N-Glycolylneuraminic Acid in Animal Models for Human Influenza A Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Cells
2.2. Expression and Purification of HA
2.3. Protein Histochemistry
2.4. Hemagglutination Assay
2.5. Glycan Microarray Binding of HA
2.6. Virus Challenge in Mice
2.7. Preparation of Lung Homogenates
2.8. Virus Titration of Lung Homogenates
3. Results
3.1. Animal Models for Human Influenza
3.2. Physical Characteristics of Animal Models
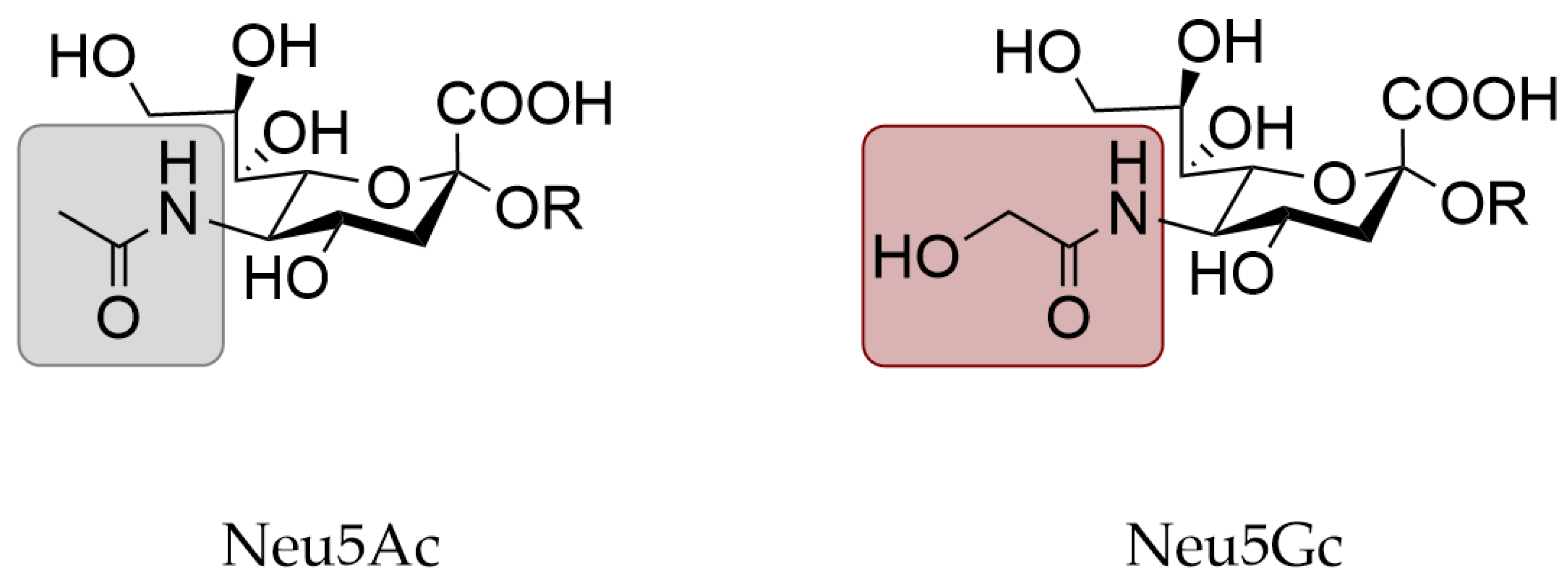
3.3. Expression of N-Glycolylneuraminic Acid in Animal Models
3.4. Neu5Gc as a Functional Receptor
4. Discussion
4.1. Relevance of Neu5Gc in Animal Models for Human Influenza
4.2. Remaining Questions on the Role of Neu5Gc and Neu5Gc Binding
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ge, S.; Wang, Z. An overview of influenza A virus receptors. Crit. Rev. Microbiol. 2011, 37, 157–165. [Google Scholar] [CrossRef]
- Gambaryan, A.S.; Matrosovich, T.Y.; Philipp, J.; Munster, V.J.; Fouchier, R.A.; Cattoli, G.; Capua, I.; Krauss, S.L.; Webster, R.G.; Banks, J.; et al. Receptor-binding profiles of H7 subtype influenza viruses in different host species. J. Virol. 2012, 86, 4370–4379. [Google Scholar] [CrossRef] [PubMed]
- Broszeit, F.; Tzarum, N.; Zhu, X.; Nemanichvili, N.; Eggink, D.; Leenders, T.; Li, Z.; Liu, L.; Wolfert, M.A.; Papanikolaou, A.; et al. N-glycolylneuraminic acid as a receptor for influenza A viruses. Cell Rep. 2019, 27, 3284–3294.e6. [Google Scholar] [CrossRef] [PubMed]
- Peri, S.; Kulkarni, A.; Feyertag, F.; Berninsone, P.M.; Alvarez-Ponce, D. Phylogenetic distribution of CMP-Neu5Ac hydroxylase (CMAH), the enzyme synthetizing the proinflammatory human xenoantigen Neu5Gc. Genome Biol. Evol. 2018, 10, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Altman, M.O.; Gagneux, P. Absence of Neu5Gc and presence of anti-Neu5Gc antibodies in humans-an evolutionary perspective. Front. Immunol. 2019, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Furukawa, J.I.; Naito, S.; Higashino, K.; Numata, Y.; Shinohara, Y. Quantitative analysis of total serum glycome in human and mouse. Proteomics 2016, 16, 2747–2758. [Google Scholar] [CrossRef]
- Varki, A. Loss of N-glycolylneuraminic acid in humans: Mechanisms, consequences, and implications for hominid evolution. Am. J. Phys. Anthropol. 2001, 116 (Suppl. 33), 54–69. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Thomson, P.C.; Wynn, P.C.; Wang, B. The non-human glycan, N-glycolylneuraminic acid (Neu5Gc), is not expressed in all organs and skeletal muscles of nine animal species. Food Chem. 2021, 343, 128439. [Google Scholar] [CrossRef]
- Raju, T.S.; Briggs, J.B.; Borge, S.M.; Jones, A.J. Species-specific variation in glycosylation of IgG: Evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 2000, 10, 477–486. [Google Scholar] [CrossRef]
- Bleckmann, C.; Geyer, H.; Lieberoth, A.; Splittstoesser, F.; Liu, Y.; Feizi, T.; Schachner, M.; Kleene, R.; Reinhold, V.; Geyer, R. O-glycosylation pattern of CD24 from mouse brain. Biol. Chem. 2009, 390, 627–645. [Google Scholar] [CrossRef]
- Gizaw, S.T.; Koda, T.; Amano, M.; Kamimura, K.; Ohashi, T.; Hinou, H.; Nishimura, S. A comprehensive glycome profiling of Huntington’s disease transgenic mice. Biochim. Biophys. Acta 2015, 1850, 1704–1718. [Google Scholar] [CrossRef]
- Diaz, S.L.; Padler-Karavani, V.; Ghaderi, D.; Hurtado-Ziola, N.; Yu, H.; Chen, X.; Brinkman-Van der Linden, E.C.; Varki, A.; Varki, N.M. Sensitive and specific detection of the non-human sialic acid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS ONE 2009, 4, e4241. [Google Scholar] [CrossRef] [PubMed]
- Reiding, K.R.; Hipgrave Ederveen, A.L.; Rombouts, Y.; Wuhrer, M. Murine plasma N-Glycosylation traits associated with sex and strain. J. Proteome Res. 2016, 15, 3489–3499. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, D.; Springer, S.A.; Ma, F.; Cohen, M.; Secrest, P.; Taylor, R.E.; Varki, A.; Gagneux, P. Sexual selection by female immunity against paternal antigens can fix loss of function alleles. Proc. Natl. Acad. Sci. USA 2011, 108, 17743–17748. [Google Scholar] [CrossRef]
