Diagnostic Performance of Ag-RDTs and NAAT for SARS-CoV2 Identification in Symptomatic Patients in Catalonia
Abstract
1. Introduction
2. Aim
3. Materials and Methods
4. Results
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Winnett, A.; Cooper, M.M.; Shelby, N.; Romano, A.E.; Reyes, J.A.; Ji, J.; Porter, M.K.; Savela, E.S.; Barlow, J.T.; Akana, R.; et al. SARS-CoV-2 viral load in saliva rises gradually and to moderate levels in some humans. medRxiv 2020. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays. Available online: https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays (accessed on 28 April 2021).
- European Centre for Disease Prevention and Control Options for the us.e of rap.id anti..gen tes.ts for COVID-19 in the EU/EEA and the UK. Available online: https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-and-uk (accessed on 28 April 2021).
- Mina, M.J.; Parker, R.; Larremore, D.B. Rethinking Covid-19 Test Sensitivity—A Strategy for Containment. N. Engl. J. Med. 2020, 383, e120. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Council Recommendation on a Common Framework for the Use and Validation of Rapid Antigen Tests and the Mutual Recognition of COVID-19 Test Results in the EU 2021/C 24/01. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32021H0122(01) (accessed on 28 April 2021).
- Prince-Guerra, J.L.; Almendares, O.; Nolen, L.D.; Gunn, J.K.L.; Dale, A.P.; Buono, S.A.; Deutsch-Feldman, M.; Suppiah, S.; Hao, L.; Zeng, Y.; et al. Evaluation of Abbott BinaxNOW Rapid Antigen Test for SARS-CoV-2 Infection at Two Community-Based Testing Sites—Pima County, Arizona, November 3–17, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.; Larsdatter Storm, M.; Toverus Landaas, K.; Bakken Kran, A. Evaluation of Abbot’s Panbio COVID-19 Rapid Antigen Test in Norway. Available online: https://www.helsedirektoratet.no/rapporter/evaluation-of-abbots-panbio-covid-19-rapid-antigen-test-in-norway/EvaluationofAbbotsPanbioCOVID-19rapidantigentestinNorway.pdf/_/attachment/inline/b3306b98-c0e0-4e96-aa62-3ca5a99f5367:10fe6f072721ece7aee (accessed on 28 April 2021).
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [PubMed]
- PanAmerican Health Organization Interpretation of Laboratory Results for COVID-19 Diagnosis-PAHO/WHO | Pan American Health Organization. Available online: https://www.paho.org/en/documents/interpretation-laboratory-results-covid-19-diagnosis (accessed on 28 April 2021).
- Toptan, T.; Eckermann, L.; Pfeiffer, A.E.; Hoehl, S.; Ciesek, S.; Drosten, C.; Corman, V.M. Evaluation of a SARS-CoV-2 rapid antigen test: Potential to help reduce community spread? J. Clin. Virol. 2021, 135. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Torres, I.; Bueno, F.; Huntley, D.; Molla, E.; Fernández-Fuentes, M.Á.; Martínez, M.; Poujois, S.; Forqué, L.; Valdivia, A.; et al. Field evaluation of a rapid antigen test (PanbioTM COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Arons, M.M.; Hatfield, K.M.; Reddy, S.C.; Kimball, A.; James, A.; Jacobs, J.R.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N. Engl. J. Med. 2020, 382, 2081–2090. [Google Scholar] [CrossRef]
