Abstract
Mosquito-borne viral infections are responsible for a significant degree of morbidity and mortality across the globe due to the severe diseases these infections cause, and they continue to increase each year. These viruses are dependent on the mosquito vector as the primary means of transmission to new vertebrate hosts including avian, livestock, and human populations. Due to the dynamic host environments that mosquito-borne viruses pass through as they are transmitted between vector and vertebrate hosts, there are various host factors that control the response to infection over the course of the pathogen’s life cycle. In this review, we discuss these host factors that are present in either vector or vertebrate models during infection, how they vary or are conserved between hosts, and their implications in future research pertaining to disease prevention and treatment.
1. Introduction
Vector-borne diseases pose a significant global health burden. Global climate changes have enabled various arthropod vectors to expand into previously uninhabitable regions which increases potential exposure to at-risk populations [1,2]. In particular, mosquito populations have expanded, and this has resulted in increasing occurrence of mosquito-borne disease [3,4], notably viruses such as West Nile virus (WNV), dengue virus (DENV), Zika virus (ZIKV), and chikungunya virus (CHIKV) [5]. While mosquitoes are also responsible for transmission of hazardous parasitic diseases such as plasmodia and filariasis parasites, their role as transmitters of viral pathogens is of particular interest to epidemiologists and infectious disease monitoring groups. Recent reports by the World Health Organization (WHO) have indicated a steady or downward trend of global malaria cases [6], while viral cases have appeared to increase in both frequency and severity [5,7,8]. This has resulted in the WHO to designate DENV and related mosquito-borne viruses as significant concerns that need to be addressed at the global level in 2020 [9].
Mosquito-borne viruses utilize the mosquito as a natural reservoir for replication and vector for transmission into vertebrate populations. Depending on the virus, transmission can occur in both sylvatic and urban settings during the bloodmeal between mosquito and vertebrate hosts such as primates, birds, and humans [10,11]. Once infected, some vertebrate hosts are unable to clear acute infection and can develop viremia which results in infectious virions circulating in the blood and lymphatic fluid. This would, in turn, result in potential virus transmission from vertebrate to mosquito in subsequent bloodmeals [10]. The transmission cycle between mosquito and vertebrate populations requires constant monitoring to identify new infections in each host type as this would indicate active viral transmission within a community or region.
Viruses that undergo this transmission cycle encounter various environmental and host factors as virions move between mosquito and vertebrate hosts. Immune responses to mosquito-borne viruses vary between vector and vertebrate hosts as the sophistication and complexity in the cellular environment vary between species. This does not, however, mean that there are no evolutionarily conserved responses that are shared between organisms. In fact, at the level of innate immunity, there are many shared antiviral mechanisms that are conserved between species. In the following review, we provide a summary of conserved host factors responsible for initiating antiviral responses against mosquito-borne viruses in mosquito and human systems, how these factors vary between organisms, and how these responses provide a foundational understanding for future vector control and therapeutic research.
4. Outlook
As evident in the gradual increase in the number and severity of clinical cases as well as the expansion of mosquito activity across the globe, the looming threat that mosquito-borne viruses pose is of significant concern and must be addressed at both the vector and clinical levels. Understanding the host immune responses and how they are varied between organisms is an important step in identifying more effective targets for vector control and therapeutics. More importantly, understanding how the responses are similar between mosquito-borne viruses is of great value as it permits research in broad or virus-specific targeting. As summarized in Table 1, the host factors involved during viral infection in mosquitoes and humans are well conserved with some variability regarding host physical barriers and adaptive immunity.
