Cytokines and Leukocytes Subpopulations Profile in SARS-CoV-2 Patients Depending on the CT Score Severity
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
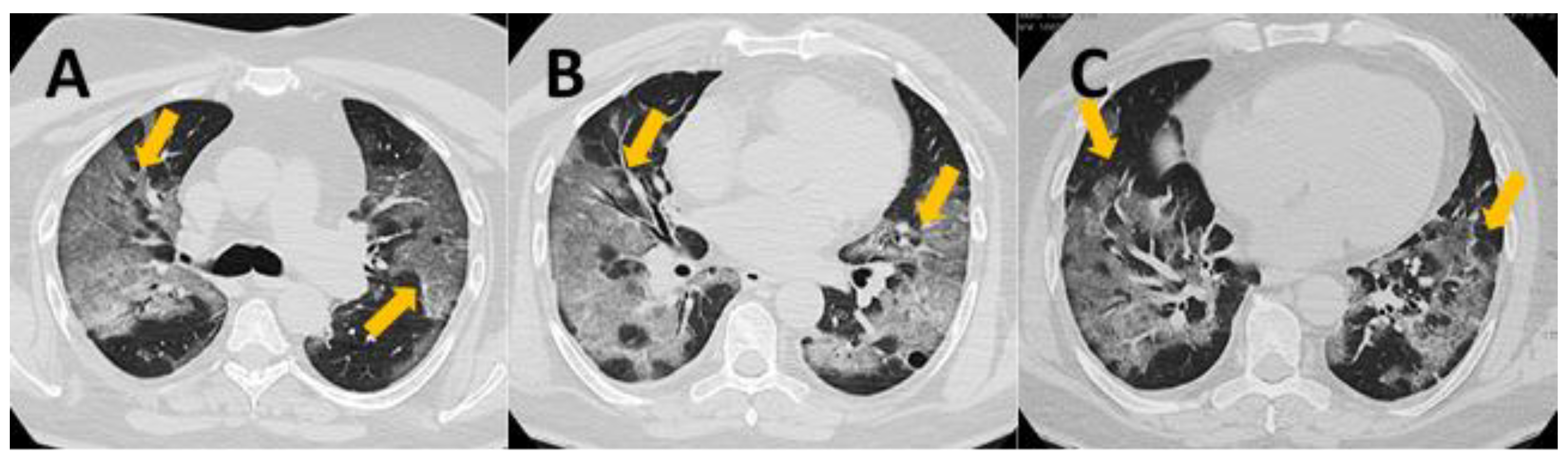
2.2. CT Severity Score
- 0 points—normal lung
- 1–5 points—mild changes
- 6–10 points—moderate changes
- 11–15 points—severe changes
- 16–25 points—critical changes
2.3. Peripheral Blood Samples
2.4. Cytokine Measurement
2.5. Flow Cytometry
2.6. Statistical Analysis
3. Results
3.1. Basic Leukocytes Profile
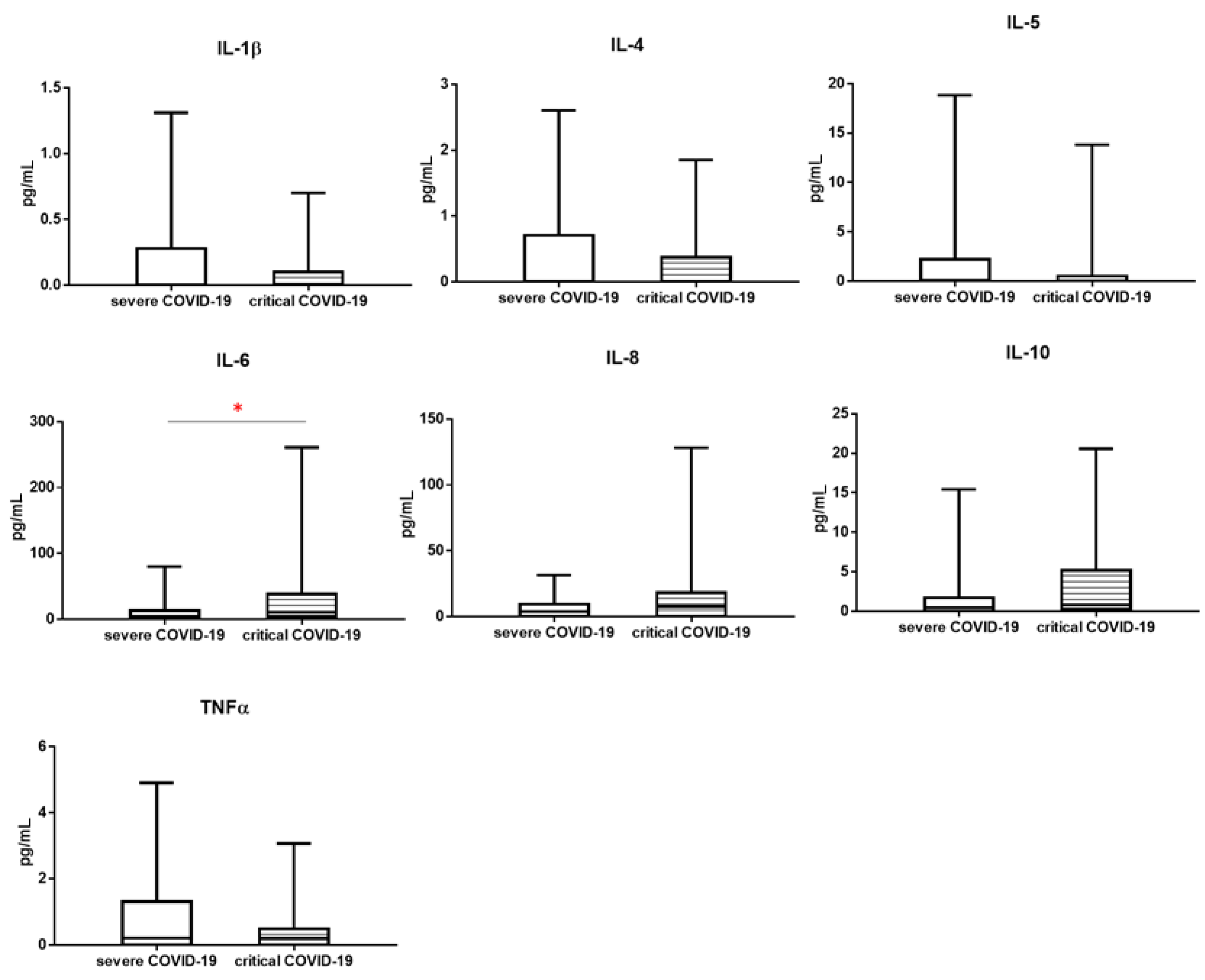
3.2. Cytokines Profile
3.3. Correlation between CT Severity Score and Study Parameters
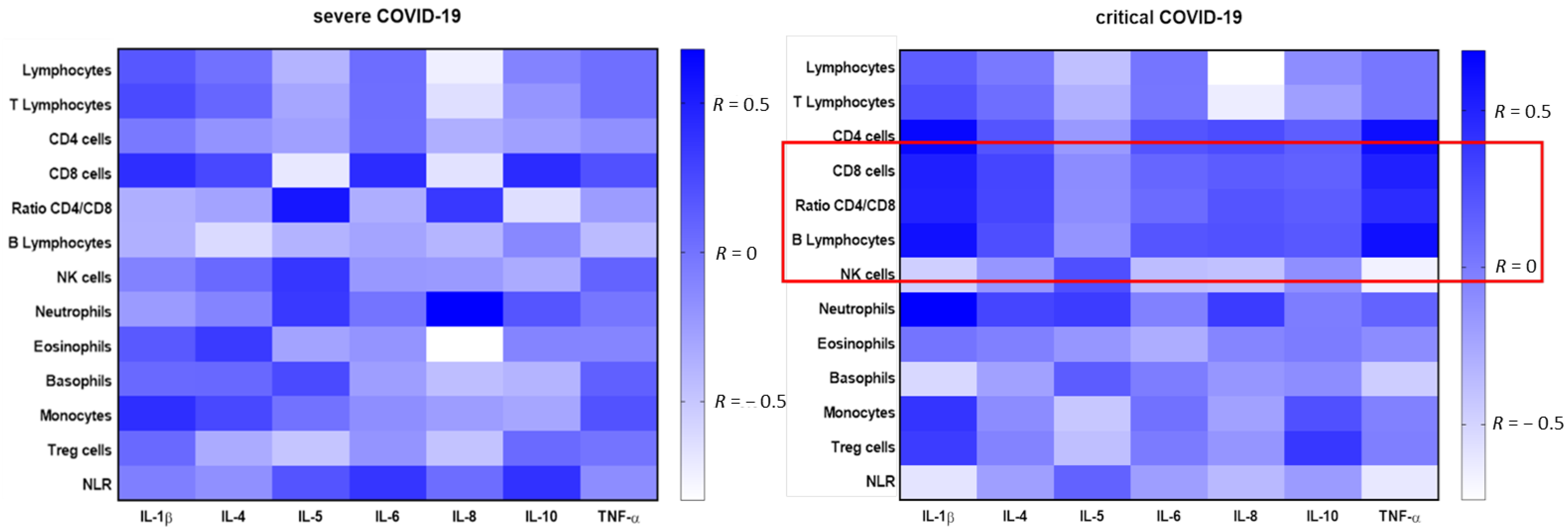
3.4. Correlation between Cytokines Concentration and Leukocyte Subpopulations Depending on Severity of COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A

References
- Chauhan, S. Comprehensive review of coronavirus disease 2019 (COVID-19). Biomed. J. 2020, 43, 334–340. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Alnor, A.; Sandberg, M.B.; Toftanes, B.E.; Vinholt, P.J. Platelet parameters and leukocyte morphology is altered in COVID-19 patients compared to non-COVID-19 patients with similar symptomatology. Scand. J. Clin. Lab. Investig. 2021, 81, 213–217. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. Mild versus severe COVID-19: Laboratory markers. Int. J. Infect. Dis. 2020, 95, 304–307. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, E.; Kwiecien, I.; Kulik, K.; Chelstowska, B.; Klos, K.; Rzepecki, P.; Chcialowski, A. Usefulness of the New Hematological Parameter: Reactive Lymphocytes RE-LYMP with Flow Cytometry Markers of Inflammation in COVID-19. Cells 2021, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.U.; Kim, M.J.; Ra, S.H.; Lee, J.; Bae, S.; Jung, J.; Kim, S.H. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin. Microbiol. Infect. 2020, 26, 948.e1–948.e3. [Google Scholar] [CrossRef]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef]
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A.; et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef]