- Buitrago-Garcia, D.; Egli-Gany, D.; Counotte, M.J.; Hossmann, S.; Imeri, H.; Ipekci, A.M.; Salanti, G.; Low, N. Occurrence and transmission potential of asymptomatic and presymptomatic SARSCoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020, 17. [Google Scholar] [CrossRef]
- Pray, I.W.; Gibbons-Burgener, S.N.; Rosenberg, A.Z.; Cole, D.; Borenstein, S.; Bateman, A.; Pevzner, E.; Westergaard, R.P. COVID-19 Outbreak at an Overnight Summer School Retreat ― Wisconsin, July–August 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1600–1604. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, J.; Sun, R.; Tang, X.; Liang, C.; Lin, H.; Zeng, L.; Hu, J.; Yuan, R.; Zhou, P.; et al. Prolonged persistence of SARS-CoV-2 RNA in body fluids. Emerg. Infect. Dis. 2020, 26, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.G.; Lee, K.M.; Hsiao, M.J.; Yang, S.L.; Huang, P.N.; Gong, Y.N.; Hsieh, T.H.; Huang, P.W.; Lin, Y.J.; Liu, Y.C.; et al. Culture-based virus isolation to evaluate potential infectivity of clinical specimens tested for COVID-19. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- González-Donapetry, P.; García-Clemente, P.; Bloise, I.; García-Sánchez, C.; Sánchez Castellano, M.Á.; Romero, M.P.; Gutiérrez Arroyo, A.; Mingorance, J.; de Ceano-Vivas La Calle, M.; García-Rodriguez, J.; et al. Think of the Children: Evaluation of SARS-CoV-2 Rapid Antigen Test in Pediatric Population. Pediatr. Infect. Dis. J. 2021. [Google Scholar] [CrossRef]
- Villaverde, S.; Domínguez-Rodríguez, S.; Sabrido, G.; Pérez-Jorge, C.; Plata, M.; Romero, M.P.; Grasa, C.; Jiménez, A.B.; Heras, E.; Broncano, A.; et al. Diagnostic Accuracy of the Panbio SARS-CoV-2 Antigen Rapid Test Compared with Rt-Pcr Testing of Nasopharyngeal Samples in the Pediatric Population. J. Pediatr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.; Pearson, C.A.B.; Quilty, B.J.; Kucharski, A.J.; Gibbs, H.; Clifford, S.; et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020, 26, 1205–1211. [Google Scholar] [CrossRef]
- Marks, M.; Millat-Martinez, P.; Ouchi, D.; Roberts, C.h.; Alemany, A.; Corbacho-Monné, M.; Ubals, M.; Tobias, A.; Tebé, C.; Ballana, E.; et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: A cohort study. Lancet Infect. Dis. 2021, 21, 629–665. [Google Scholar] [CrossRef]
- van Kampen, J.J.A.; van de Vijver, D.A.M.C.; Fraaij, P.L.A.; Haagmans, B.L.; Lamers, M.M.; Okba, N.; van den Akker, J.P.C.; Endeman, H.; Gommers, D.A.M.P.J.; Cornelissen, J.J.; et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat. Commun. 2021, 12. [Google Scholar] [CrossRef]
- de la Calle, C.; Lalueza, A.; Mancheño-Losa, M.; Maestro-de la Calle, G.; Lora-Tamayo, J.; Arrieta, E.; García-Reyne, A.; Losada, I.; de Miguel, B.; Díaz-Simón, R.; et al. Impact of viral load at admission on the development of respiratory failure in hospitalized patients with SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Discontinuation of Transmission-Based Precautions and Disposition of Patients with SARS-CoV-2 Infection in Healthcare Settings | CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html (accessed on 28 April 2021).
