SARS-CoV-2 Antigenemia as a Confounding Factor in Immunodiagnostic Assays: A Case Study
Abstract
1. Background
2. Methods
2.1. Serum Isolation and Handling
2.2. Serum Characteristics
2.3. Monoclonal Antibody Production
2.4. Double Antigen ELISA for Antibody Detection
2.5. Sandwich ELISA for SARS-CoV-2 Antigen Detection
2.6. Immunohistochemistry
2.7. RNA Extraction and Real-Time qPCR
2.8. Next Generation Sequencing
2.9. Bioinformatics
2.10. Data and Statistical Analysis
2.11. Ethical Statement
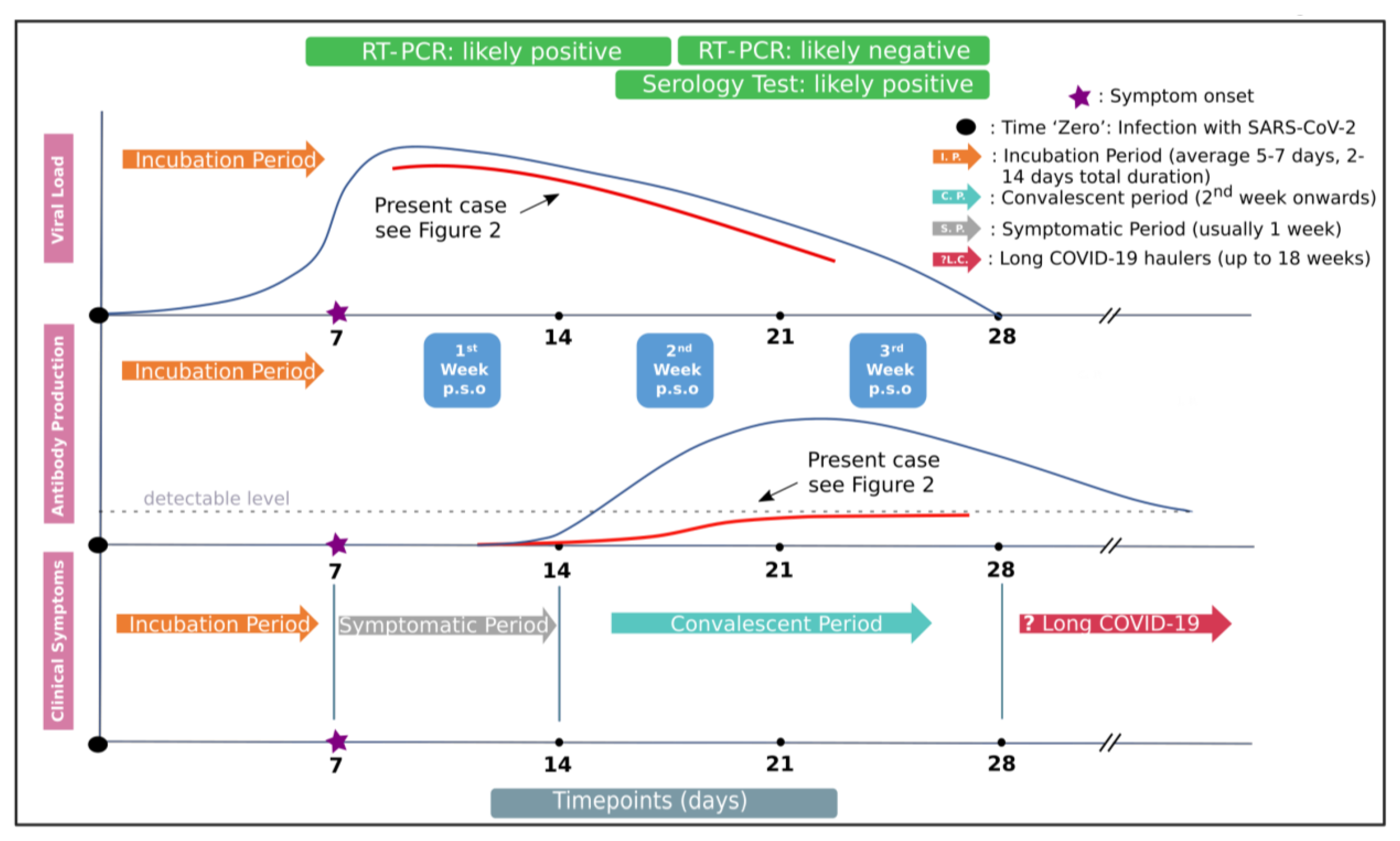
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Symptoms of Coronavirus|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 12 January 2021).