- Francone, M.; Iafrate, F.; Masci, G.M.; Coco, S.; Cilia, F.; Manganaro, L.; Panebianco, V.; Andreoli, C.; Colaiacomo, M.C.; Zingaropoli, M.A.; et al. Chest CT score in COVID-19 patients: Correlation with disease severity and short-term prognosis. Eur. Radiol. 2020, 30, 6808–6817. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fang, Y.; Li, W.; Pan, C.; Qin, P.; Zhong, Y.; Liu, X.; Huang, M.; Liao, Y.; Li, S. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur. Radiol. 2020, 30, 4407–4416. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawlowska, M.; Parczewski, M.; Piekarska, A.; Simon, K.; Tomasiewicz, K.; Zarebska-Michaluk, D. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of March 31, 2020. Pol. Arch. Intern. Med. 2020, 130, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Deng, Y.; Weng, Z.; Yang, L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. 2020, 96, 131–135. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, M.; Zhu, T.; Zhili, N.; Liu, Z.; Xiang, R.; Zhang, W.; Xu, Y. Dynamic changes in peripheral blood lymphocyte subsets in adult patients with COVID-19. Int. J. Infect. Dis. 2020, 98, 353–358. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Luo, M.; Liu, J.; Wu, L.; Lin, X.; Li, R.; Wang, Z.; Zhong, H.; Zheng, W.; et al. Lymphopenia predicted illness severity and recovery in patients with COVID-19: A single-center, retrospective study. PLoS ONE 2020, 15, e0241659. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wang, Z.; Yin, Y.; Zhao, Y.; Tao, P.; Zhong, P. Association of Peripheral Lymphocyte and the Subset Levels with the Progression and Mortality of COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 558545. [Google Scholar] [CrossRef]
- Kwiecien, I.; Rutkowska, E.; Klos, K.; Wiesik-Szewczyk, E.; Jahnz-Rozyk, K.; Rzepecki, P.; Chcialowski, A. Maturation of T and B Lymphocytes in the Assessment of the Immune Status in COVID-19 Patients. Cells 2020, 9, 2615. [Google Scholar] [CrossRef]
- Tavakolpour, S.; Rakhshandehroo, T.; Wei, E.X.; Rashidian, M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020, 225, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Jafarzadeh, S.; Nozari, P.; Mokhtari, P.; Nemati, M. Lymphopenia an important immunological abnormality in patients with COVID-19: Possible mechanisms. Scand. J. Immunol. 2021, 93, e12967. [Google Scholar] [CrossRef]