- Hedlund, M.; Tangvoranuntakul, P.; Takematsu, H.; Long, J.M.; Housley, G.D.; Kozutsumi, Y.; Suzuki, A.; Wynshaw-Boris, A.; Ryan, A.F.; Gallo, R.L.; et al. N-glycolylneuraminic acid deficiency in mice: Implications for human biology and evolution. Mol. Cell Biol. 2007, 27, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, P.; Cheriyan, M.; Hurtado-Ziola, N.; van der Linden, E.C.; Anderson, D.; McClure, H.; Varki, A.; Varki, N.M. Human-specific regulation of α2-6-linked sialic acids. J. Biol. Chem. 2003, 278, 48245–48250. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Barclay, W.S.; Roberts, K.; Yen, H.L.; Chan, R.W.; Lam, A.K.; Air, G.; Peiris, J.S.; Dell, A.; Nicholls, J.M.; et al. Glycomic characterization of respiratory tract tissues of ferrets: Implications for its use in influenza virus infection studies. J. Biol. Chem. 2014, 289, 28489–28504. [Google Scholar] [CrossRef]
- Ng, P.S.; Bohm, R.; Hartley-Tassell, L.E.; Steen, J.A.; Wang, H.; Lukowski, S.W.; Hawthorne, P.L.; Trezise, A.E.; Coloe, P.J.; Grimmond, S.M.; et al. Ferrets exclusively synthesize Neu5Ac and express naturally humanized influenza A virus receptors. Nat. Commun. 2014, 5, 5750. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Kosikova, M.; Kim, S.R.; Kotian, S.; Wu, W.W.; Shen, R.; Powers, D.N.; Agarabi, C.; Xie, H.; Ju, T. Comprehensive analysis of N-glycans in IgG purified from ferrets with or without influenza A virus infection. J. Biol. Chem. 2018, 293, 19277–19289. [Google Scholar] [CrossRef]
- Naito, Y.; Takematsu, H.; Koyama, S.; Miyake, S.; Yamamoto, H.; Fujinawa, R.; Sugai, M.; Okuno, Y.; Tsujimoto, G.; Yamaji, T.; et al. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol. Cell Biol. 2007, 27, 3008–3022. [Google Scholar] [CrossRef]
- Harbury, P.B.; Zhang, T.; Kim, P.S.; Alber, T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science 1993, 262, 1401–1407. [Google Scholar] [CrossRef]
- Nemanichvili, N.; Tomris, I.; Turner, H.L.; McBride, R.; Grant, O.C.; van der Woude, R.; Aldosari, M.H.; Pieters, R.J.; Woods, R.J.; Paulson, J.C.; et al. Fluorescent trimeric hemagglutinins reveal multivalent receptor binding properties. J. Mol. Biol. 2019, 431, 842–856. [Google Scholar] [CrossRef]
- Shaner, N.C.; Lin, M.Z.; McKeown, M.R.; Steinbach, P.A.; Hazelwood, K.L.; Davidson, M.W.; Tsien, R.Y. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat. Methods 2008, 5, 545–551. [Google Scholar] [CrossRef]
- De Vries, R.P.; de Vries, E.; Bosch, B.J.; de Groot, R.J.; Rottier, P.J.; de Haan, C.A. The influenza A virus hemagglutinin glycosylation state affects receptor-binding specificity. Virology 2010, 403, 17–25. [Google Scholar] [CrossRef]
- Zeng, Q.; Langereis, M.A.; van Vliet, A.L.; Huizinga, E.G.; de Groot, R.J. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc. Natl. Acad. Sci. USA 2008, 105, 9065–9069. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, K.M.; Parsons, L.M.; Berends, A.J.; de Vries, R.P.; Cipollo, J.F.; Verheije, M.H. Three amino acid changes in avian coronavirus spike protein allow binding to kidney tissue. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Wickramasinghe, I.N.; de Vries, R.P.; Grone, A.; de Haan, C.A.; Verheije, M.H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J. Virol. 2011, 85, 8903–8912. [Google Scholar] [CrossRef]
- Yasue, S.; Handa, S.; Miyagawa, S.; Inoue, J.; Hasegawa, A.; Yamakawa, T. Difference in form of sialic acid in red blood cell glycolipids of different breeds of dogs. J. Biochem. 1978, 83, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Hiono, T.; Okamatsu, M.; Yamamoto, N.; Ogasawara, K.; Endo, M.; Kuribayashi, S.; Shichinohe, S.; Motohashi, Y.; Chu, D.H.; Suzuki, M.; et al. Experimental infection of highly and low pathogenic avian influenza viruses to chickens, ducks, tree sparrows, jungle crows, and black rats for the evaluation of their roles in virus transmission. Vet. Microbiol. 2016, 182, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Radigan, K.A.; Misharin, A.V.; Chi, M.; Budinger, G.S. Modeling human influenza infection in the laboratory. Infect. Drug Resist. 2015, 8, 311–320. [Google Scholar] [CrossRef]
