Progression and Trends in Virus from Influenza A to COVID-19: An Overview of Recent Studies
Abstract
:1. Introduction
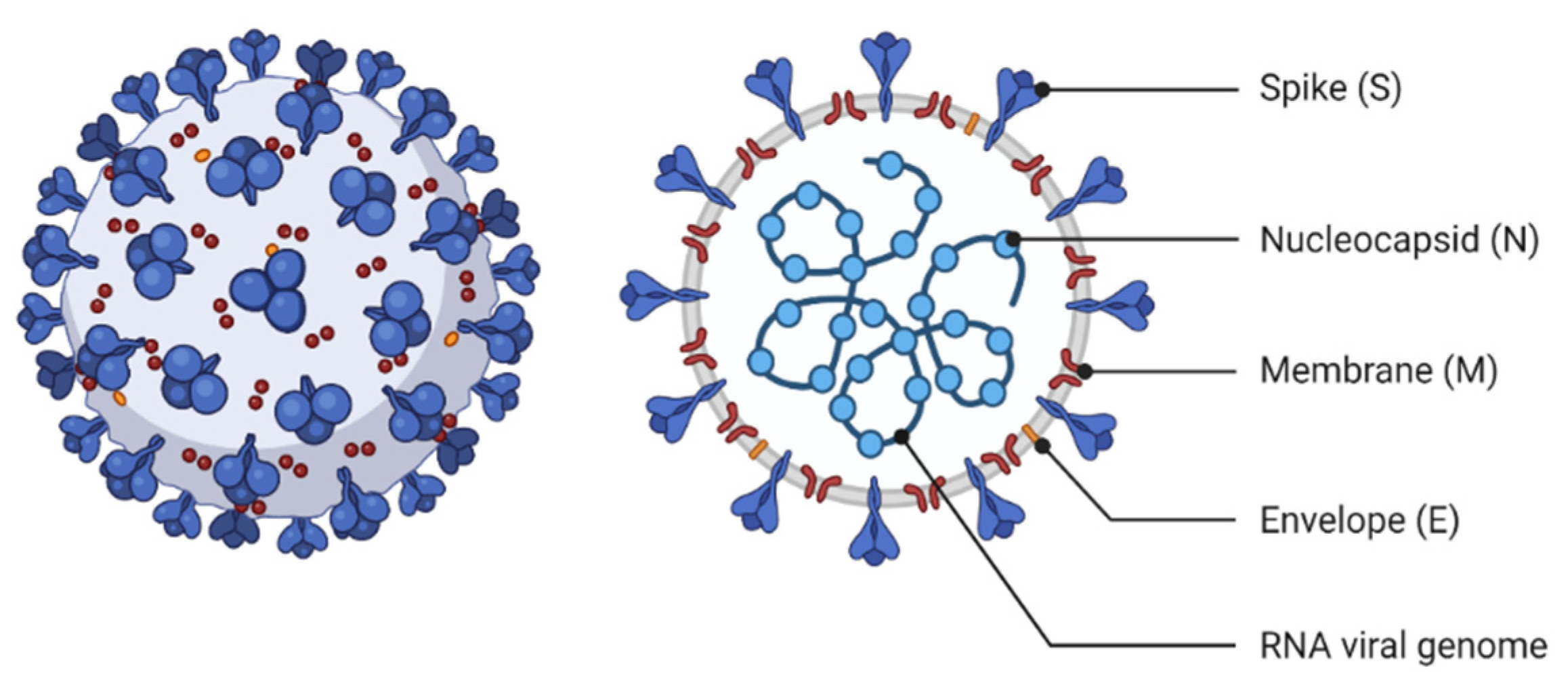
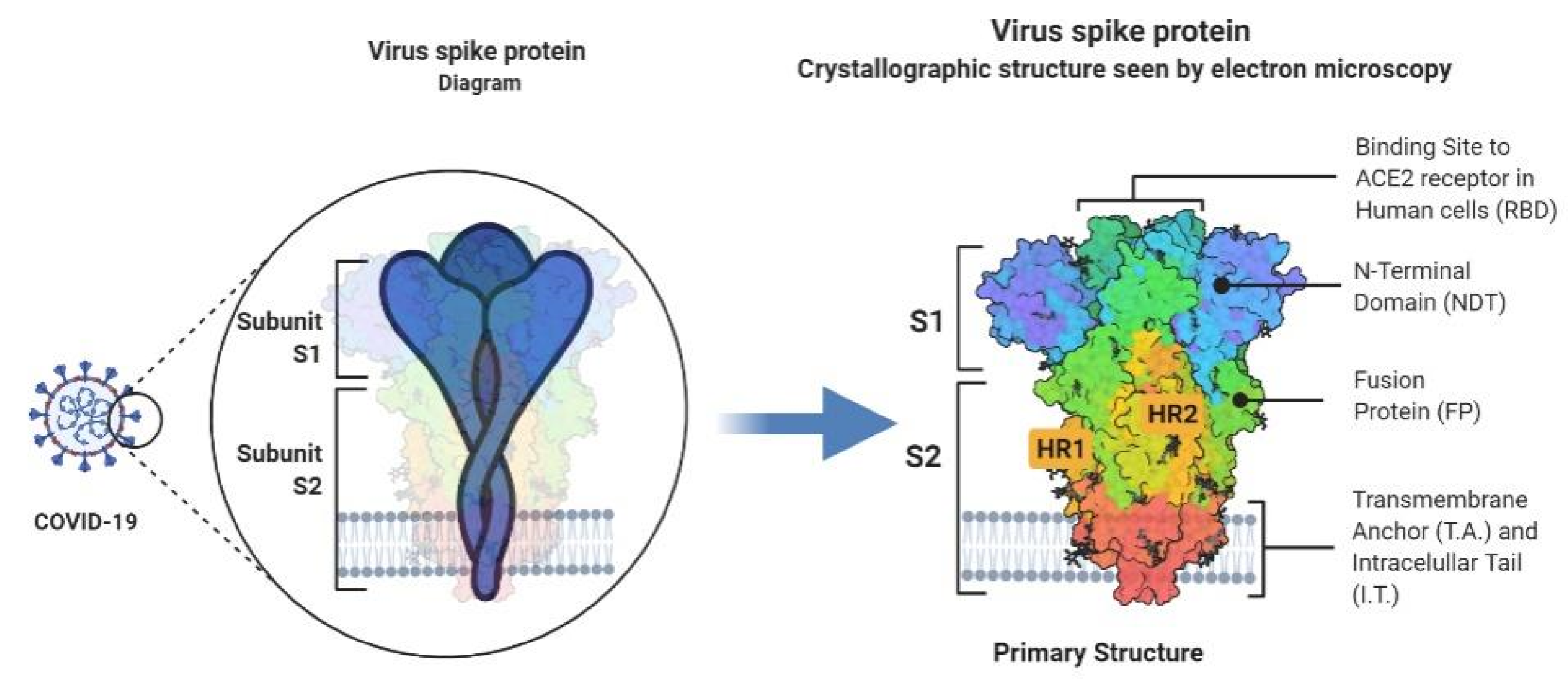
2. Virology and Structural Characteristics
2.1. Coronavirus (COVID-19)
2.2. Influenza A (H1N1)
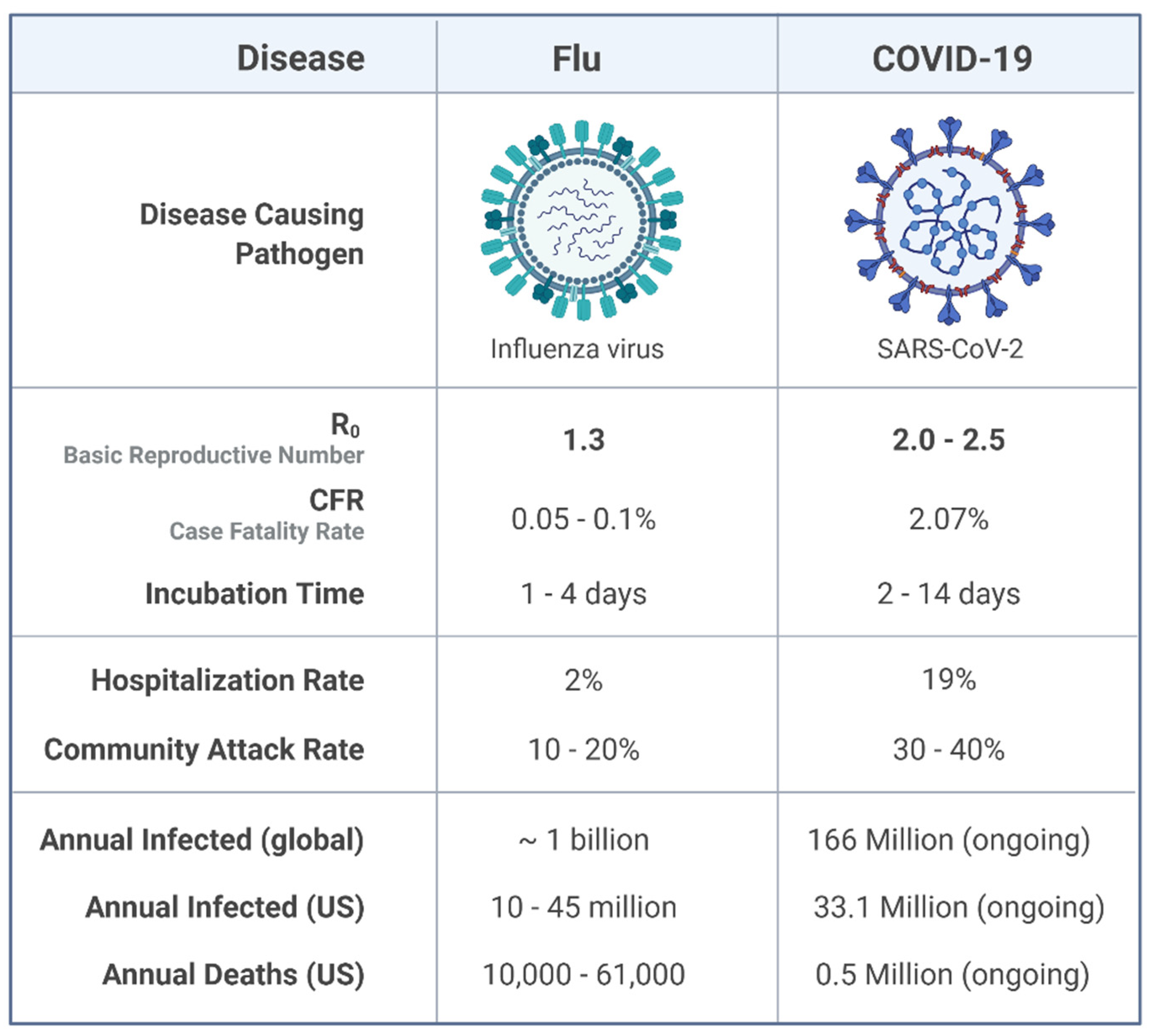
3. Epidemiology
3.1. Epidemiological Prospective of COVID-19
3.1.1. Death Rate by Age Group
3.1.2. Death Rate by Gender Ratio
3.1.3. Death Rate by Health Conditions
3.2. Epidemiological Prospective of Influenza A (H1N1)
3.2.1. Death Rate by Age Group
3.2.2. Death Rate by Sex Ratio
3.2.3. Death Rate by Health Conditions
4. Transmission and Replication
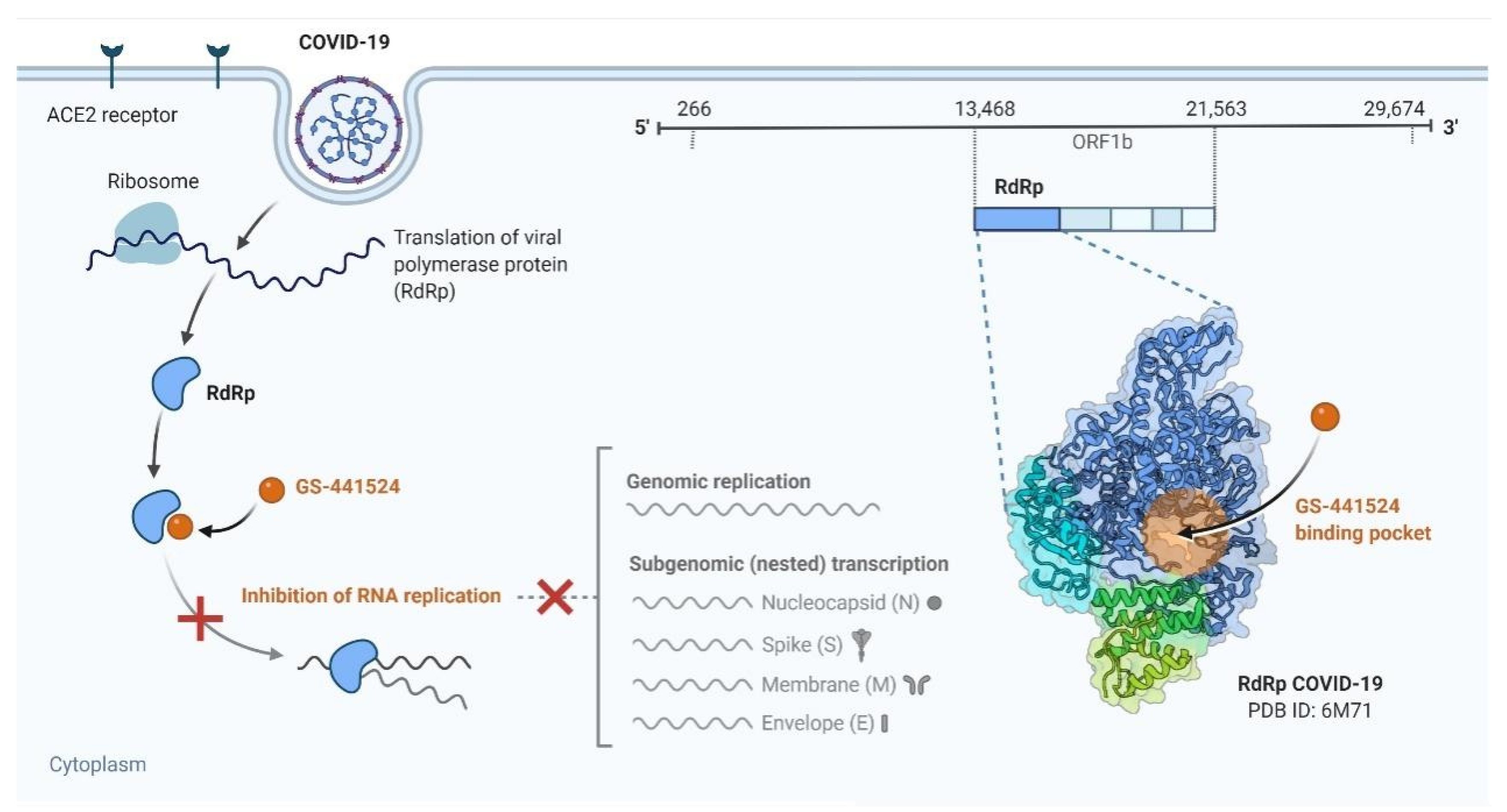
4.1. Transmission and Replication of COVID-19
4.1.1. Transmission
4.1.2. Replication
4.2. Transmission and Replication of Influenza A
4.2.1. Transmission
4.2.2. Replication
5. Clinical Manifestation
5.1. Clinical Manifestation of COVID-19
5.1.1. Cardiovascular
5.1.2. Gastrointestinal (GI)
5.1.3. Lung
5.2. Clinical Manifestation of Influenza A (H1N1)
5.2.1. How Obesity Impacts Viral Infections?
5.2.2. Why Women Have Less Tendency to Be Affected by Viral Infections?
6. Diagnosis
6.1. Diagnosis of COVID-19
6.1.1. Molecular Tests
6.1.2. Computed Tomography (CT) Scan
6.1.3. Lymphopenia
6.1.4. Gut Microbiome
6.2. Diagnosis of Influenza A (H1N1)
6.2.1. Conventional Culture
6.2.2. Antigen Detection
6.2.3. Molecular Tests
7. Treatment
7.1. Availability of Treatment against COVID-19
7.2. Availability of Treatment against Influenza A (H1N1)
8. Prevention and Control
8.1. Prevention and Control of COVID-19
8.2. Prevention and Control of Influenza A(H1N1)
9. Vaccine Production Strategies
9.1. COVID-19
9.2. Inactive and Live-Attenuated Vaccines
9.3. Live-Attenuated Vaccines (LAVs)
9.4. Inactivated Vaccines (IVs)
9.5. Designing Nucleic Acid-Based Vaccines
9.6. Viral Vectors
9.7. Protein Vaccines
10. Vaccine Production Strategies of Influenza A (H1N1)
10.1. Vaccines Produced against Influenza A (H1N1)
10.1.1. Egg-Based Vaccines
10.1.2. Attenuated Vaccines
10.1.3. Recombinant Vaccines
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezabakhsh, A.; Ala, A.; Khodaei, S.H. Novel coronavirus (COVID-19): A new emerging pandemic threat. J. Res. Clin. Med. 2020, 8, 5. [Google Scholar] [CrossRef]
- Bolsen, T.; Palm, R.; Kingsland, J.T. Framing the Origins of COVID-19. Sci. Commun. 2020, 42, 562–585. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Kuo, R.-L.; Shih, S.-R. COVID-19: The first documented coronavirus pandemic in history. Biomed. J. 2020, 43, 328–333. [Google Scholar] [CrossRef]
- Forster, P.; Forster, L.; Renfrew, C.; Forster, M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. USA 2020, 117, 9241–9243. [Google Scholar] [CrossRef] [Green Version]
- Patient, A. Swine influenza A (H1N1) infection in two children—Southern California, March–April 2009. Morb. Mortal. Wkly. Rep. 2009, 58, 400–402. [Google Scholar]
- Echevarría-Zuno, S.; Mejía-Aranguré, J.M.; Mar-Obeso, A.J.; Grajales-Muñiz, C.; Robles-Pérez, E.; González-León, M.; Ortega-Alvarez, M.C.; Gonzalez-Bonilla, C.; Rascón-Pacheco, R.A.; Borja-Aburto, V.H. Infection and death from influenza A H1N1 virus in Mexico: A retrospective analysis. Lancet 2009, 374, 2072–2079. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Weekly Epidemiological Record; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Shao, W.; Li, X.; Goraya, M.U.; Wang, S.; Chen, J.-L. Evolution of influenza a virus by mutation and re-assortment. Int. J. Mol. Sci. 2017, 18, 1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.S.H.; Banner, D.; Fang, Y.; Ng, D.C.K.; Kanagasabai, T.; Kelvin, D.J.; Kelvin, A.A. Comparative analyses of pandemic H1N1 and seasonal H1N1, H3N2, and influenza B infections depict distinct clinical pictures in ferrets. PLoS ONE 2011, 6, e27512. [Google Scholar] [CrossRef] [Green Version]
- Mohebbi, A.; Fotouhi, F.; Jamali, A.; Yaghobi, R.; Farahmand, B.; Mohebbi, R. Molecular epidemiology of the hemagglutinin gene of prevalent influenza virus A/H1N1/pdm09 among patient in Iran. Virus Res. 2019, 259, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Higginson, R. Distinguishing the novel coronavirus from influenza. J. Paramed. Pract. 2020, 12, 136–137. [Google Scholar] [CrossRef] [Green Version]