- Schmier, J.; Halpern, M.T. Patient recall and recall bias of health state and health status. Expert Rev. Pharm. Outcomes Res. 2004, 4, 159–163. [Google Scholar] [CrossRef]
- Singanayagam, A.; Patel, M.; Charlett, A.; Lopez Bernal, J.; Saliba, V.; Ellis, J.; Ladhani, S.; Zambon, M.; Gopal, R. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Eurosurveillance 2020, 25, 2001483. [Google Scholar] [CrossRef] [PubMed]
| Total | Symptomatic | Asymptomatic | p-Value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Diagnosed patients | 219,138 | 149,026 | 68.0% | 70,112 | 32.0% | ||
| Sex | |||||||
| Female | 117,220 | 53.5% | 81,034 | 54.4% | 36,186 | 51.6% | <0.001 |
| Male | 101,918 | 46.5% | 67,992 | 45.6% | 33,926 | 48.4% | <0.001 |
| Age groups | |||||||
| 0–14 | 28,225 | 12.9% | 12,723 | 8.5% | 15,502 | 22.1% | <0.001 |
| 15–44 | 87,108 | 39.8% | 63,977 | 42.9% | 23,131 | 33.0% | <0.001 |
| 45–64 | 63,103 | 28.8% | 47,437 | 31.8% | 15,666 | 22.3% | <0.001 |
| 65–80 | 24,831 | 11.3% | 16,796 | 11.3% | 8035 | 11.5% | 0.194 |
| >80 | 15,871 | 7.2% | 8093 | 5.4% | 7778 | 11.1% | <0.001 |
| Residents of nursing home | 7977 | 3.6% | 2186 | 1.5% | 5791 | 8.3% | <0.001 |
| Health care workers | 4539 | 2.1% | 3682 | 2.5% | 857 | 1.2% | <0.001 |
| Clinical evolution | |||||||
| Hospitalized | 8361 | 3.8% | 7021 | 4.7% | 1340 | 1.9% | <0.001 |
| Exitus | 4109 | 1.9% | 2313 | 1.6% | 1796 | 2.6% | <0.001 |
| Diagnostic tests | |||||||
| Ag-RDTs | 97,576 | 44.5% | 78,560 | 52.7% | 19,016 | 27.1% | |
| With any negative NAAT | 5796 | 7.4% | |||||
| NAAT | 106,484 | 48.6% | 58,667 | 39.4% | 47,817 | 68.2% | |
| With any negative Ag-RDTs | 14,522 | 24.8% | |||||
| Ag-RDTs + NAAT | 14,561 | 6.6% | 11,741 | 7.9% | 2820 | 4.0% | |
| ELISA IgM | 517 | 0.2% | 58 | 0.0% | 459 | 0.7% | |
| Total tests done | |||||||
| Ag-RDTs * | 181,276 | 32.60% | 139,462 | 38.90% | 41,814 | 21.10% | |
| Before SO * (Positives) | 31,063 (3212) | 22.3% (10.3%) | |||||
| After SO * (Positives) | 108,399 (88,743) | 77.7% (81.9%) | |||||