- Thangavel, R.R.; Bouvier, N.M. Animal models for influenza virus pathogenesis, transmission, and immunology. J. Immunol. Methods 2014, 410, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki-Horimoto, K.; Nakajima, N.; Ichiko, Y.; Sakai-Tagawa, Y.; Noda, T.; Hasegawa, H.; Kawaoka, Y. Syrian hamster as an animal model for the study of human influenza virus infection. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Samet, S.J.; Tompkins, S.M. Influenza pathogenesis in genetically defined resistant and susceptible murine strains. Yale J. Biol. Med. 2017, 90, 471–479. [Google Scholar]
- Mifsud, E.J.; Tai, C.M.; Hurt, A.C. Animal models used to assess influenza antivirals. Expert Opin. Drug Discov. 2018, 13, 1131–1139. [Google Scholar] [CrossRef]
- Albrecht, R.A.; Liu, W.C.; Sant, A.J.; Tompkins, S.M.; Pekosz, A.; Meliopoulos, V.; Cherry, S.; Thomas, P.G.; Schultz-Cherry, S. Moving forward: Recent developments for the ferret biomedical research model. mBio 2018, 9. [Google Scholar] [CrossRef]
- Belser, J.A.; Eckert, A.M.; Huynh, T.; Gary, J.M.; Ritter, J.M.; Tumpey, T.M.; Maines, T.R. A guide for the use of the ferret model for influenza virus infection. Am. J. Pathol. 2020, 190, 11–24. [Google Scholar] [CrossRef]
- Belser, J.A.; Eckert, A.M.; Tumpey, T.M.; Maines, T.R. Complexities in ferret influenza virus pathogenesis and transmission models. Microbiol. Mol. Biol. Rev. 2016, 80, 733–744. [Google Scholar] [CrossRef]
- Maher, J.A.; DeStefano, J. The ferret: An animal model to study influenza virus. Lab. Anim. N. Y. 2004, 33, 50–53. [Google Scholar] [CrossRef]
- Ottolini, M.G.; Blanco, J.C.G.; Eichelberger, M.C.; Porter, D.D.; Pletneva, L.; Richardson, J.Y.; Prince, G.A. The cotton rat provides a useful small-animal model for the study of influenza virus pathogenesis. J. Gen. Virol. 2005, 86, 2823–2830. [Google Scholar] [CrossRef]
- Green, M.G.; Huey, D.; Niewiesk, S. The cotton rat (Sigmodon hispidus) as an animal model for respiratory tract infections with human pathogens. Lab. Anim. N. Y. 2013, 42, 170–176. [Google Scholar] [CrossRef]
- Bouvier, N.M. Animal models for influenza virus transmission studies: A historical perspective. Curr. Opin. Virol. 2015, 13, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yuan, B.; Xia, X.; Zhang, S.; Du, Q.; Yang, C.; Li, N.; Zhao, J.; Zhang, Y.; Zhang, R.; et al. Tree shrew as a new animal model to study the pathogenesis of avian influenza (H9N2) virus infection. Emerg. Microbes Infect. 2018, 7, 166. [Google Scholar] [CrossRef]
- Yang, Z.F.; Zhao, J.; Zhu, Y.T.; Wang, Y.T.; Liu, R.; Zhao, S.S.; Li, R.F.; Yang, C.G.; Li, J.Q.; Zhong, N.S. The tree shrew provides a useful alternative model for the study of influenza H1N1 virus. Virol. J. 2013, 10, 111. [Google Scholar] [CrossRef]
- Holzer, B.; Martini, V.; Edmans, M.; Tchilian, E. T and B cell immune responses to influenza viruses in pigs. Front. Immunol. 2019, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki-Horimoto, K.; Nakajima, N.; Kiso, M.; Takahashi, K.; Ito, M.; Inoue, T.; Horiuchi, M.; Okahara, N.; Sasaki, E.; Hasegawa, H.; et al. The marmoset as an animal model of influenza: Infection with A(H1N1)pdm09 and highly pathogenic A(H5N1) viruses via the conventional or tracheal spray route. Front. Microbiol. 2018, 9, 844. [Google Scholar] [CrossRef]
- Davis, A.S.; Taubenberger, J.K.; Bray, M. The use of nonhuman primates in research on seasonal, pandemic and avian influenza, 1893-2014. Antivir. Res. 2015, 117, 75–98. [Google Scholar] [CrossRef]
- Manickam, C.; Shah, S.V.; Lucar, O.; Ram, D.R.; Reeves, R.K. Cytokine-mediated tissue injury in non-human primate models of viral infections. Front. Immunol. 2018, 9, 2862. [Google Scholar] [CrossRef]
- Moncla, L.H.; Ross, T.M.; Dinis, J.M.; Weinfurter, J.T.; Mortimer, T.D.; Schultz-Darken, N.; Brunner, K.; Capuano, S.V., 3rd; Boettcher, C.; Post, J.; et al. A novel nonhuman primate model for influenza transmission. PLoS ONE 2013, 8, e78750. [Google Scholar] [CrossRef]
- Oh, D.Y.; Hurt, A.C. Using the ferret as an animal model for investigating influenza antiviral effectiveness. Front. Microbiol. 2016, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Carlin, D.J.; McMurray, D.N.; Hickey, A.J. The guinea pig as a model of infectious diseases. Comp. Med. 2008, 58, 324–340. [Google Scholar] [PubMed]