- Salzberger, B.; Glück, T.; Ehrenstein, B. Successful containment of COVID-19: The WHO-Report on the COVID-19 outbreak in China. Infection 2020, 48, 151–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, S. Family Coronaviridae. Viruses 2017, 149–158. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Pöhlmann, S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell 2020, 78, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Satarker, S.; Nampoothiri, M. Structural proteins in severe acute respiratory syndrome coronavirus-2. Arch. Med. Res. 2020, 51, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Dehcheshmeh, M.; Moghbeli, S.M.; Rahimirad, S.; Alanazi, I.; Al Shehri, Z.S.; Ebrahimie, E. Achilles’ heel of SARS-CoV-2: Transcription regulatory sequence and leader sequence in 5’untranslated region have unique evolutionary patterns and are vital for virus replication in infected human cells. Preprints 2020. [Google Scholar] [CrossRef]
- Christman, M.C.; Kedwaii, A.; Xu, J.; Donis, R.O.; Lu, G. Pandemic (H1N1) 2009 virus revisited: An evolutionary retrospective. Infect. Genet. Evol. 2011, 11, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Taubenberger, J.K.; Morens, D.M. The pathology of influenza virus infections. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 499–522. [Google Scholar] [CrossRef]
- Fouchier, R.A.M.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D.M.E. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef] [Green Version]
- Roxas, M.; Jurenka, J. Colds and influenza: A review of diagnosis and conventional, botanical, and nutritional considerations. Altern. Med. Rev. 2007, 12, 25–48. [Google Scholar]
- Hamilton, B.S.; Whittaker, G.R.; Daniel, S. Influenza virus-mediated membrane fusion: Determinants of hemagglutinin fusogenic activity and experimental approaches for assessing virus fusion. Viruses 2012, 4, 1144–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arab-Mazar, Z.; Sah, R.; Rabaan, A.A.; Dhama, K.; Rodriguez-Morales, A.J. Mapping the incidence of the COVID-19 hotspot in Iran–Implications for Travellers. Travel Med. Infect. Dis. 2020, 34, 101630. [Google Scholar] [CrossRef] [PubMed]
- Marson, F.A.L.; Ortega, M.M. COVID-19 in Brazil. Pulmonology 2020, 26, 241–244. [Google Scholar] [CrossRef]
- Betancourt, W.Q.; Schmitz, B.W.; Innes, G.K.; Prasek, S.M.; Brown, K.M.P.; Stark, E.R.; Foster, A.R.; Sprissler, R.S.; Harris, D.T.; Sherchan, S.P.; et al. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci. Total Environ. 2021, 779, 146408. [Google Scholar] [CrossRef]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses—A statement of the Coronavirus Study Group. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 2020, 20, 669–677. [Google Scholar] [CrossRef]
- Undurraga, E.A.; Chowell, G.; Mizumoto, K. COVID-19 case fatality risk by age and gender in a high testing setting in Latin America: Chile, March–August 2020. Infect. Dis. Poverty 2021, 10, 11. [Google Scholar] [CrossRef]
- Bhopal, S.S.; Bhopal, R. Sex differential in COVID-19 mortality varies markedly by age. Lancet 2020, 396, 532–533. [Google Scholar] [CrossRef]
- Guilmoto, C.Z.Z. COVID-19 death rates by age and sex and the resulting mortality vulnerability of countries and regions in the world. medRxiv 2020. [Google Scholar] [CrossRef]
- Scully, E.P.; Schumock, G.; Fu, M.; Massaccesi, G.; Muschelli, J.; Betz, J.; Klein, E.Y.; West, N.E.; Robinson, M.; Garibaldi, B.T.; et al. Sex and gender differences in COVID testing, hospital admission, presentation, and drivers of severe outcomes in the DC/Maryland region. medRxiv 2021. [Google Scholar] [CrossRef]
- Takahashi, T.; Iwasaki, A. Sex differences in immune responses: Biological sex differences in immunity potentially underlie male bias for severe COVID-19. Science 2021, 371, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Moghadami, M.; Moattari, A.; Tabatabaee, H.R.; Mirahmadizadeh, A.; Rezaianzadeh, A.; Hasanzadeh, J.; Ebrahimi, M.; Zamiri, N.; Alborzi, A.; Bagheri Lankarani, K. High titers of hemagglutination inhibition antibodies against 2009 H1N1 influenza virus in Southern Iran. Iran. J. Immunol. 2010, 7, 39–48. [Google Scholar] [PubMed]