- Vedder, V.; Schildgen, V.; Lusebrink, J.; Tillmann, R.L.; Domscheit, B.; Windisch, W.; Karagiannidis, C.; Brockmann, M.; Schildgen, O. Differential cytology profiles in bronchoalveolar lavage (BAL) in COVID-19 patients: A descriptive observation and comparison with other corona viruses, Influenza virus, Haemophilus influenzae, and Pneumocystis jirovecii. Medicine 2021, 100, e24256. [Google Scholar] [CrossRef]
- Wang, W.; Su, B.; Pang, L.; Qiao, L.; Feng, Y.; Ouyang, Y.; Guo, X.; Shi, H.; Wei, F.; Su, X.; et al. High-dimensional immune profiling by mass cytometry revealed immunosuppression and dysfunction of immunity in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 650–652. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Wang, F.; Hou, H.; Luo, Y.; Tang, G.; Wu, S.; Huang, M.; Liu, W.; Zhu, Y.; Lin, Q.; Mao, L.; et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight 2020, 5, e137799. [Google Scholar] [CrossRef]
- Broos, C.E.; van Nimwegen, M.; Kleinjan, A.; ten Berge, B.; Muskens, F.; in’t Veen, J.C.; Annema, J.T.; Lambrecht, B.N.; Hoogsteden, H.C.; Hendriks, R.W.; et al. Impaired survival of regulatory T cells in pulmonary sarcoidosis. Respir. Res. 2015, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Mao, Z.; Xiao, M.; Wang, L.; Qi, S.; Zhou, F. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: A systematic review and meta-analysis. Crit. Care 2020, 24, 647. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, S.; Ventura, P.S.; Castellano, J.M.; Garcia-Adasme, S.I.; Miranda, M.; Touza, P.; Lllana, I.; Lopez-Escobar, A. Prognostic implications of neutrophil-lymphocyte ratio in COVID-19. Eur. J. Clin. Investig. 2021, 51, e13404. [Google Scholar] [CrossRef]
- Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; Lima, E.A.; Galvao, J.; da Silva, J.S.F.; Sales-Neto, J.M.; Rodrigues-Mascarenhas, S. Neutrophils and COVID-19: The road so far. Int. Immunopharmacol. 2021, 90, 107233. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagunduz, P.; Tabak, F.; Atagunduz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]