- Miao, J.; Chard, L.S.; Wang, Z.; Wang, Y. Syrian hamster as an animal model for the study on infectious diseases. Front. Immunol. 2019, 10, 2329. [Google Scholar] [CrossRef]
- Yao, Y.G. Creating animal models, why not use the Chinese tree shrew (Tupaia belangeri chinensis)? Zool Res. 2017, 38, 118–126. [Google Scholar] [CrossRef]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef]
- Wong, J.; Layton, D.; Wheatley, A.K.; Kent, S.J. Improving immunological insights into the ferret model of human viral infectious disease. Influenza Other Respir. Viruses 2019, 13, 535–546. [Google Scholar] [CrossRef]
- Tripp, R.A.; Tompkins, S.M. Animal models for evaluation of influenza vaccines. Curr. Top Microbiol. Immunol. 2009, 333, 397–412. [Google Scholar] [CrossRef]
- Rajao, D.S.; Vincent, A.L. Swine as a model for influenza A virus infection and immunity. ILAR J. 2015, 56, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.A.; Royer, C.M.; Pinkerton, K.E.; Schelegle, E.S. Nonhuman primate models of respiratory disease: Past, present, and future. ILAR J. 2017, 58, 269–280. [Google Scholar] [CrossRef]
- Belser, J.A.; Katz, J.M.; Tumpey, T.M. The ferret as a model organism to study influenza A virus infection. Dis. Model Mech. 2011, 4, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.R.; Desrosiers, A.; Terzaghi, M.; Little, J.B. Morphometric and histological analysis of the lungs of Syrian golden hamsters. J. Anat. 1978, 125, 527–553. [Google Scholar] [PubMed]
- Ye, L.; He, M.; Huang, Y.; Zhao, G.; Lei, Y.; Zhou, Y.; Chen, X. Tree shrew as a new animal model for the study of lung cancer. Oncol. Lett. 2016, 11, 2091–2095. [Google Scholar] [CrossRef][Green Version]
- Canning, B.J.; Chou, Y. Using guinea pigs in studies relevant to asthma and COPD. Pulm. Pharmacol. Ther. 2008, 21, 702–720. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Deutsch, G.H.; Wert, S.E.; On behalf of the Ontology Subcommittee; NHLBI Molecular Atlas of Lung Development Program Consortium. Comprehensive anatomic ontologies for lung development: A comparison of alveolar formation and maturation within mouse and human lung. J. Biomed. Semant. 2019, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, H.; Bi, Y.; Long, Q.; Gong, Y.; Dai, J.; Sun, M.; Cun, W. Characteristics of the tree shrew humoral immune system. Mol. Immunol. 2020, 127, 175–185. [Google Scholar] [CrossRef]
- Tchilian, E.; Holzer, B. Harnessing local immunity for an effective universal swine influenza vaccine. Viruses 2017, 9, 98. [Google Scholar] [CrossRef]
- Chua, S.; Tan, H.Q.; Engelberg, D.; Lim, L.H.K. Alternative experimental models for studying influenza proteins, host-virus interactions and anti-influenza drugs. Pharmaceuticals 2019, 12, 147. [Google Scholar] [CrossRef]
- Messaoudi, I.; Estep, R.; Robinson, B.; Wong, S.W. Nonhuman primate models of human immunology. Antioxid Redox Signal 2011, 14, 261–273. [Google Scholar] [CrossRef]
- Barreiro, L.B.; Marioni, J.C.; Blekhman, R.; Stephens, M.; Gilad, Y. Functional comparison of innate immune signaling pathways in primates. PLoS Genet. 2010, 6, e1001249. [Google Scholar] [CrossRef] [PubMed]
- Varki, N.M.; Strobert, E.; Dick, E.J., Jr.; Benirschke, K.; Varki, A. Biomedical differences between human and nonhuman hominids: Potential roles for uniquely human aspects of sialic acid biology. Annu. Rev. Pathol. 2011, 6, 365–393. [Google Scholar] [CrossRef]
- Redelinghuys, P.; Antonopoulos, A.; Liu, Y.; Campanero-Rhodes, M.A.; McKenzie, E.; Haslam, S.M.; Dell, A.; Feizi, T.; Crocker, P.R. Early murine T-lymphocyte activation is accompanied by a switch from N-glycolyl- to N-acetyl-neuraminic acid and generation of ligands for siglec-E. J. Biol. Chem. 2011, 286, 34522–34532. [Google Scholar] [CrossRef]
- Naito-Matsui, Y.; Takada, S.; Kano, Y.; Iyoda, T.; Sugai, M.; Shimizu, A.; Inaba, K.; Nitschke, L.; Tsubata, T.; Oka, S.; et al. Functional evaluation of activation-dependent alterations in the sialoglycan composition of T cells. J. Biol. Chem. 2014, 289, 1564–1579. [Google Scholar] [CrossRef]