- Webster, R.G.; Kendal, A.P.; Gerhard, W. Analysis of antigenic drift in recently isolated influenza A (H1N1) viruses using monoclonal antibody preparations. Virology 1979, 96, 258–264. [Google Scholar] [CrossRef]
- Kilbourne, E.D. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 2006, 12, 9–14. [Google Scholar] [CrossRef]
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Balish, A.; Warnes, C.M.; Wu, K.; Barnes, N.; Emery, S.; Berman, L.; Shu, B.; Lindstrom, S.; Xu, X.; Uyeki, T.; et al. Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) Virus-United States, 2009. Morb. Mortal. Wkly. Rep. 2009, 58, 826–829. [Google Scholar]
- Lange, E.; Kalthoff, D.; Blohm, U.; Teifke, J.P.; Breithaupt, A.; Maresch, C.; Starick, E.; Fereidouni, S.; Hoffmann, B.; Mettenleiter, T.C.; et al. Pathogenesis and transmission of the novel swine-origin influenza virus A/H1N1 after experimental infection of pigs. J. Gen. Virol. 2009, 90, 2119–2123. [Google Scholar] [CrossRef]
- Malveiro, D.; Flores, P.; Sousa, E.; Guimarães, J.C. The 2009 pandemic influenza A (H1N1) virus infection: Experience of a paediatric service at a third-level hospital in Lisbon, Portugal. Rev. Port. Pneumol. Engl. Ed. 2012, 18, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Al-Tawfiq, J.A.; Abed, M.; Saadeh, B.M.; Ghandour, J.; Shaltaf, M.; Babiker, M.M. Pandemic influenza A (2009 H1N1) in hospitalized patients in a Saudi Arabian hospital: Epidemiology and clinical comparison with H1N1-negative patients. J. Infect. Public Health 2011, 4, 228–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brockwell-Staats, C.; Webster, R.G.; Webby, R.J. Diversity of influenza viruses in swine and the emergence of a novel human pandemic influenza A (H1N1). Influenza Respir. Viruses 2009, 3, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hillyard, R.D. Novel swine-origin influenza A (H1N1) virus investigation team emergence of a novel swime origin-inf A (H1N1) virus in humans. N. Engl. J. Med. 2009, 360, 2605–2615. [Google Scholar]
- Lemaitre, M.; Carrat, F. Comparative age distribution of influenza morbidity and mortality during seasonal influenza epidemics and the 2009 H1N1 pandemic. BMC Infect. Dis. 2010, 10, 162. [Google Scholar] [CrossRef] [Green Version]
- Bhat, N.; Wright, J.G.; Broder, K.R.; Murray, E.L.; Greenberg, M.E.; Glover, M.J.; Likos, A.M.; Posey, D.L.; Klimov, A.; Lindstrom, S.E.; et al. Influenza-associated deaths among children in the United States, 2003–2004. N. Engl. J. Med. 2005, 353, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.P.; Mackay, I.M. Age-specific and sex-specific morbidity and mortality from avian influenza A (H7N9). J. Clin. Virol. 2013, 58, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Paskoff, T.; Sattenspiel, L. Sex-and age-based differences in mortality during the 1918 influenza pandemic on the island of Newfoundland. Am. J. Hum. Biol. 2019, 31, e23198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noymer, A.; Garenne, M. The 1918 influenza epidemic’s effects on sex differentials in mortality in the United States. Popul. Dev. Rev. 2000, 26, 565–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Rieiro, C.; Carrasco-Garrido, P.; Hernández-Barrera, V.; de Andres, A.; Jimenez-Trujillo, I.; de Miguel, A.; Jiménez-García, R. Pandemic influenza hospitalization in Spain (2009): Incidence, in-hospital mortality, comorbidities and costs. Hum. Vaccines Immunother. 2012, 8, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Q.; Fan, L.; Liu, W.; Li, J.; Yue, J.; Wang, M.; Ke, X.; Yin, Y.; Chen, Q.; Jiang, C. Direct evidence of active SARS-CoV-2 replication in the intestine. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.-Y.; Chen, L.; Wang, M. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, A.; Yi, B.; Ding, K.; Wang, H.; Wang, J.; Shi, H.; Wang, S.; Xu, G. The epidemiological characteristics of infection in close contacts of COVID-19 in Ningbo city. Chin. J. Epidemiol. 2020, 41, 668–672. [Google Scholar]
- Smith, J.C.; Sausville, E.L.; Girish, V.; Yuan, M.L.; Vasudevan, A.; John, K.M.; Sheltzer, J.M. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev. Cell 2020, 53, 514–529. [Google Scholar] [CrossRef]