Further investigation is required into identifying and evaluating the importance of certain antiviral immune responses in both humans and mosquitoes. For example, vector-control mechanisms such as the endosymbiont Wolbachia, while reducing viral load in mosquitoes infected with ZIKV and DENV [110,111], may be pro-viral for other related viruses such as WNV [164]. This indicates that there may be virus-specific variations among related pathogens that result in potential, broad antiviral preventatives being less effective. This is also the case in humans as responses that are important against one virus may be insignificant or detrimental for another [157]. One example of a potential cross-species antiviral target, as previously discussed, is how insulin/IGF-1 signaling regulates mosquito and human immunity. Since this pathway possesses a broad effect on homeostatic activity in both organisms, insulin-mediated immunity may be achieved by targeting different downstream host factors or pathways. While studies into insulin-mediated arboviral immunity are not well established in humans, there is an established effect of dysfunctional insulin signaling on patient survival and virus activity for ZIKV and WNV [114,160]. Research in and implementation of more effective antivirals in both mosquitoes and humans are necessary and require a greater understanding regarding the conserved and differing host factors that respond to these zoonotic infections.
Author Contributions
C.E.T. wrote the manuscript in consultation with A.G.G. All authors have read and agreed to the published version of the manuscript.
Funding
Research in the Goodman Lab is supported by NIH/NIAID Grant R01 AI139051 to A.G.G. C.E.T. is supported by the NIH/NIGMS pre-doctoral fellowship T32 GM008336 and a fellowship from the Poncin Scholarship Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
We thank S. Allen Whiles and Y.B. Rodger for critical review of this manuscript.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Morin, C.W.; Comrie, A.C. Modeled Response of the West Nile Virus Vector Culex Quinquefasciatus to Changing Climate Using the Dynamic Mosquito Simulation Model. Int. J. Biometeorol. 2010, 54, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A.M. Mapping the Global Potential Distributions of Two Arboviral Vectors Aedes Aegypti and Ae. Albopictus under Changing Climate. PLoS ONE 2018, 13, e0210122. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes Aegypti and Ae. Albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global Risk Mapping for Major Diseases Transmitted by Aedes Aegypti and Aedes Albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.J.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital Signs: Trends in Reported Vectorborne Disease Cases—United States and Territories, 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67. [Google Scholar] [CrossRef]
- Guglielmi, G. Malaria Cases Are Falling Worldwide. Nature 2019. [Google Scholar] [CrossRef]