| NAAT * | 375,388 | 67.40% | 218,724 | 61.10% | 156,664 | 78.90% | |
| Before SO * (Positives) | 127,517 (21,568) | 58.3% (16.9%) | |||||
| After SO * (Positives) | 91,207 (67,722) | 41.7% (74.3%) | |||||
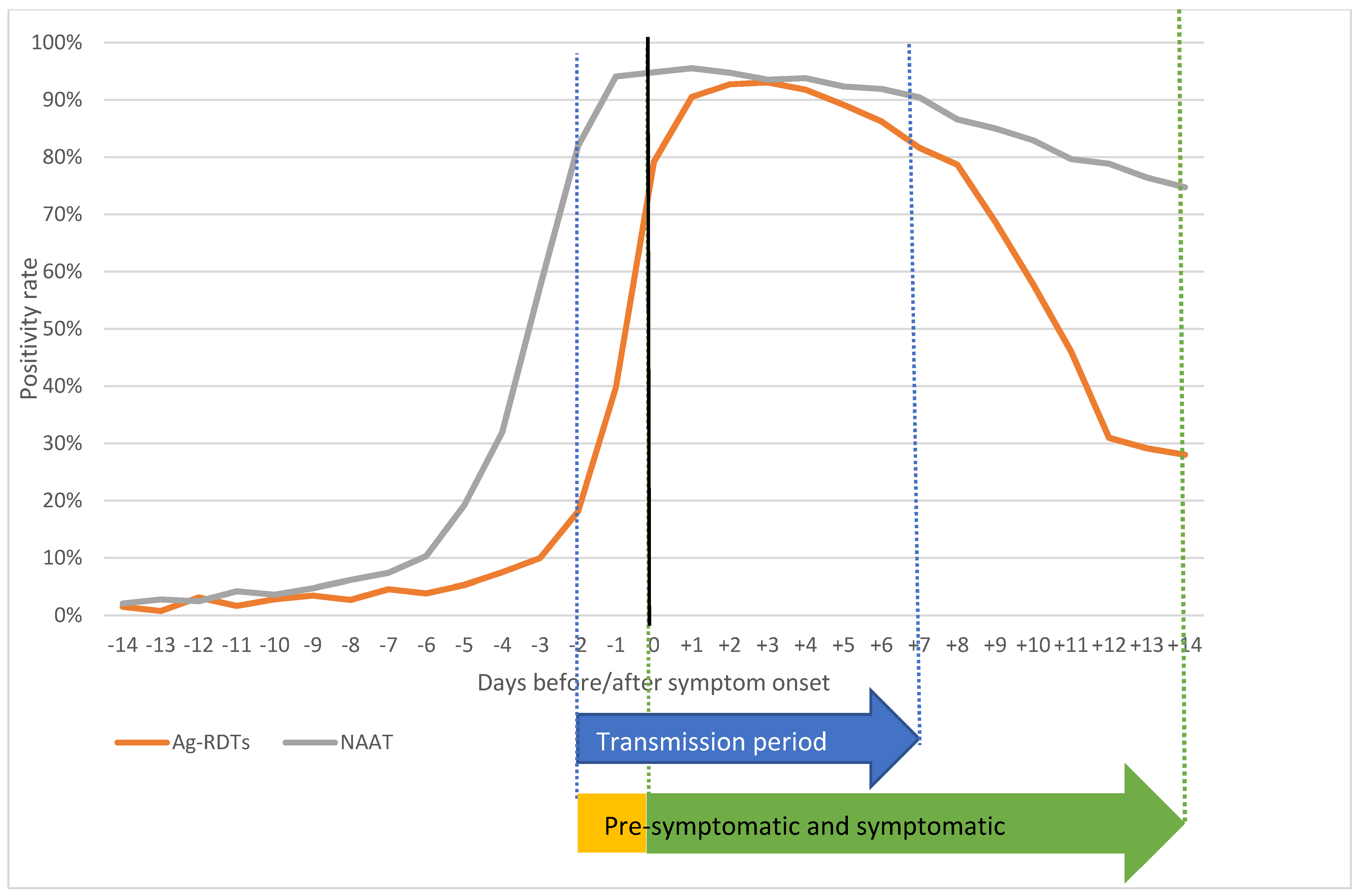
| Days | Ag-RDTs | NAAT | ▲% | CI− | CI+ | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tests | Positives | % | Tests | Positives | % | |||||
| −14 | 396 | 6 | 1.5% | 1100 | 23 | 2.1% | −0.6% | −2.2% | 1.1% | 0.617 |
| −13 | 384 | 3 | 0.8% | 1072 | 30 | 2.8% | −2.0% | −3.5% | −0.5% | 0.038 |
| −12 | 445 | 14 | 3.1% | 1132 | 28 | 2.5% | 0.7% | −1.3% | 2.7% | 0.567 |
| −11 | 538 | 9 | 1.7% | 1352 | 57 | 4.2% | −2.5% | −4.2% | −0.9% | 0.010 |
| −10 | 602 | 17 | 2.8% | 1272 | 46 | 3.6% | −0.8% | −2.6% | 1.0% | 0.452 |
| −9 | 666 | 23 | 3.5% | 1420 | 67 | 4.7% | −1.3% | −3.1% | 0.6% | 0.226 |
| −8 | 852 | 23 | 2.7% | 1461 | 90 | 6.2% | −3.5% | −5.2% | −1.7% | 0.000 |
| −7 | 1163 | 53 | 4.6% | 1729 | 128 | 7.4% | −2.8% | −4.6% | −1.1% | 0.003 |
| −6 | 1326 | 51 | 3.8% | 1688 | 174 | 10.3% | −6.5% | −8.3% | −4.6% | 0.000 |
| −5 | 1624 | 86 | 5.3% | 1962 | 376 | 19.2% | −13.9% | −16.0% | −11.8% | 0.000 |
| −4 | 1884 | 141 | 7.5% | 2249 | 718 | 31.9% | −24.4% | −26.8% | −22.1% | 0.000 |
| −3 | 2538 | 253 | 10.0% | 3162 | 1814 | 57.4% | −47.4% | −49.5% | −45.3% | 0.000 |
| −2 | 3266 | 592 | 18.1% | 5167 | 4235 | 82.0% | −63.8% | −65.5% | −62.1% | 0.000 |
| −1 | 4647 | 1849 | 39.8% | 14,360 | 13,512 | 94.1% | −54.3% | −55.8% | −52.8% | 0.000 |
| Symptom onset | 20,806 | 16,476 | 79.2% | 12,190 | 11,559 | 94.8% | −15.6% | −16.3% | −15.0% | 0.000 |
| +1 | 26,770 | 24,232 | 90.5% | 8800 | 8406 | 95.5% | −5.0% | −5.6% | −4.4% | 0.000 |
| +2 | 20,285 | 18,806 | 92.7% | 6267 | 5937 | 94.7% | −2.0% | −2.7% | −1.4% | 0.000 |
| +3 | 12,731 | 11,845 | 93.0% | 4555 | 4260 | 93.5% | −0.5% | −1.3% | 0.4% | 0.257 |
| +4 | 7223 | 6629 | 91.8% | 3388 | 3178 | 93.8% | −2.0% | −3.1% | −1.0% | 0.000 |