- de Celles, M.D.; Casalegno, J.-S.; Lina, B.; Opatowski, L. Influenza may facilitate the spread of SARS-CoV-2. medRxiv 2020. [Google Scholar] [CrossRef]
- Linton, N.M.; Kobayashi, T.; Yang, Y.; Hayashi, K.; Akhmetzhanov, A.R.; Jung, S.; Yuan, B.; Kinoshita, R.; Nishiura, H. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: A statistical analysis of publicly available case data. J. Clin. Med. 2020, 9, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, K.; Karousis, E.D.; Jomaa, A.; Scaiola, A.; Echeverria, B.; Gurzeler, L.-A.; Leibundgut, M.; Thiel, V.; Mühlemann, O.; Ban, N. SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to inhibit translation. Nat. Struct. Mol. Biol. 2020, 27, 959–966. [Google Scholar] [CrossRef]
- Asghari, A.; Naseri, M.; Safari, H.; Saboory, E.; Parsamanesh, N. The Novel Insight of SARS-CoV-2 Molecular Biology and Pathogenesis and Therapeutic Options. DNA Cell Biol. 2020, 39, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, D.; Zhou, J.; Pan, T.; Chen, J.; Yang, Y.; Lv, M.; Ye, X.; Peng, G.; Fang, L.; et al. Porcine deltacoronavirus nsp5 antagonizes type I interferon signaling by cleaving STAT2. J. Virol. 2017, 91, e00003-17. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, L.; Zhang, J.; Ge, X.; Zhou, R.; Zheng, H.; Geng, G.; Guo, X.; Yang, H. Nsp9 and Nsp10 contribute to the fatal virulence of highly pathogenic porcine reproductive and respiratory syndrome virus emerging in China. PLoS Pathog. 2014, 10, e1004216. [Google Scholar] [CrossRef] [PubMed]
- Kirchdoerfer, R.N.; Ward, A.B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 2019, 10, 2342. [Google Scholar] [CrossRef] [Green Version]
- Shu, T.; Huang, M.; Wu, D.; Ren, Y.; Zhang, X.; Han, Y.; Mu, J.; Wang, R.; Qiu, Y.; Zhang, D.-Y.; et al. SARS-Coronavirus-2 Nsp13 possesses NTPase and RNA helicase activities that can be inhibited by bismuth salts. Virol. Sin. 2020, 35, 321–329. [Google Scholar] [CrossRef]
- Yuen, C.-K.; Lam, J.-Y.; Wong, W.-M.; Mak, L.-F.; Wang, X.; Chu, H.; Cai, J.-P.; Jin, D.-Y.; To, K.K.-W.; Chan, J.F.-W.; et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 2020, 9, 1418–1428. [Google Scholar] [CrossRef]
- Wolff, G.; Limpens, R.W.A.L.; Zevenhoven-Dobbe, J.C.; Laugks, U.; Zheng, S.; de Jong, A.W.M.; Koning, R.I.; Agard, D.A.; Grünewald, K.; Koster, A.J.; et al. A molecular pore spans the double membrane of the coronavirus replication organelle. Science 2020, 369, 1395–1398. [Google Scholar] [CrossRef]
- Robson, F.; Khan, K.S.; Le, T.K.; Paris, C.; Demirbag, S.; Barfuss, P.; Rocchi, P.; Ng, W.-L. Coronavirus RNA proofreading: Molecular basis and therapeutic targeting. Mol. Cell 2020, 79, 710–727. [Google Scholar] [CrossRef]
- Asadi, S.; ben Hnia, N.G.; Barre, R.S.; Wexler, A.S.; Ristenpart, W.D.; Bouvier, N.M. Influenza A virus is transmissible via aerosolized fomites. Nat. Commun. 2020, 11, 4062. [Google Scholar] [CrossRef]
- Wu, Z.; Harrich, D.; Li, Z.; Hu, D.; Li, D. The unique features of SARS-CoV-2 transmission: Comparison with SARS-CoV, MERS-CoV and 2009 H1N1 pandemic influenza virus. Rev. Med. Virol. 2021, 31, e2171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, taaa021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Wang, Q.; Chen, S.; Gao, S.; Song, L.; Liu, P.; Huang, W. Influenza A virus NS1 induces G0/G1 cell cycle arrest by inhibiting the expression and activity of RhoA protein. J. Virol. 2013, 87, 3039–3052. [Google Scholar] [CrossRef] [Green Version]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A virus cell entry, replication, virion assembly and movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, C.; Hu, Y.; Lei, E.; Lin, X.; Zhao, L.; Zou, Z.; Zhang, A.; Zhou, H.; Chen, H.; et al. hisT1h1c regulates interferon-$β$ and inhibits influenza Virus replication by interacting with irF3. Front. Immunol. 2017, 8, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yang, C.; Sun, X.; Lin, X.; Zhao, L.; Chen, H.; Jin, M. Evidence for a novel mechanism of influenza A virus host adaptation modulated by PB 2-627. FEBS J. 2019, 286, 3389–3400. [Google Scholar] [CrossRef]
