV lagoviruses in a challenge trial. Vet. Microbiol. 2018, 219, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Muller, C.; Ulrich, R.; Franzke, K.; Muller, M.; Kollner, B. Crude extracts of recombinant baculovirus expressing rabbit hemorrhagic disease virus 2 VLPs from both insect and rabbit cells protect rabbits from rabbit hemorrhagic disease caused by RHDV2. Arch. Virol. 2019, 164, 137–148. [Google Scholar] [CrossRef]
- Muller, C.; Ulrich, R.; Schinkothe, J.; Muller, M.; Kollner, B. Characterization of protective humoral and cellular immune responses against RHDV2 induced by a new vaccine based on recombinant baculovirus. Vaccine 2019, 37, 4195–4203. [Google Scholar] [CrossRef]
- Dalton, K.P.; Balseiro, A.; Juste, R.A.; Podadera, A.; Nicieza, I.; Del Llano, D.; Gonzalez, R.; Martin Alonso, J.M.; Prieto, J.M.; Parra, F.; et al. Clinical course and pathogenicity of variant rabbit haemorrhagic disease virus in experimentally infected adult and kit rabbits: Significance towards control and spread. Vet. Microbiol. 2018, 220, 24–32. [Google Scholar] [CrossRef]
- Neimanis, A.; Larsson Pettersson, U.; Huang, N.; Gavier-Widen, D.; Strive, T. Elucidation of the pathology and tissue distribution of Lagovirus europaeus GI.2/RHDV2 (rabbit haemorrhagic disease virus 2) in young and adult rabbits (Oryctolagus cuniculus). Vet. Res. 2018, 49, 46. [Google Scholar] [CrossRef] [Green Version]
- Hall, R.N.; Mahar, J.E.; Haboury, S.; Stevens, V.; Holmes, E.C.; Strive, T. Emerging rabbit hemorrhagic disease virus 2 (RHDVb), Australia. Emerg. Infect. Dis. 2015, 21, 2276–2278. [Google Scholar] [CrossRef]
- Lopes, A.M.; Blanco-Aguiar, J.; Martin-Alonso, A.; Leitao, M.; Foronda, P.; Mendes, M.; Goncalves, D.; Abrantes, J.; Esteves, P.J. Full genome sequences are key to disclose RHDV2 emergence in the Macaronesian islands. Virus Genes 2018, 54, 1–4. [Google Scholar] [CrossRef]
- Dalton, K.P.; Arnal, J.L.; Benito, A.A.; Chacon, G.; Martin Alonso, J.M.; Parra, F. Conventional and real time RT-PCR assays for the detection and differentiation of variant rabbit hemorrhagic disease virus (RHDVb) and its recombinants. J. Virol. Methods 2018, 251, 118–122. [Google Scholar] [CrossRef]
- Fitzner, A.; Niedbalski, W. Detection of rabbit haemorrhagic disease virus 2 (GI.2) in Poland. Pol. J. Vet. Sci. 2018, 21, 451–458. [Google Scholar]
- Liu, J.; Kerr, P.J.; Wright, J.D.; Strive, T. Serological assays to discriminate rabbit haemorrhagic disease virus from Australian non-pathogenic rabbit calicivirus. Vet. Microbiol. 2012, 157, 345–354. [Google Scholar] [CrossRef]
- Cooke, B.D.; Robinson, A.J.; Merchant, J.C.; Nardin, A.; Capucci, L. Use of ELISAs in field studies of rabbit haemorrhagic disease (RHD) in Australia. Epidemiol. Infect. 2000, 124, 563–576. [Google Scholar] [CrossRef]
- Strive, T.; Piper, M.; Huang, N.; Mourant, R.; Kovaliski, J.; Capucci, L.; Cox, T.E.; Smith, I. Retrospective serological analysis reveals presence of the emerging lagovirus RHDV2 in Australia in wild rabbits at least five months prior to its first detection. Transbound. Emerg. Dis. 2020, 67, 822–833. [Google Scholar] [CrossRef]
- Read, A.J.; Kirkland, P.D. Efficacy of a commercial vaccine against different strains of rabbit haemorrhagic disease virus. Aust. Vet. J. 2017, 95, 223–226. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. Ggpubr: ‘Ggplot2’ Based Publication Ready Plots. Available online: https://cran.r-project.org/package=ggpubr (accessed on 4 April 2021).
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘Ggplot2’. Available online: https://cran.r-project.org/package=survminer (accessed on 4 April 2021).
- Wickham, H.; Bryan, J. Readxl: Read Excel Files. Available online: https://cran.r-project.org/package=readxl (accessed on 4 April 2021).