- Sun, H.; Guo, P.; Zhang, L.; Wang, F. Serum Interleukin-6 Concentrations and the Severity of COVID-19 Pneumonia: A Retrospective Study at a Single Center in Bengbu City, Anhui Province, China, in January and February 2020. Med. Sci. Monit. 2020, 26, e926941. [Google Scholar] [CrossRef]
- Dhar, S.K.; Vishnupriyan, K.; Damodar, S.; Gujar, S.; Das, M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: Results from meta-analysis and regression. Heliyon 2021, 7, e06155. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Y.; Ou, W.; Ming, F.; Liang, G.; Qian, Y.; Cai, Q.; Dong, S.; Hu, S.; Wang, W.; et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: A cohort study. J. Transl. Med. 2020, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Jin, M.; Miao, L.; Yang, X.; Huang, P.; Xiong, H.; Huang, P.; Zhao, Q.; Du, J.; Hong, J. Early chest computed tomography to diagnose COVID-19 from suspected patients: A multicenter retrospective study. Am. J. Emerg. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, P.G.; Mruk, B.; Mazur, S.; Poltorak-Szymczak, G.; Sklinda, K.; Walecki, J. COVID-19 severity scoring systems in radiological imaging—A review. Pol. J. Radiol. 2020, 85, e361–e368. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, H.; Dauphars, D.J.; He, Y.W. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol. 2021, 42, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Glickman, J.W.; Pavel, A.B.; Guttman-Yassky, E.; Miller, R.L. The role of circulating eosinophils on COVID-19 mortality varies by race/ethnicity. Allergy 2021, 76, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Z.; Liu, S.; Gong, C.; Chen, L.; Ai, G.; Zhu, X.; Zhang, C.; Li, D. Absolute Eosinophil Count Predicts Intensive Care Unit Transfer Among Elderly COVID-19 Patients from General Isolation Wards. Front. Med. 2020, 7, 585222. [Google Scholar] [CrossRef]
- Xia, Z. Eosinopenia as an early diagnostic marker of COVID-19 at the time of the epidemic. EClinicalMedicine 2020, 23, 100398. [Google Scholar] [CrossRef]