- Song, L.; Liu, H.; Gao, S.; Jiang, W.; Huang, W. Cellular microRNAs inhibit replication of the H1N1 influenza A virus in infected cells. J. Virol. 2010, 84, 8849–8860. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaiwarith, R.; Prommee, N.; Liwsrisakun, C.; Oberdorfer, P.; Nuntachit, N.; Pothirat, C. A novel influenza A H1N1 clinical manifestations in patients at Chiang Mai University Hospital. J. Med. Assoc. Thail. 2011, 94, 908–915. [Google Scholar]
- Riquelme, A.; Alvarez-Lobos, M.; Pavez, C.; Hasbun, P.; Dabanch, J.; Cofre, C.; Jimenez, J.; Calvo, M. Gastrointestinal manifestations among Chilean patients infected with novel influenza A (H1N1) 2009 virus. Gut 2009, 58, 1567–1568. [Google Scholar] [CrossRef] [PubMed]
- Fezeu, L.; Julia, C.; Henegar, A.; Bitu, J.; Hu, F.B.; Grobbee, D.E.; Kengne, A.-P.; Hercberg, S.; Czernichow, S. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Kassir, R. Risk of COVID-19 for patients with obesity. Obes. Rev. 2020, 21, e13034. [Google Scholar] [CrossRef] [Green Version]
- Long, C.; Xu, H.; Shen, Q.; Zhang, X.; Fan, B.; Wang, C.; Zeng, B.; Li, Z.; Li, X.; Li, H. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur. J. Radiol. 2020, 126, 108961. [Google Scholar] [CrossRef] [PubMed]
- Dara, M.; Talebzadeh, M. CRISPR/Cas as a potential diagnosis technique for COVID-19. Avicenna J. Med. Biotechnol. 2020, 12, 201–202. [Google Scholar]
- Panning, M.; Eickmann, M.; Landt, O.; Monazahian, M.; Ölschläger, S.; Baumgarte, S.; Reischl, U.; Wenzel, J.J.; Niller, H.H.; Günther, S.; et al. Detection of influenza A (H1N1) v virus by real-time RT-PCR. Eurosurveillance 2009, 14, 19329. [Google Scholar] [CrossRef]
- Huang, I.; Pranata, R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. J. Intensive Care 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, H.; Song, P.; Zhang, Z.; Chen, J.; Zhou, Y.-H. Dynamic changes of lymphocyte counts in adult patients with severe pandemic H1N1 influenza A. J. Infect. Public Health 2019, 12, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. 2020, 71, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Deriu, E.; Boxx, G.M.; He, X.; Pan, C.; Benavidez, S.D.; Cen, L.; Rozengurt, N.; Shi, W.; Cheng, G. Influenza virus affects intestinal microbiota and secondary salmonella infection in the gut through type I interferons. PLoS Pathog. 2016, 12, e1005572. [Google Scholar] [CrossRef]
- Smart, L.; Fawkes, N.; Goggin, P.; Pennick, G.; Rainsford, K.D.; Charlesworth, B.; Shah, N. A narrative review of the potential pharmacological influence and safety of ibuprofen on coronavirus disease 19 (COVID-19), ACE2, and the immune system: A dichotomy of expectation and reality. Inflammopharmacology 2020, 28, 1141–1152. [Google Scholar] [CrossRef]
- Deng, Q.; Hu, B.; Zhang, Y.; Wang, H.; Zhou, X.; Hu, W.; Cheng, Y.; Yan, J.; Ping, H.; Zhou, Q. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020, 311, 116–121. [Google Scholar] [CrossRef]
- Zhong, P.; Xu, J.; Yang, D.; Shen, Y.; Wang, L.; Feng, Y.; Du, C.; Song, Y.; Wu, C.; Hu, X.; et al. COVID-19-associated gastrointestinal and liver injury: Clinical features and potential mechanisms. Signal Transduct. Target. Ther. 2020, 5, 256. [Google Scholar] [CrossRef]
- Aziz, M.; Haghbin, H.; Lee-Smith, W.; Goyal, H.; Nawras, A.; Adler, D.G. Gastrointestinal predictors of severe COVID-19: Systematic review and meta-analysis. Ann. Gastroenterol. 2020, 33, 615–630. [Google Scholar] [CrossRef]
- Leng, L.; Cao, R.; Ma, J.; Mou, D.; Zhu, Y.; Li, W.; Lv, L.; Gao, D.; Zhang, S.; Gong, F.; et al. Pathological features of COVID-19-associated lung injury: A preliminary proteomics report based on clinical samples. Signal Transduct. Target. Ther. 2020, 5, 240. [Google Scholar] [CrossRef]
- Puzelli, S.; Buonaguro, F.M.; Facchini, M.; Palmieri, A.; Calzoletti, L.; De Marco, M.A.; Arace, P.; De Campora, E.; Esposito, C.; Cassone, A.; et al. Cardiac tamponade and heart failure due to myopericarditis as a presentation of infection with the pandemic H1N1 2009 influenza A virus. J. Clin. Microbiol. 2010, 48, 2298–2300. [Google Scholar] [CrossRef] [Green Version]