- Buchlis, G.; Odorizzi, P.; Soto, P.C.; Pearce, O.M.; Hui, D.J.; Jordan, M.S.; Varki, A.; Wherry, E.J.; High, K.A. Enhanced T cell function in a mouse model of human glycosylation. J. Immunol. 2013, 191, 228–237. [Google Scholar] [CrossRef]
- Kreijtz, J.H.; Bodewes, R.; van Amerongen, G.; Kuiken, T.; Fouchier, R.A.; Osterhaus, A.D.; Rimmelzwaan, G.F. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine 2007, 25, 612–620. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Choi, A.; Hirsh, A.; Margine, I.; Iida, S.; Barrera, A.; Ferres, M.; Albrecht, R.A.; Garcia-Sastre, A.; Bouvier, N.M.; et al. Defining the antibody cross-reactome directed against the influenza virus surface glycoproteins. Nat. Immunol. 2017, 18, 464–473. [Google Scholar] [CrossRef]
- Allen, J.D.; Jang, H.; DiNapoli, J.; Kleanthous, H.; Ross, T.M. Elicitation of protective antibodies against 20 years of future H3N2 cocirculating influenza virus variants in ferrets preimmune to historical H3N2 influenza viruses. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Music, N.; Tzeng, W.P.; Liaini Gross, F.; Levine, M.Z.; Xu, X.; Shieh, W.J.; Tumpey, T.M.; Katz, J.M.; York, I.A. Repeated vaccination against matched H3N2 influenza virus gives less protection than single vaccination in ferrets. NPJ Vaccines 2019, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Skarlupka, A.L.; Ross, T.M. Immune imprinting in the influenza ferret model. Vaccines 2020, 8, 173. [Google Scholar] [CrossRef]
- Karlsson, E.A.; Engel, G.A.; Feeroz, M.M.; San, S.; Rompis, A.; Lee, B.P.; Shaw, E.; Oh, G.; Schillaci, M.A.; Grant, R.; et al. Influenza virus infection in nonhuman primates. Emerg. Infect. Dis. 2012, 18, 1672–1675. [Google Scholar] [CrossRef]
- Collaborative Cross, C. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics 2012, 190, 389–401. [Google Scholar] [CrossRef]
- Leist, S.R.; Pilzner, C.; van den Brand, J.M.; Dengler, L.; Geffers, R.; Kuiken, T.; Balling, R.; Kollmus, H.; Schughart, K. Influenza H3N2 infection of the collaborative cross founder strains reveals highly divergent host responses and identifies a unique phenotype in CAST/EiJ mice. BMC Genom. 2016, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Boon, A.C.; deBeauchamp, J.; Hollmann, A.; Luke, J.; Kotb, M.; Rowe, S.; Finkelstein, D.; Neale, G.; Lu, L.; Williams, R.W.; et al. Host genetic variation affects resistance to infection with a highly pathogenic H5N1 influenza A virus in mice. J. Virol. 2009, 83, 10417–10426. [Google Scholar] [CrossRef] [PubMed]
- Boon, A.C.; deBeauchamp, J.; Krauss, S.; Rubrum, A.; Webb, A.D.; Webster, R.G.; McElhaney, J.; Webby, R.J. Cross-reactive neutralizing antibodies directed against pandemic H1N1 2009 virus are protective in a highly sensitive DBA/2 mouse influenza model. J. Virol. 2010, 84, 7662–7667. [Google Scholar] [CrossRef]
- Pica, N.; Iyer, A.; Ramos, I.; Bouvier, N.M.; Fernandez-Sesma, A.; Garcia-Sastre, A.; Lowen, A.C.; Palese, P.; Steel, J. The DBA.2 mouse is susceptible to disease following infection with a broad, but limited, range of influenza A and B viruses. J. Virol. 2011, 85, 12825–12829. [Google Scholar] [CrossRef]
- Srivastava, B.; Blazejewska, P.; Hessmann, M.; Bruder, D.; Geffers, R.; Mauel, S.; Gruber, A.D.; Schughart, K. Host genetic background strongly influences the response to influenza a virus infections. PLoS ONE 2009, 4, e4857. [Google Scholar] [CrossRef]
- Trammell, R.A.; Liberati, T.A.; Toth, L.A. Host genetic background and the innate inflammatory response of lung to influenza virus. Microbes Infect. 2012, 14, 50–58. [Google Scholar] [CrossRef]
- Everitt, A.R.; Clare, S.; Pertel, T.; John, S.P.; Wash, R.S.; Smith, S.E.; Chin, C.R.; Feeley, E.M.; Sims, J.S.; Adams, D.J.; et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature 2012, 484, 519–523. [Google Scholar] [CrossRef]
- Shin, D.L.; Hatesuer, B.; Bergmann, S.; Nedelko, T.; Schughart, K. Protection from severe influenza virus infections in mice carrying the Mx1 influenza virus resistance gene strongly depends on genetic background. J. Virol. 2015, 89, 9998–10009. [Google Scholar] [CrossRef]