- Giovanetti, M.; de Mendonça, M.C.L.; Fonseca, V.; Mares-Guia, M.A.; Fabri, A.; Xavier, J.; de Jesus, J.G.; Gräf, T.; Dos Santos Rodrigues, C.D.; Dos Santos, C.C.; et al. Yellow Fever Virus Reemergence and Spread in Southeast Brazil, 2016–2019. J. Virol. 2019, 94, e01623-19. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.A. PAHO/WHO Data—Dengue Cases | PAHO/WHO. Available online: https://www.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/dengue-nacional-en/252-dengue-pais-anoen.html?showall=&start=2 (accessed on 2 March 2021).
- Ten Health Issues WHO Will Tackle This Year. Available online: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 (accessed on 21 January 2019).
- Ahlers, L.R.H.; Goodman, A.G. The Immune Responses of the Animal Hosts of West Nile Virus: A Comparison of Insects, Birds, and Mammals. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Bugallo, G.; Piedra, L.A.; Rodriguez, M.; Bisset, J.A.; Lourenço-de-Oliveira, R.; Weaver, S.C.; Vasilakis, N.; Vega-Rúa, A. Vector-Borne Transmission and Evolution of Zika Virus. Nat. Ecol. Evol. 2019, 3, 561. [Google Scholar] [CrossRef]
- Harrington, T.; Kuehnert, M.J.; Kamel, H.; Lanciotti, R.S.; Hand, S.; Currier, M.; Chamberland, M.E.; Petersen, L.R.; Marfin, A.A. West Nile Virus Infection Transmitted by Blood Transfusion. Transfusion (Paris) 2003, 43, 1018–1022. [Google Scholar] [CrossRef]
- Tambyah, P.A.; Koay, E.S.C.; Poon, M.L.M.; Lin, R.V.T.P.; Ong, B.K.C. Dengue Hemorrhagic Fever Transmitted by Blood Transfusion. N. Engl. J. Med. 2008, 359, 1526–1527. [Google Scholar] [CrossRef]
- D’Ortenzio, E.; Matheron, S.; de Lamballerie, X.; Hubert, B.; Piorkowski, G.; Maquart, M.; Descamps, D.; Damond, F.; Yazdanpanah, Y.; Leparc-Goffart, I. Evidence of Sexual Transmission of Zika Virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.M.; Antony, K.M.; Dudley, D.M.; Kohn, S.; Simmons, H.A.; Wolfe, B.; Salamat, M.S.; Teixeira, L.B.C.; Wiepz, G.J.; Thoong, T.H.; et al. Highly Efficient Maternal-Fetal Zika Virus Transmission in Pregnant Rhesus Macaques. PLoS Pathog. 2017, 13, e1006378. [Google Scholar] [CrossRef]
- Armstrong, P.M.; Ehrlich, H.Y.; Magalhaes, T.; Miller, M.R.; Conway, P.J.; Bransfield, A.; Misencik, M.J.; Gloria-Soria, A.; Warren, J.L.; Andreadis, T.G.; et al. Successive Blood Meals Enhance Virus Dissemination within Mosquitoes and Increase Transmission Potential. Nat. Microbiol. 2020, 5, 239–247. [Google Scholar] [CrossRef]
- Comeau, G.; Zinna, R.A.; Scott, T.; Ernst, K.; Walker, K.; Carrière, Y.; Riehle, M.A. Vertical Transmission of Zika Virus in Aedes Aegypti Produces Potentially Infectious Progeny. Am. J. Trop. Med. Hyg. 2020, 103, 876–883. [Google Scholar] [CrossRef]
- Merwaiss, F.; Filomatori, C.V.; Susuki, Y.; Bardossy, E.S.; Alvarez, D.E.; Saleh, M.-C. Chikungunya Virus Replication Rate Determines the Capacity of Crossing Tissue Barriers in Mosquitoes. J. Virol. 2021, 95. [Google Scholar] [CrossRef]
- Sim, S.; Ramirez, J.L.; Dimopoulos, G. Dengue Virus Infection of the Aedes Aegypti Salivary Gland and Chemosensory Apparatus Induces Genes That Modulate Infection and Blood-Feeding Behavior. PLoS Pathog. 2012, 8, e1002631. [Google Scholar] [CrossRef] [PubMed]
- Göertz, G.P.; Vogels, C.B.F.; Geertsema, C.; Koenraadt, C.J.M.; Pijlman, G.P. Mosquito Co-Infection with Zika and Chikungunya Virus Allows Simultaneous Transmission without Affecting Vector Competence of Aedes Aegypti. PLoS Negl. Trop. Dis. 2017, 11, e0005654. [Google Scholar] [CrossRef] [PubMed]