- Maines, T.R.; Jayaraman, A.; Belser, J.A.; Wadford, D.A.; Pappas, C.; Zeng, H.; Gustin, K.M.; Pearce, M.B.; Viswanathan, K.; Shriver, Z.H.; et al. Transmission and pathogenesis of swine-origin 2009 A (H1N1) influenza viruses in ferrets and mice. Science 2009, 325, 484–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, H.; Hao, Q.; Idell, S.; Tang, H. Transcription factor Runx3 is induced by influenza A virus and double-strand RNA and mediates airway epithelial cell apoptosis. Sci. Rep. 2015, 5, 17916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Siqueira, J.V.V.; Almeida, L.G.; Zica, B.O.; Brum, I.B.; Barceló, A.; de Siqueira Galil, A.G. Impact of obesity on hospitalizations and mortality, due to COVID-19: A systematic review. Obes. Res. Clin. Pract. 2020, 14, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Younes, A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: Clinical response to viral infection. J. Biol. Regul. Homeost. Agents 2020, 34, 339–343. [Google Scholar] [PubMed]
- Sama, I.E.; Ravera, A.; Santema, B.T.; van Goor, H.; ter Maaten, J.M.; Cleland, J.G.F.; Rienstra, M.; Friedrich, A.W.; Samani, N.J.; Ng, L.L.; et al. Circulating plasma concentrations of ACE2 in men and women with heart failure and effects of renin-angiotensin-aldosterone-inhibitors. Eur. Heart J. 2020, 41, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, A.; Ardebili, A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef] [Green Version]
- Gronowski, A.M. Who or what is SHERLOCK? Ejifcc 2018, 29, 201–204. [Google Scholar]
- Awulachew, E.; Diriba, K.; Anja, A.; Getu, E.; Belayneh, F. Computed tomography (CT) imaging features of patients with COVID-19: Systematic review and meta-analysis. Radiol. Res. Pract. 2020, 2020, 1023506. [Google Scholar] [CrossRef] [PubMed]
- Onigbinde, S.O.; Ojo, A.S.; Fleary, L.; Hage, R. Chest computed tomography findings in COVID-19 and influenza: A narrative review. BioMed Res. Int. 2020, 2020, 6928368. [Google Scholar] [CrossRef]
- Peaper, D.R.; Landry, M.L. Rapid diagnosis of influenza: State of the art. Clin. Lab. Med. 2014, 34, 365–385. [Google Scholar] [CrossRef]
- Sharma, V.; Kaushik, S. Comparative analysis of molecular methods for detection of influenza viruses. Microbiol. Res. J. Int. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Roa, P.L.; Catalán, P.; Giannella, M.; de Viedma, D.G.; Sandonis, V.; Bouza, E. Comparison of real-time RT-PCR, shell vial culture, and conventional cell culture for the detection of the pandemic influenza A (H1N1) in hospitalized patients. Diagn. Microbiol. Infect. Dis. 2011, 69, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Al-Khikani, F.H.O. Amphotericin B as antiviral drug: Possible efficacy against COVID-19. Ann. Thorac. Med. 2020, 15, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Liu, F.; Jiang, R.; Yang, X.; You, T.; Liu, X.; Xiao, C.Q.; Shi, Z.; Jiang, H.; Rao, Z.; et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature 2020. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Pizzini, A.; Aichner, M.; Sahanic, S.; Böhm, A.; Egger, A.; Hoermann, G.; Kurz, K.; Widmann, G.; Bellmann-Weiler, R.; Weiss, G.; et al. Impact of vitamin d deficiency on COVID-19—A prospective analysis from the CovILD registry. Nutrients 2020, 12, 2775. [Google Scholar] [CrossRef]
- Rojas, M.; Rodríguez, Y.; Monsalve, D.M.; Acosta-Ampudia, Y.; Camacho, B.; Gallo, J.E.; Rojas-Villarraga, A.; Ramírez-Santana, C.; Díaz-Coronado, J.C.; Manrique, R.; et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun. Rev. 2020, 19, 102554. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of theCytokine Storm’in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Durand, N.; Mallea, J.; Zubair, A.C. Insights into the use of mesenchymal stem cells in COVID-19 mediated acute respiratory failure. NPJ Regen. Med. 2020, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.S.; Khan, M.A. Mesenchymal Stem Cells: A new front emerge in COVID19 treatment: Mesenchymal Stem Cells therapy for SARS-CoV2 viral infection. Cytotherapy 2020, in press. [Google Scholar] [CrossRef]
- Barik, S. New treatments for influenza. BMC Med. 2012, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Duwe, S. Influenza viruses–antiviral therapy and resistance. GMS Infect. Dis. 2017, 5, 1–10. [Google Scholar]