- Perlman, S. COVID-19 poses a riddle for the immune system. Nat. Cell Biol. 2020, 584, 345–346. [Google Scholar] [CrossRef]
- Russell, M.W.; Moldoveanu, Z.; Ogra, P.L.; Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 611337. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Schurink, B.; Roos, E.; Radonic, T.; Barbe, E.; Bouman, C.S.C.; de Boer, H.H.; de Bree, G.J.; Bulle, E.B.; Aronica, E.M.; Florquin, S.; et al. Viral presence and immunopathology in patients with lethal COVID-19: A prospective autopsy cohort study. Lancet Microbe 2020, 1, e290–e299. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Di Cristanziano, V.; Meyer-Schwickerath, C.; Eberhardt, K.A.; Rybniker, J.; Heger, E.; Knops, E.; Hallek, M.; Klein, F.; Holtick, U.; Jung, N. Detection of SARS-CoV-2 viremia before onset of COVID-19 symptoms in an allo-transplanted patient with acute leukemia. Bone Marrow Transplant. 2021, 56, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Martin, J.F.; González-Rivera, M.; Almansa, R.; Micheloud, D.; Tedim, A.P.; Domínguez-Gil, M.; Resino, S.; Martín-Fernández, M.; Murua, P.R.; Pérez-García, F.; et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit. Care 2020, 24, 1–13. [Google Scholar] [CrossRef]
- Le Hingrat, Q.; Visseaux, B.; Laouenan, C.; Tubiana, S.; Bouadma, L.; Yazdanpanah, Y.; Duval, X.; Burdet, C.; Ichou, H.; Damond, F.; et al. Detection of SARS-CoV-2 N-antigen in blood during acute COVID-19 provides a sensitive new marker and new testing alternatives. Clin. Microbiol. Infect. 2021, 27, 789.e1–789.e5. [Google Scholar] [CrossRef] [PubMed]
- Kellam, P.; Barclay, W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J. Gen. Virol. 2020, 101, 791–797. [Google Scholar] [CrossRef]
- Campbell, C.M.P.; Viscidi, R.P.; Torres, B.N.; Lin, H.-Y.; Fulp, W.; Abrahamsen, M.; Lazcano-Ponce, E.; Villa, L.L.; Kreimer, A.R.; Giuliano, A.R. Human Papillomavirus (HPV) L1 Serum Antibodies and the Risk of Subsequent Oral HPV Acquisition in Men: The HIM Study. J. Infect. Dis. 2016, 214, 45–48. [Google Scholar] [CrossRef]