- Rückert, C.; Weger-Lucarelli, J.; Garcia-Luna, S.M.; Young, M.C.; Byas, A.D.; Murrieta, R.A.; Fauver, J.R.; Ebel, G.D. Impact of Simultaneous Exposure to Arboviruses on Infection and Transmission by Aedes Aegypti Mosquitoes. Nat. Commun. 2017, 8, 15412. [Google Scholar] [CrossRef] [PubMed]
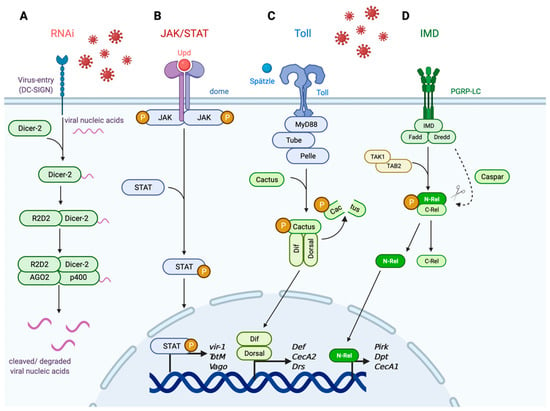
- Schuster, S.; Miesen, P.; van Rij, R.P. Antiviral RNAi in Insects and Mammals: Parallels and Differences. Viruses 2019, 11, 448. [Google Scholar] [CrossRef]
- Saleh, M.-C.; Tassetto, M.; van Rij, R.P.; Goic, B.; Gausson, V.; Berry, B.; Jacquier, C.; Antoniewski, C.; Andino, R. Antiviral Immunity in Drosophila Requires Systemic RNAi Spread. Nature 2009, 458, 346–350. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hanley, K.A. RNA Interference Modulates Replication of Dengue Virus in Drosophila Melanogaster Cells. BMC Microbiol. 2010, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.L.; Keene, K.M.; Brackney, D.E.; Olson, K.E.; Blair, C.D.; Wilusz, J.; Foy, B.D. Aedes Aegypti Uses RNA Interference in Defense against Sindbis Virus Infection. BMC Microbiol. 2008, 8, 47. [Google Scholar] [CrossRef]
- Lee, Y.S.; Nakahara, K.; Pham, J.W.; Kim, K.; He, Z.; Sontheimer, E.J.; Carthew, R.W. Distinct Roles for Drosophila Dicer-1 and Dicer-2 in the SiRNA/MiRNA Silencing Pathways. Cell 2004, 117, 69–81. [Google Scholar] [CrossRef]
- Galiana-Arnoux, D.; Dostert, C.; Schneemann, A.; Hoffmann, J.A.; Imler, J.-L. Essential Function in Vivo for Dicer-2 in Host Defense against RNA Viruses in Drosophila. Nat. Immunol. 2006, 7, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Rand, T.A.; Kalidas, S.; Du, F.; Kim, H.-E.; Smith, D.P.; Wang, X. R2D2, a Bridge between the Initiation and Effector Steps of the Drosophila RNAi Pathway. Science 2003, 301, 1921–1925. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Ishizuka, A.; Siomi, H.; Siomi, M.C. Distinct Roles for Argonaute Proteins in Small RNA-Directed RNA Cleavage Pathways. Genes Dev. 2004, 18, 1655–1666. [Google Scholar] [CrossRef]
- Van Rij, R.P.; Saleh, M.-C.; Berry, B.; Foo, C.; Houk, A.; Antoniewski, C.; Andino, R. The RNA Silencing Endonuclease Argonaute 2 Mediates Specific Antiviral Immunity in Drosophila Melanogaster. Genes Dev. 2006, 20, 2985–2995. [Google Scholar] [CrossRef]
- McFarlane, M.; Almire, F.; Kean, J.; Donald, C.L.; McDonald, A.; Wee, B.; Lauréti, M.; Varjak, M.; Terry, S.; Vazeille, M.; et al. The Aedes Aegypti Domino Ortholog P400 Regulates Antiviral Exogenous Small Interfering RNA Pathway Activity and Ago-2 Expression. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Scott, J.C.; Brackney, D.E.; Campbell, C.L.; Bondu-Hawkins, V.; Hjelle, B.; Ebel, G.D.; Olson, K.E.; Blair, C.D. Comparison of Dengue Virus Type 2-Specific Small RNAs from RNA Interference-Competent and –Incompetent Mosquito Cells. PLoS Negl. Trop. Dis. 2010, 4, e848. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Duchemin, J.-B.; Voysey, R.; Walker, P.J. Dicer-2-Dependent Activation of Culex Vago Occurs via the TRAF-Rel2 Signaling Pathway. PLoS Negl. Trop. Dis. 2014, 8, e2823. [Google Scholar] [CrossRef]
- Brackney, D.E.; Beane, J.E.; Ebel, G.D. RNAi Targeting of West Nile Virus in Mosquito Midguts Promotes Virus Diversification. PLoS Pathog. 2009, 5, e1000502. [Google Scholar] [CrossRef]