- Anafu, A.A.; Bowen, C.H.; Chin, C.R.; Brass, A.L.; Holm, G.H. Interferon-inducible transmembrane protein 3 (IFITM3) restricts reovirus cell entry. J. Biol. Chem. 2013, 288, 17261–17271. [Google Scholar] [CrossRef] [Green Version]
- Khalili, H.; Nasiripour, S.; Etminiani-Esfahani, M. Serum vitamin D concentration in pandemic 2009 H1N1 influenza infected patients. J. Diabetes Metab. Disord. 2010, 9, 19. [Google Scholar]
- Hung, I.F.N.; To, K.K.W.; Lee, C.-K.; Lee, K.-L.; Chan, K.; Yan, W.-W.; Liu, R.; Watt, C.-L.; Chan, W.-M.; Lai, K.-Y.; et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 2011, 52, 447–456. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Pizzol, D.; Marotta, C.; Antunes, M.; Racalbuto, V.; Veronese, N.; Smith, L. Coronavirus Diseases (COVID-19) Current Status and Future Perspectives: A Narrative Review. Int. J. Environ. Res. Public Health 2020, 17, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafeez, A.; Ahmad, S.; Siddqui, S.A.; Ahmad, M.; Mishra, S. A review of COVID-19 (Coronavirus Disease-2019) diagnosis, treatments and prevention. EJMO 2020, 4, 116–125. [Google Scholar]
- Williams, A.M.; Kalra, G.; Commiskey, P.W.; Bowers, E.M.R.; Rudolph, B.R.; Pitcher, M.D.; Dansingani, K.K.; Jhanji, V.; Nischal, K.K.; Sahel, J.-A.; et al. Ophthalmology practice during the coronavirus disease 2019 pandemic: The University of Pittsburgh experience in promoting clinic safety and embracing video visits. Ophthalmol. Ther. 2020, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Hirsch, M.S.; Bloom, A. Coronavirus disease 2019 (COVID-19): Epidemiology, virology, and prevention. Lancet. Infect. Dis 2020, 1, 2019–2020. [Google Scholar]
- Stockwell, M.S.; Broder, K.R.; Lewis, P.; Jakob, K.; Iqbal, S.; Fernandez, N.; Sharma, D.; Barrett, A.; LaRussa, P. Assessing fever frequency after pediatric live attenuated versus inactivated influenza vaccination. J. Pediat. Infect. Dis. Soc. 2017, 6, e7–e14. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Kawaoka, Y. Current and future influenza vaccines. Nat. Med. 2019, 25, 212–220. [Google Scholar] [CrossRef]
- Kang, S.-M.; Yao, Q.; Guo, L.; Compans, R.W. Mucosal immunization with virus-like particles of simian immunodeficiency virus conjugated with cholera toxin subunit B. J. Virol. 2003, 77, 9823–9830. [Google Scholar] [CrossRef] [Green Version]
- Dormitzer, P.R.; Galli, G.; Castellino, F.; Golding, H.; Khurana, S.; Del Giudice, G.; Rappuoli, R. Influenza vaccine immunology. Immunol. Rev. 2011, 239, 167–177. [Google Scholar] [CrossRef]
- Belshe, R.B.; Edwards, K.M.; Vesikari, T.; Black, S.V.; Walker, R.E.; Hultquist, M.; Kemble, G.; Connor, E.M. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 2007, 356, 685–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccines Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Xu, Y.; Bao, L.; Zhang, L.; Yu, P.; Qu, Y.; Zhu, H.; Zhao, W.; Han, Y.; Qin, C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enjuanes, L.; Zuñiga, S.; Castaño-Rodriguez, C.; Gutierrez-Alvarez, J.; Canton, J.; Sola, I. Molecular basis of coronavirus virulence and vaccine development. Adv. Virus Res. 2016, 96, 245–286. [Google Scholar]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 2020, 181, 894–904. [Google Scholar] [CrossRef]
- Bull, J.J. Evolutionary reversion of live viral vaccines: Can genetic engineering subdue it? Virus Evol. 2015, 1, vev005. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Xu, H.; Zhou, X.; Zhang, Z.; Tian, Z.; Wang, Y.; Wu, Y.; Zhang, B.; Niu, Z.; Zhang, C.; et al. Generation of influenza A viruses as live but replication-incompetent virus vaccines. Science 2016, 354, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E. China Coronavirus: Hong Kong Researchers have Already Developed Vaccine but Need Time to Test it, Expert Reveals: South China Morning Post. 2020. Available online: https://www.scmp.com/news/hong-kong/health-environment/article/3047956/china-coronavirus-hong-kong-researchers-have (accessed on 7 June 2021).
- Shieber, J. Codagenix Raises $20 Million for a New Flu Vaccine and Other Therapies; Tech Crunch. 2020. Available online: https://techcrunch.com/2020/01/13/codagenix-raises-20-million-for-a-new-flu-vaccine-and-other-therapies/ (accessed on 7 June 2021).