| +5 | 4024 | 3587 | 89.1% | 2673 | 2468 | 92.3% | −3.2% | −4.6% | −1.8% | 0.000 |
| +6 | 2410 | 2078 | 86.2% | 2555 | 2348 | 91.9% | −5.7% | −7.5% | −3.9% | 0.000 |
| +7 | 1876 | 1531 | 81.6% | 2227 | 2014 | 90.4% | −8.8% | −11.0% | −6.6% | 0.000 |
| +8 | 962 | 757 | 78.7% | 2148 | 1860 | 86.6% | −7.9% | −10.9% | −4.9% | 0.000 |
| +9 | 728 | 500 | 68.7% | 2844 | 2417 | 85.0% | −16.3% | −20.0% | −12.6% | 0.000 |
| +10 | 656 | 379 | 57.8% | 2989 | 2479 | 82.9% | −25.2% | −29.3% | −21.1% | 0.000 |
| +11 | 554 | 255 | 46.0% | 2816 | 2243 | 79.7% | −33.6% | −38.1% | −29.1% | 0.000 |
| +12 | 507 | 157 | 31.0% | 2434 | 1919 | 78.8% | −47.9% | −52.3% | −43.4% | 0.000 |
| +13 | 436 | 127 | 29.1% | 2490 | 1903 | 76.4% | −47.3% | −52.0% | −42.6% | 0.000 |
| +14 | 414 | 116 | 28.0% | 2141 | 1600 | 74.7% | −46.7% | −51.6% | −41.9% | 0.000 |
| Pre-Symptomatic Period (−2; −1) | Symptomatic Period (0; +14) | Transmission Period (−2; +7) | Post-Transmission Period (+8; +14) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag-RDTs | NAAT | ▲% | Ag-RDTs | NAAT | ▲% | Ag-RDTs | NAAT | ▲% | Ag-RDTs | NAAT | ▲% | |
| Total | 30.8% | 90.9% | −60.0% | 87.1% | 90.2% | −3.1% | 84.2% | 93.1% | −8.9% | 53.8% | 80.7% | −26.9% |
| 0–4 | 32.1% | 95.8% | −63.7% | 90.0% | 93.5% | −3.5% | 84.3% | 94.7% | −10.4% | 59.0% | 84.3% | −25.3% |
| 5–14 | 42.6% | 95.3% | −52.7% | 90.8% | 94.7% | −3.9% | 87.4% | 95.7% | −8.3% | 49.2% | 80.6% | −31.5% |
| 15–44 | 28.1% | 90.5% | −62.3% | 86.7% | 89.6% | −2.8% | 83.8% | 93.4% | −9.6% | 43.8% | 79.3% | −35.5% |
| 45–64 | 29.4% | 90.1% | −60.7% | 86.7% | 90.2% | −3.5% | 83.9% | 93.2% | −9.3% | 53.5% | 81.0% | −27.6% |
| 65–80 | 37.0% | 91.3% | −54.2% | 87.8% | 92.2% | −4.4% | 85.3% | 93.5% | −8.1% | 70.6% | 85.3% | −14.7% |
| +80 | 37.1% | 88.5% | −51.4% | 87.8% | 87.0% | 0.9% | 84.3% | 88.3% | −4.0% | 77.1% | 83.4% | −6.3% |
| Health workers | 22.3% | 86.1% | −63.8% | 81.1% | 82.6% | −1.5% | 79.9% | 89.7% | −9.9% | 29.5% | 76.7% | −47.1% |