- Harsh, S.; Ozakman, Y.; Kitchen, S.M.; Paquin-Proulx, D.; Nixon, D.F.; Eleftherianos, I. Dicer-2 Regulates Resistance and Maintains Homeostasis against Zika Virus Infection in Drosophila. J. Immunol. 2018, 201, 3058–3072. [Google Scholar] [CrossRef]
- Chotkowski, H.L.; Ciota, A.T.; Jia, Y.; Puig-Basagoiti, F.; Kramer, L.D.; Shi, P.-Y.; Glaser, R.L. West Nile Virus Infection of Drosophila Melanogaster Induces a Protective RNAi Response. Virology 2008, 377, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Cirimotich, C.M.; Scott, J.C.; Phillips, A.T.; Geiss, B.J.; Olson, K.E. Suppression of RNA Interference Increases Alphavirus Replication and Virus-Associated Mortality in Aedes Aegypti Mosquitoes. BMC Microbiol. 2009, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Siu, R.W.C.; Fragkoudis, R.; Simmonds, P.; Donald, C.L.; Chase-Topping, M.E.; Barry, G.; Attarzadeh-Yazdi, G.; Rodriguez-Andres, J.; Nash, A.A.; Merits, A.; et al. Antiviral RNA Interference Responses Induced by Semliki Forest Virus Infection of Mosquito Cells: Characterization, Origin, and Frequency-Dependent Functions of Virus-Derived Small Interfering RNAs. J. Virol. 2011, 85, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.R. Viruses and RNA Interference: Issues and Controversies. J. Virol. 2014, 88, 12934–12936. [Google Scholar] [CrossRef]
- Schnettler, E.; Sterken, M.G.; Leung, J.Y.; Metz, S.W.; Geertsema, C.; Goldbach, R.W.; Vlak, J.M.; Kohl, A.; Khromykh, A.A.; Pijlman, G.P. Noncoding Flavivirus RNA Displays RNA Interference Suppressor Activity in Insect and Mammalian Cells. J. Virol. 2012, 86, 13486–13500. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Han, Y.; Fan, X.; Ding, S.-W. RNA Interference Functions as an Antiviral Immunity Mechanism in Mammals. Science 2013, 342, 231–234. [Google Scholar] [CrossRef]
- BioRender. Available online: https://app.biorender.com/illustrations/edit/604466dd34d48400a7c5f5bb (accessed on 7 March 2021).
- Osman, D.; Buchon, N.; Chakrabarti, S.; Huang, Y.-T.; Su, W.-C.; Poidevin, M.; Tsai, Y.-C.; Lemaitre, B. Autocrine and Paracrine Unpaired Signaling Regulate Intestinal Stem Cell Maintenance and Division. J. Cell Sci. 2012, 125, 5944–5949. [Google Scholar] [CrossRef]
- Wright, V.M.; Vogt, K.L.; Smythe, E.; Zeidler, M.P. Differential Activities of the Drosophila JAK/STAT Pathway Ligands Upd, Upd2 and Upd3. Cell Signal. 2011, 23, 920–927. [Google Scholar] [CrossRef]
- Wittes, J.; Schüpbach, T. A Gene Expression Screen in Drosophila Melanogaster Identifies Novel JAK/STAT and EGFR Targets During Oogenesis. G3 Genes Genomes Genet. 2019, 9, 47–60. [Google Scholar] [CrossRef]
- Ghiglione, C.; Devergne, O.; Georgenthum, E.; Carballès, F.; Médioni, C.; Cerezo, D.; Noselli, S. The Drosophila Cytokine Receptor Domeless Controls Border Cell Migration and Epithelial Polarization during Oogenesis. Development 2002, 129, 5437–5447. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.C.; Bach, E.A. JAK/STAT Signaling in Stem Cells and Regeneration: From Drosophila to Vertebrates. Dev. Camb. Engl. 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E. Physiological Significance of STAT Proteins: Investigations through Gene Disruption in Vivo. Cell. Mol. Life Sci. CMLS 1999, 55, 1559–1567. [Google Scholar] [CrossRef]