- Le Page, C.; Genin, P.; Baines, M.G.; Hiscott, J. Interferon activation and innate immunity. Rev. Immunogenet. 2000, 2, 374–386. [Google Scholar]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; on behalf of the HLH across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Hosseini, A.; Hashemi, V.; Shomali, N.; Asghari, F.; Gharibi, T.; Akbari, M.; Gholizadeh, S.; Jafari, A. Innate and adaptive immune responses against coronavirus. Biomed. Pharmacother. 2020, 132, 110859. [Google Scholar] [CrossRef] [PubMed]
- Braciale, T.J.; Sun, J.; Kim, T.S. Regulating the adaptive immune response to respiratory virus infection. Nat. Rev. Immunol. 2012, 12, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- He, S.; Zhou, C.; Lu, D.; Yang, H.; Xu, H.; Wu, G.; Pan, W.; Zhu, R.; Jia, H.; Tang, X.; et al. Relationship between chest CT manifestations and immune response in COVID-19 patients. Int. J. Infect. Dis. 2020, 98, 125–129. [Google Scholar] [CrossRef] [PubMed]
- McClain, M.T.; Park, L.P.; Nicholson, B.; Veldman, T.; Zaas, A.K.; Turner, R.; Lambkin-Williams, R.; Gilbert, A.S.; Ginsburg, G.S.; Woods, C.W. Longitudinal analysis of leukocyte differentials in peripheral blood of patients with acute respiratory viral infections. J. Clin. Virol. 2013, 58, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lutgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schroder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; van der Most, R.G.; Akondy, R.S.; Glidewell, J.T.; Albott, S.; Masopust, D.; Murali-Krishna, K.; Mahar, P.L.; Edupuganti, S.; Lalor, S.; et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 2008, 28, 710–722. [Google Scholar] [CrossRef]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; van den Akker, J.P.C.; Molenkamp, R.; Koopmans, M.P.G.; van Gorp, E.C.M.; et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020, 5, eabd2071. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Y.; Zhang, M.; Yang, C.X.; Zhang, N.; Wang, X.C.; Yang, X.P.; Dong, X.Q.; Zheng, Y.T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef] [PubMed]


| Severe COVID-19 n = 23 | Critical COVID-19 n = 15 | |
|---|---|---|
| Sex: f/m (n) | 15/8 | 1/14 |