- Ciabattini, A.; Nardini, C.; Santoro, F.; Garagnani, P.; Franceschi, C.; Medaglini, D. Vaccination in the elderly: The challenge of immune changes with aging. Proc. Semin. Immunol. 2018, 40, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.M.; Simon, J.K.; Baker, J.R., Jr. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Pfizer vaccine efficacy was 52% after first dose and 95% after second dose, paper shows. BMJ 2020, 371, m4826. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Moderna applies for US and EU approval as vaccine trial reports 94.1% efficacy. BMJ Br. Med. J. 2020, 371. [Google Scholar] [CrossRef]
- Dicks, M.D.J.; Spencer, A.J.; Edwards, N.J.; Wadell, G.; Bojang, K.; Gilbert, S.C.; Hill, A.V.S.; Cottingham, M.G. A novel chimpanzee adenovirus vector with low human seroprevalence: Improved systems for vector derivation and comparative immunogenicity. PLoS ONE 2012, 7, e40385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccines Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoll, M.D.; Wonodi, C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
- Chen, W.-H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. The SARS-CoV-2 vaccine pipeline: An overview. Curr. Trop. Med. Rep. 2020, 7, 61–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.-H.; Chag, S.M.; Poongavanam, M.V.; Biter, A.B.; Ewere, E.A.; Rezende, W.; Seid, C.A.; Hudspeth, E.M.; Pollet, J.; McAtee, C.P.; et al. Optimization of the production process and characterization of the yeast-expressed SARS-CoV recombinant receptor-binding domain (RBD219-N1), a SARS vaccine candidate. J. Pharm. Sci. 2017, 106, 1961–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, M.M.; DeStefano, F. Vaccine-associated hypersensitivity. J. Allergy Clin. Immunol. 2018, 141, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Walter, E.B.; Fry, A.M.; Jernigan, D.B. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2019–2020 influenza season. MMWR Recomm. Rep. 2019, 68, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.-C.; Wang, H.; Fang, H.-H.; Yang, J.G.; Lin, X.J.; Liang, X.-F.; Zhang, X.-F.; Pan, H.-X.; Meng, F.-Y.; Hu, Y.M.; et al. A novel influenza A (H1N1) vaccine in various age groups. N. Engl. J. Med. 2009, 361, 2414–2423. [Google Scholar] [CrossRef] [Green Version]
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar] [PubMed]
| Gender proportion of COVID-19 cases | Age (Years) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| Male (%) | 47.5 | 50 | 52.8 | 52.3 | 51.8 | 53 | 49.7 | 39.2 | |
| Female (%) | 52.5 | 50 | 47.2 | 47.7 | 48.2 | 47 | 50.3 | 60.8 | |
| Gender proportion of COVID-19 deaths | Age (Years) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| Male (%) | 65 | 63.9 | 65.5 | 64.6 | 67.1 | 64.22 | 60.7 | 46.9 | |
| Female (%) | 35 | 36.1 | 34.5 | 35.4 | 32.9 | 35.8 | 39.3 | 53.1 | |
| Gender proportion of COVID-19 morbidity | Age (Years) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| Male (%) | 0.94 | 3.17 | 4.16 | 3.38 | 3.32 | 3.02 | 2.55 | 2.85 | |
| Female (%) | 1.08 | 3.22 | 3.67 | 2.93 | 2.84 | 2.36 | 2.04 | 2.56 |
| Feature | COVID-19 | Influenza A(H1N1) | References | ||
|---|---|---|---|---|---|
| Epidemiology and Transmission | Fecal-oral transmission | Proved | Not proved | [51,62] | |
| Age composition | Most patients were older than 50 | Most patients were younger than 60 | |||
| Transmission mode | Asymptomatic/ symptomatic | Symptomatic | [2] | ||
| Reproduction number | 3 | 1.5 | [64] | ||
| Incubation period | 4.9 | 1.4 | [63] | ||
| Treatment | Anti-viral drug | N3/ebselen/Remdesivir | Oseltamivir and zanamivir | [72,74,75] | |
| CP therapy | * | * | [76,77] | ||
| Vitamin D | * | * | [69,70] | ||
| MSC therapy | * | Not effective | [78] | ||
| Diagnosis | CRISPR-based SHERLOCK technique | * | Not develop | [79] | |
| qPCR | * | * | [80] | ||
| Gut microbiome | * | * | [81] | ||
| Lymphopenia | * | * | [82] | ||
| CT scan | Ground-glass opacities have frequently been placed in the periphery of lower lobes | Ground-glass opacities has a central, peripheral, or random distribution | [83] | ||
| Clinical manifestation | Acute lung injury | * | * | [56] | |
| Cardiovascular | * | * | [50] | ||
| Gastrointestinal | diarrhea | * | * | [84] | |
| nausea | * | * | |||
| vomiting | * | * | |||
| Molecular biology | Receptor for virus-cell entrance | ACE2 | Sialic acid receptor | [85,86] | |
| Genetic material | Just one positive-sense single-stranded RNA | Eight negative-sense single-stranded RNA | [87] | ||
| Location of replication | DMV (cytoplasm) | Nucleus | [31] | ||
| Vaccine Type | Vaccine | Producing Company |
|---|---|---|
| Inactivated vaccine | A full inactivated virus with formalin and alum adjuvant | Sinovac |
| Inactivated virus | Inactivated SARS-CoV-2 | Beijing Institute of Biological Products, Sinopharm |
| Inactivated virus | Inactivated SARS-CoV-2 | Wuhan Institute of Biological Products, Sinopharm |
| Inactivated virus | Inactivated SARS-CoV-2 | Institute of Medical Biology, Chinese Academy of Medical Sciences |
| Subunit vaccine | S protein fusion with adjuvant and M-matrix | Novavax |
| Non-amplifiable viral vector vaccine | Intramuscular recombination vaccine on adenovirus type 5 (Ad5-nCoV) vector | CanSino Biological Incorporation, Beijing Institute of Biotechnology, Canadian Center for Vaccinology |
| Non-amplifiable viral vector vaccine | chimpanzee adenovirus-based vector (ChAdOx1) vaccine | University of Oxford, AstraZeneca |
| Non-amplifiable viral vector vaccine | Approach 1: Dendritic cells expressing SARS-CoV-2 minigene Approach 2: Artificial supply cells expressing SARS-CoV-2 minigene | Shenzhen Geno-Immune Medical Institute |
| DNA vaccine | Optimized DNA vaccine prescribed with electroporation | Inovio Pharmaceuticals |
| DNA vaccine | Aural DNA vaccine (bacTRL-Spike) coding SARS-CoV-2 S protein | Symvivo |
| RNA vaccine | mRNA vaccine for S2 region of S protein of virus enclosed by nano lipid | Moderna |
| RNA vaccine | mRNA vaccine with lipid nanoparticle | BioNTech, Pfizer, Fosun Pharma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daemi, H.B.; Kulyar, M.F.-e.-A.; He, X.; Li, C.; Karimpour, M.; Sun, X.; Zou, Z.; Jin, M. Progression and Trends in Virus from Influenza A to COVID-19: An Overview of Recent Studies. Viruses 2021, 13, 1145. https://doi.org/10.3390/v13061145
Daemi HB, Kulyar MF-e-A, He X, Li C, Karimpour M, Sun X, Zou Z, Jin M. Progression and Trends in Virus from Influenza A to COVID-19: An Overview of Recent Studies. Viruses. 2021; 13(6):1145. https://doi.org/10.3390/v13061145
Chicago/Turabian StyleDaemi, Hakimeh Baghaei, Muhammad Fakhar-e-Alam Kulyar, Xinlin He, Chengfei Li, Morteza Karimpour, Xiaomei Sun, Zhong Zou, and Meilin Jin. 2021. "Progression and Trends in Virus from Influenza A to COVID-19: An Overview of Recent Studies" Viruses 13, no. 6: 1145. https://doi.org/10.3390/v13061145
APA StyleDaemi, H. B., Kulyar, M. F.-e.-A., He, X., Li, C., Karimpour, M., Sun, X., Zou, Z., & Jin, M. (2021). Progression and Trends in Virus from Influenza A to COVID-19: An Overview of Recent Studies. Viruses, 13(6), 1145. https://doi.org/10.3390/v13061145