- Doyle, W.J.; Casselbrant, M.L.; Li-Korotky, H.; Doyle, A.P.C.; Lo, C.; Turner, R.; Cohen, S. The Interleukin 6 −174 C/C Genotype Predicts Greater Rhinovirus Illness. J. Infect. Dis. 2010, 201, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.; Ward, G. 5-3 Interferences in immunoassay—Google Scholar. Clin. Biochem. Rev. 2004, 25, 105–120. [Google Scholar]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nat. Cell Biol. 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Vlachoyiannopoulos, P.; Alexopoulos, H.; Apostolidi, I.; Bitzogli, K.; Barba, C.; Athanasopoulou, E.; Dalakas, M.; Tzioufas, A. Anti-SARS-CoV-2 antibody detection in healthcare workers of two tertiary hospitals in Athens, Greece. Clin. Immunol. 2020, 221, 108619. [Google Scholar] [CrossRef]
- Gorgoulis, V.G.; Vassiliou, L.-V.F.; Karakaidos, P.; Zacharatos, P.; Kotsinas, A.; Liloglou, T.; Venere, M.; Ditullio, R.A., Jr.; Kastrinakis, N.G.; Levy, B.; et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nat. Cell Biol. 2005, 434, 907–913. [Google Scholar] [CrossRef]
- Evangelou, K.; Veroutis, D.; Foukas, P.G.; Paschalaki, K.; Kittas, C.; Tzioufas, A.G. Alveolar type II cells harbouring SARS-CoV-2 show senescence with a proinflammatory phenotype. BioRxiv 2021, 2021, 424917. [Google Scholar] [CrossRef]
- Lagopati, N.; Tsioli, P.; Mourkioti, I.; Polyzou, A.; Papaspyropoulos, A.; Zafiropoulos, A.; Evangelou, K.; Sourvinos, G.; Gorgoulis, V.G. Sample pooling strategies for SARS-CoV-2 detection. J. Virol. Methods 2021, 289, 114044. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.F.; Maley, A.M.; Wu, C.; Gilboa, T.; Norman, M.; Lazarovits, R.; Mao, C.-P.; Newton, G.; Chang, M.; Nguyen, K.; et al. Ultra-Sensitive Serial Profiling of SARS-CoV-2 Antigens and Antibodies in Plasma to Understand Disease Progression in COVID-19 Patients with Severe Disease. Clin. Chem. 2020, 66, 1562–1572. [Google Scholar] [CrossRef]
- Li, K.; Huang, B.; Wu, M.; Zhong, A.; Li, L.; Cai, Y.; Wang, Z.; Wu, L.; Zhu, M.; Li, J.; et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.C.; Risch, L.; Weber, M.; Thiel, S.; Grossmann, K.; Wohlwend, N.; Lung, T.; Hillmann, D.; Ritzler, M.; Bigler, S.; et al. Frequency of serological non-responders and false-negative RT-PCR results in SARS-CoV-2 testing: A population-based study. Clin. Chem. Lab. Med. 2020, 58, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Marklund, E.; Leach, S.; Axelsson, H.; Nyström, K.; Norder, H.; Bemark, M.; Angeletti, D.; Lundgren, A.; Nilsson, S.; Andersson, L.-M.; et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS ONE 2020, 15, e0241104. [Google Scholar] [CrossRef]