| Age (mean ± SD years) | 54.9 ± 14.4 | 59.1 ± 12.0 |
| Clinical symptoms (n, %) | ||
| 20, 87.0% | 15, 100% |
| 10, 43.5% | 11, 73.3% |
| 8, 34.8% | 14, 93.3% |
| 5, 21.7% | 13, 86.7% |
| Saturation (mean ± SD %) | 94.0 ± 4.2% | 88.2 ± 6.7% |
| Diseases comorbidities (n, %) | ||
| 2, 8.7% | 4, 26.7% |
| 3, 13.0% | 7, 46.7% |
| 1, 4.3% | 4, 26.7% |
| 3, 13.0% | 3, 20.0% |
| 2, 8.7% | 3, 20.0% |
| COVID-19 Severe n = 23 Median (Q1–Q3) | COVID-19 Critical n = 15 Median (Q1–Q3) | Mann-Whitney U Test | |
|---|---|---|---|
| WBC [k/µL] | 5280 (4580–8620) | 9220 (4370–13010) | * 0.0382 |
| [% of leukocytes] | |||
| Lymphocytes | 23.1 (11.6–36.4) | 10.3 (6.7–20.8) | * 0.0219 |
| T Lymphocytes | 18.0 (7.8–28.0) | 6.9 (4.6–15.2) | * 0.0514 |
| CD4 cells | 11.3 (4.8–17.9) | 3.8 (2.8–6.0) | * 0.0.018 |
| CD8 cells | 5.1 (2.3–12.6) | 3.5 (0.7–7.7) | 0.2238 |
| Ratio CD4/CD8 | 2.1 (0.7–3.4) | 1.2 (0.8–4.2) | 0.8828 |
| Treg cells | 0.557 (0.346–0.947) | 0.214 (0.155–0.360) | * 0.0061 |
| Treg cells CD45RO+ CD95+ [% among Treg cells] | 76.5 (67.1–85.2) | 81.3 (79.1–89.0) | 0.0959 |
| B Lymphocytes | 2.5 (1.5–3.6) | 1.3 (1.0–2.5) | * 0.0412 |
| NK cells | 3.5 (1.0–5.4) | 1.1 (0.7–2.1) | * 0.0238 |
| Neutrophils | 67.3 (46.6–80.6) | 85.3 (64.4–88.6) | * 0.0444 |
| Eosinophils | 0.2 (0.0–0.9) | 0.1 (0.0–1.2) | 0.9295 |
| Basophils | 0.2 (0.1–0.7) | 0.1 (0.0–0.2) | 0.1143 |
| Monocytes | 7.0 (4.1–9.0) | 5.4 (2.9–9.1) | 0.1340 |
| NLR | 2.9 (1.4–6.9) | 8.4 (3.1–12.7) | * 0.0258 |
| Cytokines [pg/mL] | COVID-19 Severe n = 23 Median (Q1–Q3) | COVID-19 Critical n = 15 Median (Q1–Q3) | Mann-Whitney U Test |
|---|---|---|---|
| IL-1β | 0.000 (0.000–0.283) | 0.000 (0.000–0.100) | 0.7013 |
| IL-4 | 0.000 (0.000–0.710) | 0.000 (0.000–0.370) | 0.2861 |
| IL-5 | 0.128 (0.000–2.200) | 0.000 (0.000–0.500) | 0.2729 |
| IL-6 | 4.036 (0.474–13.000) | 10.500 (4.000–38.380) | * 0.0382 |
| IL-8 | 3.900 (0.000–9.4447) | 7.600 (0.000–18.010) | 0.3140 |
| IL-10 | 0.460 (0.000–1.730) | 0.831 (0.300–5.200) | 0.1623 |
| TNF-α | 0.209 (0.000–1.300) | 0.212 (0.000–0.490) | 0.5548 |
| CT Severity Score (n = 38 ) | ||
|---|---|---|
| r | p-Values | |