- Huang, C.H.; Chen, C.J.; Yen, C.T.; Yu, C.P.; Huang, P.N.; Kuo, R.L.; Lin, S.J.; Chang, C.K.; Shih, S.R. Caspase-1 deficient mice are more susceptible to influenza A virus infection with PA variation. J. Infect. Dis. 2013, 208, 1898–1905. [Google Scholar] [CrossRef]
- Schauer, R. Sialic acids as link to Japanese scientists. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2016, 92, 109–120. [Google Scholar] [CrossRef]
- Löfling, J.; Lyi, S.M.; Parrish, C.R.; Varki, A. Canine and feline parvoviruses preferentially recognize the non-human cell surface sialic acid N-glycolylneuraminic acid. Virology 2013, 440, 89–96. [Google Scholar] [CrossRef]
- Martin, P.T.; Golden, B.; Okerblom, J.; Camboni, M.; Chandrasekharan, K.; Xu, R.; Varki, A.; Flanigan, K.M.; Kornegay, J.N. A comparative study of N-glycolylneuraminic acid (Neu5Gc) and cytotoxic T cell (CT) carbohydrate expression in normal and dystrophin-deficient dog and human skeletal muscle. PLoS ONE 2014, 9, e88226. [Google Scholar] [CrossRef]
- Sun, Y.; Bi, Y.; Pu, J.; Hu, Y.; Wang, J.; Gao, H.; Liu, L.; Xu, Q.; Tan, Y.; Liu, M.; et al. Guinea pig model for evaluating the potential public health risk of swine and avian influenza viruses. PLoS ONE 2010, 5, e15537. [Google Scholar] [CrossRef]
- Iwersen, M.; Vandamme-Feldhaus, V.; Schauer, R. Enzymatic 4-O-acetylation of N-acetylneuraminic acid in guinea-pig liver. Glycoconj J. 1998, 15, 895–904. [Google Scholar] [CrossRef]
- Blanco, J.C.; Pletneva, L.M.; Wan, H.; Araya, Y.; Angel, M.; Oue, R.O.; Sutton, T.C.; Perez, D.R. Receptor characterization and susceptibility of cotton rats to avian and 2009 pandemic influenza virus strains. J. Virol. 2013, 87, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.N.; Yau, L.F.; Liu, L.; Zeng, X.; Chen, D.C.; Jiang, M.; Liu, J.; Wang, J.R.; Jiang, Z.H. Microfluidic chip-LC/MS-based glycomic analysis revealed distinct N-glycan profile of rat serum. Sci. Rep. 2015, 5, 12844. [Google Scholar] [CrossRef] [PubMed]
- Yau, L.F.; Chan, K.M.; Yang, C.G.; Ip, S.W.; Kang, Y.; Mai, Z.T.; Tong, T.T.; Jiang, Z.H.; Yang, Z.F.; Wang, J.R. Comprehensive glycomic profiling of respiratory tract tissues of tree shrews by TiO(2)-PGC chip mass spectrometry. J. Proteome Res. 2020, 19, 1470–1480. [Google Scholar] [CrossRef]
- Bateman, A.C.; Karamanska, R.; Busch, M.G.; Dell, A.; Olsen, C.W.; Haslam, S.M. Glycan analysis and influenza A virus infection of primary swine respiratory epithelial cells: The importance of NeuAcα2-6 glycans. J. Biol. Chem. 2010, 285, 34016–34026. [Google Scholar] [CrossRef]
- Iwatsuki-Horimoto, K.; Nakajima, N.; Shibata, M.; Takahashi, K.; Sato, Y.; Kiso, M.; Yamayoshi, S.; Ito, M.; Enya, S.; Otake, M.; et al. The microminipig as an animal model for influenza A virus infection. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Byrd-Leotis, L.; Liu, R.; Bradley, K.C.; Lasanajak, Y.; Cummings, S.F.; Song, X.; Heimburg-Molinaro, J.; Galloway, S.E.; Culhane, M.R.; Smith, D.F.; et al. Shotgun glycomics of pig lung identifies natural endogenous receptors for influenza viruses. Proc. Natl. Acad. Sci. USA 2014, 111, E2241–E2250. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; Ochonicky, K.L.; German, J.B.; Donovan, S.M.; Lebrilla, C.B. Structural determination and daily variations of porcine milk oligosaccharides. J. Agric. Food Chem. 2010, 58, 4653–4659. [Google Scholar] [CrossRef]
- Cooper, D.K. Modifying the sugar icing on the transplantation cake. Glycobiology 2016, 26, 571–581. [Google Scholar] [CrossRef]
- Kim, Y.G.; Oh, J.Y.; Gil, G.C.; Kim, M.K.; Ko, J.H.; Lee, S.; Lee, H.J.; Wee, W.R.; Kim, B.G. Identification of alpha-Gal and non-Gal epitopes in pig corneal endothelial cells and keratocytes by using mass spectrometry. Curr. Eye Res. 2009, 34, 877–895. [Google Scholar] [CrossRef]