| Nursing home residents | 24.4% | 84.9% | −60.5% | 79.7% | 82.0% | −2.3% | 74.9% | 84.7% | −9.8% | 50.8% | 77.4% | −26.6% |
| Hospitalized | 35.5% | 82.5% | −47.1% | 78.7% | 85.4% | −6.7% | 76.2% | 85.4% | −9.3% | 69.6% | 82.2% | −12.6% |
| Exitus | 38.8% | 81.8% | −43.0% | 87.3% | 85.0% | 2.3% | 83.4% | 84.2% | −0.8% | 82.4% | 82.8% | −0.5% |
| Higher incidence rate * | 31.3% | 91.0% | −59.7% | 87.4% | 90.3% | −3.0% | 84.4% | 93.2% | −8.8% | 54.5% | 80.7% | −26.2% |
| Lower incidence rate * | 28.1% | 90.3% | −62.1% | 85.8% | 89.6% | −3.8% | 83.0% | 92.6% | −9.6% | 49.9% | 80.9% | −31.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basile, L.; Guadalupe-Fernández, V.; Valdivia Guijarro, M.; Martinez Mateo, A.; Ciruela Navas, P.; Mendioroz Peña, J.; the Epidemiological Surveillance Network of Catalonia. Diagnostic Performance of Ag-RDTs and NAAT for SARS-CoV2 Identification in Symptomatic Patients in Catalonia. Viruses 2021, 13, 908. https://doi.org/10.3390/v13050908
Basile L, Guadalupe-Fernández V, Valdivia Guijarro M, Martinez Mateo A, Ciruela Navas P, Mendioroz Peña J, the Epidemiological Surveillance Network of Catalonia. Diagnostic Performance of Ag-RDTs and NAAT for SARS-CoV2 Identification in Symptomatic Patients in Catalonia. Viruses. 2021; 13(5):908. https://doi.org/10.3390/v13050908
Chicago/Turabian StyleBasile, Luca, Víctor Guadalupe-Fernández, Manuel Valdivia Guijarro, Ana Martinez Mateo, Pilar Ciruela Navas, Jacobo Mendioroz Peña, and the Epidemiological Surveillance Network of Catalonia. 2021. "Diagnostic Performance of Ag-RDTs and NAAT for SARS-CoV2 Identification in Symptomatic Patients in Catalonia" Viruses 13, no. 5: 908. https://doi.org/10.3390/v13050908
APA StyleBasile, L., Guadalupe-Fernández, V., Valdivia Guijarro, M., Martinez Mateo, A., Ciruela Navas, P., Mendioroz Peña, J., & the Epidemiological Surveillance Network of Catalonia. (2021). Diagnostic Performance of Ag-RDTs and NAAT for SARS-CoV2 Identification in Symptomatic Patients in Catalonia. Viruses, 13(5), 908. https://doi.org/10.3390/v13050908