- Wickham, H. The split-apply-combine strategy for data analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Borchers, H.W. Pracma: Practical Numerical Math Functions. Available online: https://cran.r-project.org/package=pracma (accessed on 4 April 2021).
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. Available online: https://cran.r-project.org/package=emmeans (accessed on 4 April 2021).
- Wilke, C. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘Ggplot2’. Available online: https://cran.r-project.org/package=cowplot (accessed on 4 April 2021).
- Neuwirth, E. RColorBrewer: ColorBrewer Palettes. Available online: https://cran.r-project.org/package=RColorBrewer (accessed on 4 April 2021).
- Wickham, H.; Seidel, D. Scales: Scale Functions for Visualization. Available online: https://cran.r-project.org/package=scales (accessed on 4 April 2021).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Illera, J.C.; Gonzalez Gil, A.; Silvan, G.; Illera, M. The effects of different anaesthetic treatments on the adreno-cortical functions and glucose levels in NZW rabbits. J. Physiol. Biochem. 2000, 56, 329–336. [Google Scholar] [CrossRef]
- Abrantes, J.; Droillard, C.; Lopes, A.M.; Lemaitre, E.; Lucas, P.; Blanchard, Y.; Marchandeau, S.; Esteves, P.J.; Le Gall-Recule, G. Recombination at the emergence of the pathogenic rabbit haemorrhagic disease virus Lagovirus europaeus/GI.2. Sci. Rep. 2020, 10, 14502. [Google Scholar] [CrossRef]
- Mahar, J.E.; Jenckel, M.; Huang, N.; Smertina, E.; Holmes, E.C.; Strive, T.; Hall, R.N. Frequent intergenotypic recombination between the non-structural and structural genes is a major driver of epidemiological fitness in caliciviruses. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ferreira, P.G.; Dinis, M.; Costa, E.; Silva, A.; Aguas, A.P. Adult rabbits acquire resistance to lethal calicivirus infection by adoptive transfer of sera from infected young rabbits. Vet. Immunol. Immunopathol. 2008, 121, 364–369. [Google Scholar] [CrossRef]
- Urakova, N.; Netzler, N.; Kelly, A.G.; Frese, M.; White, P.A.; Strive, T. Purification and biochemical characterisation of rabbit calicivirus RNA-dependent RNA polymerases and identification of non-nucleoside inhibitors. Viruses 2016, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.Y.; Chou, C.C.; Liu, C.I.; Shien, J.H. Impairment of renal function and electrolyte balance in rabbit hemorrhagic disease. J. Vet. Med. Sci. 2008, 70, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Tunon, M.J.; Sanchez-Campos, S.; Garcia-Ferreras, J.; Alvarez, M.; Jorquera, F.; Gonzalez-Gallego, J. Rabbit hemorrhagic viral disease: Characterization of a new animal model of fulminant liver failure. J. Lab. Clin. Med. 2003, 141, 272–278. [Google Scholar] [CrossRef]
- Fisher, P.; Brown, S.; Arrow, J. Welfare impacts of pindone poisoning in rabbits (Oryctolagus cuniculus). Animals 2016, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Balci, F.; Oakeshott, S.; Shamy, J.L.; El-Khodor, B.F.; Filippov, I.; Mushlin, R.; Port, R.; Connor, D.; Paintdakhi, A.; Menalled, L.; et al. High-throughput automated phenotyping of two genetic mouse models of Huntington’s disease. PLoS Curr. 2013, 5. [Google Scholar] [CrossRef]
- Wonderlich, E.R.; Swan, Z.D.; Bissel, S.J.; Hartman, A.L.; Carney, J.P.; O’Malley, K.J.; Obadan, A.O.; Santos, J.; Walker, R.; Sturgeon, T.J.; et al. Widespread virus replication in alveoli drives acute respiratory distress syndrome in aerosolized H5N1 influenza infection of macaques. J. Immunol. 2017, 198, 1616–1626. [Google Scholar] [CrossRef] [Green Version]