- Linssen, J.; Ermens, A.; Berrevoets, M.; Seghezzi, M.; Previtali, G.; Brugge, S.V.D.S.-V.D.; Russcher, H.; Verbon, A.; Gillis, J.M.; Riedl, J.; et al. A novel haemocytometric COVID-19 prognostic score developed and validated in an observational multicentre European hospital-based study. eLife 2020, 9, 1–37. [Google Scholar] [CrossRef]
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell 2021, 184, 169–183. [Google Scholar] [CrossRef]
- Karia, R.; Nagraj, S. A Review of Viral Shedding in Resolved and Convalescent COVID-19 Patients. SN Compr. Clin. Med. 2020, 2, 2086–2095. [Google Scholar] [CrossRef]
- Lee, S.; Channappanavar, R.; Kanneganti, T.-D. Coronaviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines. Trends Immunol. 2020, 41, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.L.; Mellors, J.W. Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) RNA in Blood of Patients With Coronavirus Disease 2019 (COVID-19): What Does It Mean? Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Perna, F.; Bruzzaniti, S.; Piemonte, E.; Maddaloni, V.; Atripaldi, L.; Sale, S.; Sanduzzi, A.; Nicastro, C.; Pepe, N.; Bifulco, M.; et al. Serum levels of SARS-CoV-2 nucleocapsid antigen associate with inflammatory status and disease severity in COVID-19 patients. Clin. Immunol. 2021, 226, 108720. [Google Scholar] [CrossRef]
- Felsenstein, S.; Herbert, J.A.; McNamara, P.S.; Hedrich, C.M. COVID-19: Immunology and treatment options. Clin. Immunol. 2020, 215, 108448. [Google Scholar] [CrossRef]
- Jääskeläinen, A.; Kuivanen, S.; Kekäläinen, E.; Ahava, M.; Loginov, R.; Kallio-Kokko, H.; Vapalahti, O.; Jarva, H.; Kurkela, S.; Lappalainen, M. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J. Clin. Virol. 2020, 129, 104512. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.; Simmons, R. Do antibody positive healthcare workers have lower SARS-CoV-2 infection rates than antibody nega-tive healthcare workers? Large multi-centre prospective cohort study (the SIREN study), England: June to November 2020. MedRxiv 2021, 2021, 21249642. [Google Scholar] [CrossRef]
| Demographic Information | ||
|---|---|---|
| Age | 19 | |
| Sex | Male | |
| Weight | 95 kg | |
| Height | 175 cm | |
| BMI | 31 kg/m2 | |
| Past Medical History | ||
| Underlying disease | Prediabetes | |
| Medication | Metformin | |