- Burlak, C.; Bern, M.; Brito, A.E.; Isailovic, D.; Wang, Z.Y.; Estrada, J.L.; Li, P.; Tector, A.J. N-linked glycan profiling of GGTA1/CMAH knockout pigs identifies new potential carbohydrate xenoantigens. Xenotransplantation 2013, 20, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Horiike, G.; Yamazaki, Y.; Kawabe, K.; Masuda, H.; Miyamoto, D.; Matsuda, M.; Nishimura, S.I.; Yamagata, T.; Ito, T.; et al. Swine influenza virus strains recognize sialylsugar chains containing the molecular species of sialic acid predominantly present in the swine tracheal epithelium. FEBS Lett. 1997, 404, 192–196. [Google Scholar] [CrossRef]
- Sriwilaijaroen, N.; Kondo, S.; Yagi, H.; Takemae, N.; Saito, T.; Hiramatsu, H.; Kato, K.; Suzuki, Y. N-glycans from porcine trachea and lung: Predominant NeuAcα2-6Gal could be a selective pressure for influenza variants in favor of human-type receptor. PLoS ONE 2011, 6, e16302. [Google Scholar] [CrossRef] [PubMed]
- Springer, S.A.; Diaz, S.L.; Gagneux, P. Parallel evolution of a self-signal: Humans and new world monkeys independently lost the cell surface sugar Neu5Gc. Immunogenetics 2014, 66, 671–674. [Google Scholar] [CrossRef]
- Li, Q.; Shaikh, S.; Iwase, H.; Long, C.; Lee, W.; Zhang, Z.; Wang, Y.; Ayares, D.; Cooper, D.K.C.; Hara, H. Carbohydrate antigen expression and anti-pig antibodies in New World capuchin monkeys: Relevance to studies of xenotransplantation. Xenotransplantation 2019, 26, e12498. [Google Scholar] [CrossRef]
- Daly, J.M.; Blunden, A.S.; Macrae, S.; Miller, J.; Bowman, S.J.; Kolodziejek, J.; Nowotny, N.; Smith, K.C. Transmission of equine influenza virus to English foxhounds. Emerg. Infect. Dis. 2008, 14, 461–464. [Google Scholar] [CrossRef]
- Barnard, K.N.; Alford-Lawrence, B.K.; Buchholz, D.W.; Wasik, B.R.; LaClair, J.R.; Yu, H.; Honce, R.; Ruhl, S.; Pajic, P.; Daugherity, E.K.; et al. Modified sialic acids on mucus and erythrocytes inhibit influenza A virus hemagglutinin and neuraminidase functions. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Yamakawa, T.; Tanabe, Y. Further studies on the red cell glycolipids of various breeds of dogs. A possible assumption about the origin of Japanese dogs. J. Biochem. 1984, 96, 1777–1782. [Google Scholar] [CrossRef]
- Wen, F.; Blackmon, S.; Olivier, A.K.; Li, L.; Guan, M.; Sun, H.; Wang, P.G.; Wan, X.F. Mutation W222L at the receptor binding site of hemagglutinin could facilitate viral adaption from equine influenza A (H3N8) virus to dogs. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ito, T.; Suzuki, T.; Holland, R.E., Jr.; Chambers, T.M.; Kiso, M.; Ishida, H.; Kawaoka, Y. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 2000, 74, 11825–11831. [Google Scholar] [CrossRef]
- Takahashi, T.; Takano, M.; Kurebayashi, Y.; Masuda, M.; Kawagishi, S.; Takaguchi, M.; Yamanaka, T.; Minami, A.; Otsubo, T.; Ikeda, K.; et al. N-glycolylneuraminic acid on human epithelial cells prevents entry of influenza A viruses that possess N-glycolylneuraminic acid binding ability. J. Virol. 2014, 88, 8445–8456. [Google Scholar] [CrossRef]
- Higa, H.H.; Rogers, G.N.; Paulson, J.C. Influenza virus hemagglutinins differentiate between receptor determinants bearing N-acetyl-, N-glycollyl-, and N,O-diacetylneuraminic acids. Virology 1985, 144, 279–282. [Google Scholar] [CrossRef]
- Spruit, C.M.; Zhu, X.; Broszeit, F.; Han, A.X.; van der Woude, R.; Bouwman, K.M.; Luu, M.M.T.; Russell, C.A.; Wilson, I.A.; Boons, G.-J.; et al. N-glycolylneuraminic acid binding of avian H7 influenza A viruses. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tumpey, T.M.; Maines, T.R.; Van Hoeven, N.; Glaser, L.; Solorzano, A.; Pappas, C.; Cox, N.J.; Swayne, D.E.; Palese, P.; Katz, J.M.; et al. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 2007, 315, 655–659. [Google Scholar] [CrossRef]
- Maines, T.R.; Chen, L.M.; Matsuoka, Y.; Chen, H.; Rowe, T.; Ortin, J.; Falcon, A.; Nguyen, T.H.; Mai le, Q.; Sedyaningsih, E.R.; et al. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc. Natl. Acad. Sci. USA 2006, 103, 12121–12126. [Google Scholar] [CrossRef]
- Gambaryan, A.; Tuzikov, A.; Pazynina, G.; Bovin, N.; Balish, A.; Klimov, A. Evolution of the receptor binding phenotype of influenza A (H5) viruses. Virology 2006, 344, 432–438. [Google Scholar] [CrossRef]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef]