- Honnold, S.P.; Mossel, E.C.; Bakken, R.R.; Fisher, D.; Lind, C.M.; Cohen, J.W.; Eccleston, L.T.; Spurgers, K.B.; Erwin-Cohen, R.; Bradfute, S.B.; et al. Eastern equine encephalitis virus in mice I: Clinical course and outcome are dependent on route of exposure. Virol. J. 2015, 12, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, T.K.; Trefry, J.C.; Marko, S.T.; Chance, T.B.; Wells, J.B.; Pratt, W.D.; Johnson, J.C.; Mucker, E.M.; Norris, S.L.; Chappell, M.; et al. Euthanasia assessment in Ebola virus infected nonhuman primates. Viruses 2014, 6, 4666–4682. [Google Scholar] [CrossRef] [Green Version]
- Hartman, A.L.; Powell, D.S.; Bethel, L.M.; Caroline, A.L.; Schmid, R.J.; Oury, T.; Reed, D.S. Aerosolized rift valley fever virus causes fatal encephalitis in African green monkeys and common marmosets. J. Virol. 2014, 88, 2235–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, T.F.; Huitron-Resendiz, S.; Weed, M.R.; Henriksen, S.J.; Fox, H.S. Early physiological abnormalities after simian immunodeficiency virus infection. PNAS 1998, 95, 15072–15077. [Google Scholar] [CrossRef] [Green Version]

| Isolate | Variant | Rabbit Type † | Inoculum Dose § | Route of Infection ‡ | Age | Time to Endpoint || | # Dead/ # Total | Case Fatality (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| France 2010 10-07 | Unknown NS; GI.2VP60 | NZW | 10% liver homogenate with >5120 HA units | IM | >10 weeks | 5–9 days | 1/5 | 20% | [14] |
| France 2010 10-28 | GI.3NS/GI.2VP60 | NZW | PO | 11/24 | 46% | ||||
| IM | 1/2 | 50% | |||||||
| Italy 2011 Ud11 | Unknown NS; GI.2VP60 | NZW | PO | 1/5 | 20% | ||||
| France 2010 10-32 | GI.3NS/GI.2VP60 | NZW | PO | 3/32 | 9% | ||||
| IV | 0/4 | 0% | |||||||
| Rural | PO | 0/5 | 0% | ||||||
| France 2012 | Unknown NS; GI.2VP60 | Unknown | Unknown | IM | >10 weeks | 2–9 days | 45/95 | 47% | [28] |
| 4 weeks | 2–3 days | 8/15 | 53% | ||||||
| Italy 2014 Ta14 | Unknown NS; GI.2VP60 | NZW | 0.5% liver homogenate | PO | Adult | Av. 75 h | 4/5 ¶ | 80% ¶ | [29] |
| Italy 2015 Ch15 | Av. 86 h | 4/5 ¶ | 80% ¶ | ||||||
| Spain 2013 Zar2013-10 | GI.3NS/GI.2VP60 | NZW | 0.2% clarified liver homogenate | PO | <6.1 weeks | Av. 53 h | 23/51 | 45% | [30] |
| PO | >10 weeks | 17/43 | 40% | ||||||
| Spain 2011 N11 | GI.3NS/GI.2VP60 | NZW | 15,000 HA units | SC | 12 weeks | NA | 0/14 | 0% | [33] |
| 4.6 weeks | 2 days | 3/14 | 21% | ||||||
| Werne | Unknown NS; GI.2VP60 | Zimmer-man | 2560 HA units | IM | 12 weeks | Av. 36 h | 4/4 | 100% | [31] |
| Australia 2015 BlMt-1 | GI.1bNS/GI.2VP60 | NZW | 2% clarified liver homogenate | PO | 5 weeks | <90 h | 5/5 | 100% | [34] |
| Adult | <96 h | 2/2 | 100% | ||||||
| Werne | Unknown NS; GI.2VP60 | ZiKa-hybrid | 2560 HA units | IM | >10 weeks | Av. 42 h | 17/20 | 85% | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, R.N.; King, T.; O'Connor, T.; Read, A.J.; Arrow, J.; Trought, K.; Duckworth, J.; Piper, M.; Strive, T. Age and Infectious Dose Significantly Affect Disease Progression after RHDV2 Infection in Naïve Domestic Rabbits. Viruses 2021, 13, 1184. https://doi.org/10.3390/v13061184
Hall RN, King T, O'Connor T, Read AJ, Arrow J, Trought K, Duckworth J, Piper M, Strive T. Age and Infectious Dose Significantly Affect Disease Progression after RHDV2 Infection in Naïve Domestic Rabbits. Viruses. 2021; 13(6):1184. https://doi.org/10.3390/v13061184
Chicago/Turabian StyleHall, Robyn N., Tegan King, Tiffany O'Connor, Andrew J. Read, Jane Arrow, Katherine Trought, Janine Duckworth, Melissa Piper, and Tanja Strive. 2021. "Age and Infectious Dose Significantly Affect Disease Progression after RHDV2 Infection in Naïve Domestic Rabbits" Viruses 13, no. 6: 1184. https://doi.org/10.3390/v13061184
APA StyleHall, R. N., King, T., O'Connor, T., Read, A. J., Arrow, J., Trought, K., Duckworth, J., Piper, M., & Strive, T. (2021). Age and Infectious Dose Significantly Affect Disease Progression after RHDV2 Infection in Naïve Domestic Rabbits. Viruses, 13(6), 1184. https://doi.org/10.3390/v13061184