| Allergies | No | |
| Smoking history | Non-smoker | |
| Alcohol consumption | Socially | |
| Family History | ||
| Father | Prediabetes | |
| Mother | Hypertension | |
| History of Present illness | ||
| Onset of COVID-19 symptoms | 12 October 2020 (Day 1) | |
| Symptoms | Fever | |
| Duration of symptoms | 12 October 2020–14 October 2020 (Day 1–3; Fever up to 38.4 °C) 25 October 2020–29 October 2020 (Day 14–18; Fever up to 37.4 °C) | |
| PCR tests (nasopharyngeal swab specimens) | 13 October 2020 (Day 2; Ct = 15) 27 October 2020 (Day 16; Ct = 30) | |
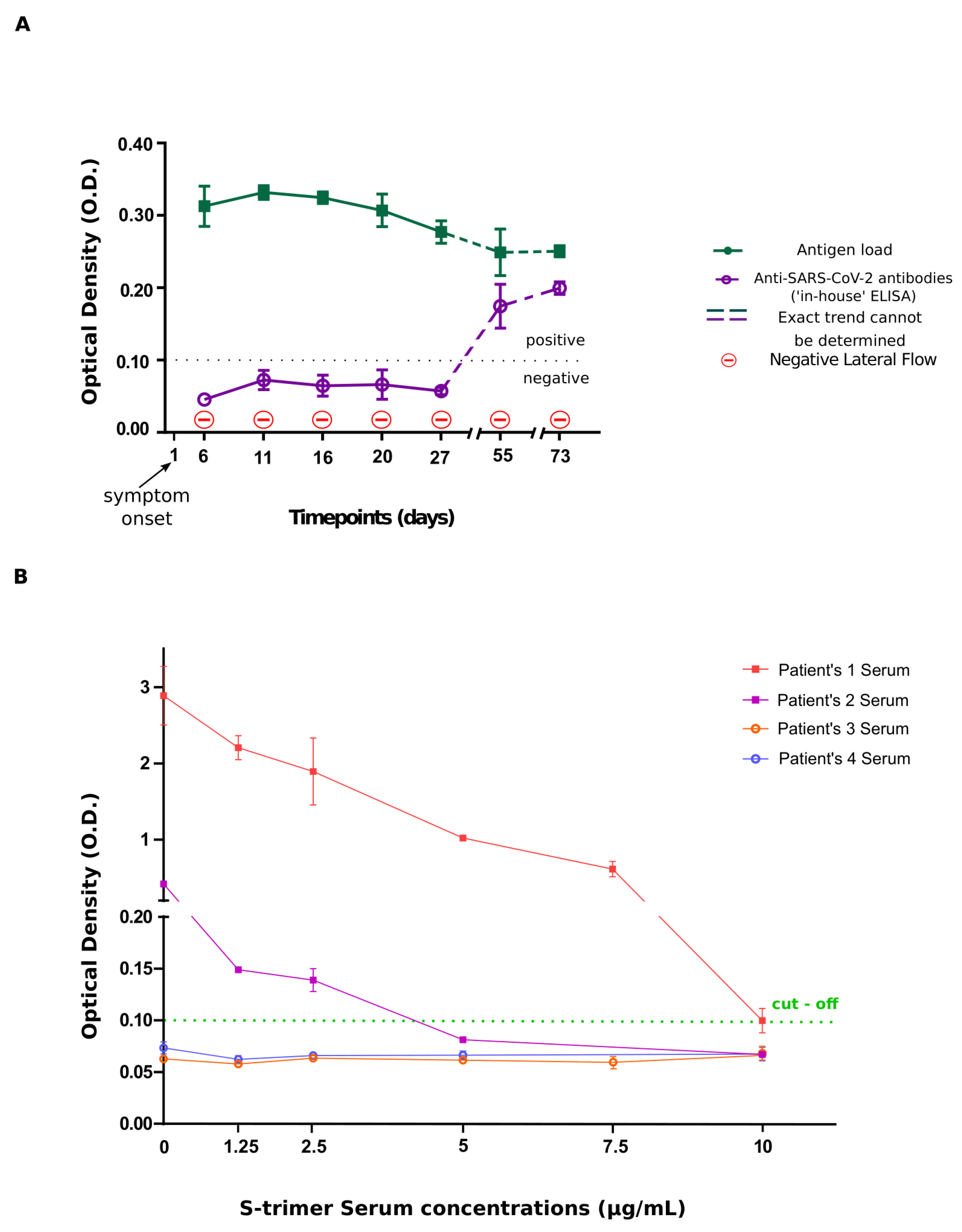
| Days of blood sampling | O.D. values | |
| Antibody assay | Antigen Assay | |
| Day 6 | 0.046 ± 0.005 | 0.313 ± 0.028 |
| Day 11 | 0.066 ± 0.011 | 0.332 ± 0.010 |
| Day 16 | 0.056 ± 0.007 | 0.325 ± 0.008 |
| Day 20 | 0.064 ± 0.019 | 0.307 ± 0.023 |
| Day 27 | 0.055 ± 0.004 | 0.277 ± 0.016 |
| Day 55 | 0.167 ± 0.024 | 0.249 ± 0.032 |
| Day 73 | 0.200 ± 0.009 | 0.233 ± 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belogiannis, K.; Florou, V.A.; Fragkou, P.C.; Ferous, S.; Chatzis, L.; Polyzou, A.; Lagopati, N.; Vassilakos, D.; Kittas, C.; Tzioufas, A.G.; et al. SARS-CoV-2 Antigenemia as a Confounding Factor in Immunodiagnostic Assays: A Case Study. Viruses 2021, 13, 1143. https://doi.org/10.3390/v13061143
Belogiannis K, Florou VA, Fragkou PC, Ferous S, Chatzis L, Polyzou A, Lagopati N, Vassilakos D, Kittas C, Tzioufas AG, et al. SARS-CoV-2 Antigenemia as a Confounding Factor in Immunodiagnostic Assays: A Case Study. Viruses. 2021; 13(6):1143. https://doi.org/10.3390/v13061143
Chicago/Turabian StyleBelogiannis, Konstantinos, Venetia A. Florou, Paraskevi C. Fragkou, Stefanos Ferous, Loukas Chatzis, Aikaterini Polyzou, Nefeli Lagopati, Demetrios Vassilakos, Christos Kittas, Athanasios G. Tzioufas, and et al. 2021. "SARS-CoV-2 Antigenemia as a Confounding Factor in Immunodiagnostic Assays: A Case Study" Viruses 13, no. 6: 1143. https://doi.org/10.3390/v13061143
APA StyleBelogiannis, K., Florou, V. A., Fragkou, P. C., Ferous, S., Chatzis, L., Polyzou, A., Lagopati, N., Vassilakos, D., Kittas, C., Tzioufas, A. G., Tsiodras, S., Sourvinos, G., & Gorgoulis, V. G. (2021). SARS-CoV-2 Antigenemia as a Confounding Factor in Immunodiagnostic Assays: A Case Study. Viruses, 13(6), 1143. https://doi.org/10.3390/v13061143