- Rosenke, K.; Meade-White, K.; Letko, M.; Clancy, C.; Hansen, F.; Liu, Y.; Okumura, A.; Tang-Huau, T.L.; Li, R.; Saturday, G.; et al. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg. Microbes Infect. 2020, 9, 2673–2684. [Google Scholar] [CrossRef]
- Padler-Karavani, V.; Yu, H.; Cao, H.; Chokhawala, H.; Karp, F.; Varki, N.; Chen, X.; Varki, A. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: Potential implications for disease. Glycobiology 2008, 18, 818–830. [Google Scholar] [CrossRef]
- Glaser, L.; Conenello, G.; Paulson, J.; Palese, P. Effective replication of human influenza viruses in mice lacking a major α2,6 sialyltransferase. Virus Res. 2007, 126, 9–18. [Google Scholar] [CrossRef]
- Long, J.S.; Mistry, B.; Haslam, S.M.; Barclay, W.S. Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2019, 17, 67–81. [Google Scholar] [CrossRef]
- Ji, Y.; White, Y.J.; Hadden, J.A.; Grant, O.C.; Woods, R.J. New insights into influenza A specificity: An evolution of paradigms. Curr. Opin. Struct Biol. 2017, 44, 219–231. [Google Scholar] [CrossRef]
- De Vries, R.P.; Zhu, X.; McBride, R.; Rigter, A.; Hanson, A.; Zhong, G.; Hatta, M.; Xu, R.; Yu, W.; Kawaoka, Y.; et al. Hemagglutinin receptor specificity and structural analyses of respiratory droplet-transmissible H5N1 viruses. J. Virol. 2014, 88, 768–773. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.P.; de Vries, E.; Moore, K.S.; Rigter, A.; Rottier, P.J.; de Haan, C.A. Only two residues are responsible for the dramatic difference in receptor binding between swine and new pandemic H1 hemagglutinin. J. Biol. Chem. 2011, 286, 5868–5875. [Google Scholar] [CrossRef]
- De Vries, R.P.; Tzarum, N.; Peng, W.; Thompson, A.J.; Ambepitiya Wickramasinghe, I.N.; de la Pena, A.T.T.; van Breemen, M.J.; Bouwman, K.M.; Zhu, X.; McBride, R.; et al. A single mutation in Taiwanese H6N1 influenza hemagglutinin switches binding to human-type receptors. EMBO Mol. Med. 2017, 9, 1314–1325. [Google Scholar] [CrossRef]
| Animal Model | Sialic Acid Linkage in the Respiratory Tract | Neu5Gc Detected | Intact CMAH Gene [4] | |
|---|---|---|---|---|
| α2,3 | α2,6 | |||
| Mouse | + [1] | - [1,16] | Yes [6,9,10,11,12,13,14,15,89,90] Yes (Figure 2) | Yes |
| Ferret | + [17] - (upper respiratory tract) [1] + (lung) [1] | + [17] + (upper respiratory tract) [1] - (lung) [1] | No [17,18,19] No (Figure 2) | No |
| Guinea pig | + (nasal tract, trachea) [91] + (lung) [91] | + (nasal tract, trachea) [91] - (lung) [91] | Yes [9,92] Yes (Figure 2) | Yes |
| Rat | + (trachea) [93] -- (lung) [93] | + (trachea) [93] - (lung) [93] | Yes [9,94] | Yes |
| Syrian hamster | + [32] | + [32] | Yes (Figure 2) | Yes |
| Tree shrew | + [95] - (nasal tract, trachea) [43] + (lung) [43] | + [95] + (nasal tract, trachea) [43] - (lung) [43] | Yes [95] | Yes |
| Swine | - [1,96] + (nasal tract) [97] - (trachea) [97] + (lung) [97,98] | + [1,96] - (nasal tract) [97] + (trachea) [97] + (lung) [97,98] | Yes [96,98,99,100,101,102] Yes, 53% [103] Yes, 9–14% [104] Yes (Figure 2) | Yes |
| Marmoset | + [45] | -- [45] | No [105] | No |
| Macaque | Unknown | Unknown | Yes [9,106] | Yes |
| Dog | + [107] | - [3,107] | No [28,88,108] Yes [88,89,109,110] | Yes |
| Horse | + [1,107,111] | -- [3,107,111] | Yes [3,8,108] | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spruit, C.M.; Nemanichvili, N.; Okamatsu, M.; Takematsu, H.; Boons, G.-J.; de Vries, R.P. N-Glycolylneuraminic Acid in Animal Models for Human Influenza A Virus. Viruses 2021, 13, 815. https://doi.org/10.3390/v13050815
Spruit CM, Nemanichvili N, Okamatsu M, Takematsu H, Boons G-J, de Vries RP. N-Glycolylneuraminic Acid in Animal Models for Human Influenza A Virus. Viruses. 2021; 13(5):815. https://doi.org/10.3390/v13050815
Chicago/Turabian StyleSpruit, Cindy M., Nikoloz Nemanichvili, Masatoshi Okamatsu, Hiromu Takematsu, Geert-Jan Boons, and Robert P. de Vries. 2021. "N-Glycolylneuraminic Acid in Animal Models for Human Influenza A Virus" Viruses 13, no. 5: 815. https://doi.org/10.3390/v13050815
APA StyleSpruit, C. M., Nemanichvili, N., Okamatsu, M., Takematsu, H., Boons, G.-J., & de Vries, R. P. (2021). N-Glycolylneuraminic Acid in Animal Models for Human Influenza A Virus. Viruses, 13(5), 815. https://doi.org/10.3390/v13050815