| Lymphocytes [%] | −0.402 | 0.0123 * |
| T Lymphocytes [%] | −0.344 | 0.0339 * |
| CD4 cells [%] | −0.387 | 0.0162 * |
| CD8 cells [%] | −0.311 | 0.0572 |
| Ratio CD4/CD8 [%] | 0.061 | 0.7156 |
| Treg cells [%] | −0.392 | 0.0148 * |
| Treg cells CD45RO+ CD95+ [%] | 0.219 | 0.2046 |
| B Lymphocytes [%] | −0.284 | 0.0829 |
| NK cells [%] | −0.374 | 0.0205 * |
| Neutrophils [%] | 0.352 | 0.0301 * |
| Eosinophils [%] | −0.030 | 0.8540 |
| Basophils [%] | −0.232 | 0.1598 |
| Monocytes [%] | −0.049 | 0.7689 |
| NLR | 0.390 | 0.0152 * |
| IL-1β [pg/mL] | 0.003 | 0.9847 |
| IL-4 [pg/mL] | −0.223 | 0.1775 |
| IL-5 [pg/mL] | −0.026 | 0.8762 |
| IL-6 [pg/mL] | 0.351 | 0.0304 * |
| IL-8 [pg/mL] | 0.124 | 0.4579 |
| IL-10 [pg/mL] | 0.105 | 0.5294 |
| TNF-α [pg/mL] | −0.150 | 0.3680 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowska, E.; Kwiecień, I.; Żabicka, M.; Maliborski, A.; Raniszewska, A.; Kłos, K.; Urbańska, W.; Klajnowicz, I.; Rzepecki, P.; Chciałowski, A. Cytokines and Leukocytes Subpopulations Profile in SARS-CoV-2 Patients Depending on the CT Score Severity. Viruses 2021, 13, 880. https://doi.org/10.3390/v13050880
Rutkowska E, Kwiecień I, Żabicka M, Maliborski A, Raniszewska A, Kłos K, Urbańska W, Klajnowicz I, Rzepecki P, Chciałowski A. Cytokines and Leukocytes Subpopulations Profile in SARS-CoV-2 Patients Depending on the CT Score Severity. Viruses. 2021; 13(5):880. https://doi.org/10.3390/v13050880
Chicago/Turabian StyleRutkowska, Elżbieta, Iwona Kwiecień, Magdalena Żabicka, Artur Maliborski, Agata Raniszewska, Krzysztof Kłos, Weronika Urbańska, Izabella Klajnowicz, Piotr Rzepecki, and Andrzej Chciałowski. 2021. "Cytokines and Leukocytes Subpopulations Profile in SARS-CoV-2 Patients Depending on the CT Score Severity" Viruses 13, no. 5: 880. https://doi.org/10.3390/v13050880
APA StyleRutkowska, E., Kwiecień, I., Żabicka, M., Maliborski, A., Raniszewska, A., Kłos, K., Urbańska, W., Klajnowicz, I., Rzepecki, P., & Chciałowski, A. (2021). Cytokines and Leukocytes Subpopulations Profile in SARS-CoV-2 Patients Depending on the CT Score Severity. Viruses, 13(5), 880. https://doi.org/10.3390/v13050880