- Ahlers, L.R.H.; Trammell, C.E.; Carrell, G.F.; Mackinnon, S.; Torrevillas, B.K.; Chow, C.Y.; Luckhart, S.; Goodman, A.G. Insulin Potentiates JAK/STAT Signaling to Broadly Inhibit Flavivirus Replication in Insect Vectors. Cell Rep. 2019, 29, 1946–1960.e5. [Google Scholar] [CrossRef] [PubMed]
- Harsh, S.; Fu, Y.; Kenney, E.; Han, Z.; Eleftherianos, I. Zika Virus Non-Structural Protein NS4A Restricts Eye Growth in Drosophila through Regulation of JAK/STAT Signaling. Dis. Model. Mech. 2020, 13, dmm040816. [Google Scholar] [CrossRef]
- Angleró-Rodríguez, Y.I.; MacLeod, H.J.; Kang, S.; Carlson, J.S.; Jupatanakul, N.; Dimopoulos, G. Aedes Aegypti Molecular Responses to Zika Virus: Modulation of Infection by the Toll and Jak/Stat Immune Pathways and Virus Host Factors. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Terradas, G.; Joubert, D.A.; McGraw, E.A. The RNAi Pathway Plays a Small Part in Wolbachia-Mediated Blocking of Dengue Virus in Mosquito Cells. Sci. Rep. 2017, 7, 43847. [Google Scholar] [CrossRef]
- Jupatanakul, N.; Sim, S.; Angleró-Rodríguez, Y.I.; Souza-Neto, J.; Das, S.; Poti, K.E.; Rossi, S.L.; Bergren, N.; Vasilakis, N.; Dimopoulos, G. Engineered Aedes Aegypti JAK/STAT Pathway-Mediated Immunity to Dengue Virus. PLOS Negl. Trop. Dis. 2017, 11, e0005187. [Google Scholar] [CrossRef] [PubMed]
- Souza-Neto, J.A.; Sim, S.; Dimopoulos, G. An Evolutionary Conserved Function of the JAK-STAT Pathway in Anti-Dengue Defense. Proc. Natl. Acad. Sci. USA 2009, 106, 17841–17846. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Jouanguy, E.; Irving, P.; Troxler, L.; Galiana-Arnoux, D.; Hetru, C.; Hoffmann, J.A.; Imler, J.-L. The Jak-STAT Signaling Pathway Is Required but Not Sufficient for the Antiviral Response of Drosophila. Nat. Immunol. 2005, 6, 946–953. [Google Scholar] [CrossRef]
- Agaisse, H.; Petersen, U.-M.; Boutros, M.; Mathey-Prevot, B.; Perrimon, N. Signaling Role of Hemocytes in Drosophila JAK/STAT-Dependent Response to Septic Injury. Dev. Cell 2003, 5, 441–450. [Google Scholar] [CrossRef]
- Harrison, D.A.; McCoon, P.E.; Binari, R.; Gilman, M.; Perrimon, N. Drosophila Unpaired Encodes a Secreted Protein That Activates the JAK Signaling Pathway. Genes Dev. 1998, 12, 3252–3263. [Google Scholar] [CrossRef]
- Brown, S.; Hu, N.; Hombría, J.C. Identification of the First Invertebrate Interleukin JAK/STAT Receptor, the Drosophila Gene Domeless. Curr. Biol. CB 2001, 11, 1700–1705. [Google Scholar] [CrossRef]
- Binari, R.; Perrimon, N. Stripe-Specific Regulation of Pair-Rule Genes by Hopscotch, a Putative Jak Family Tyrosine Kinase in Drosophila. Genes Dev. 1994, 8, 300–312. [Google Scholar] [CrossRef]
- Yan, R.; Small, S.; Desplan, C.; Dearolf, C.R.; Darnell, J.E. Identification of a Stat Gene That Functions in Drosophila Development. Cell 1996, 84, 421–430. [Google Scholar] [CrossRef]
- Hou, X.S.; Melnick, M.B.; Perrimon, N. Marelle Acts Downstream of the Drosophila HOP/JAK Kinase and Encodes a Protein Similar to the Mammalian STATs. Cell 1996, 84, 411–419. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Trinidad, L.; Voysey, R.; Duchemin, J.-B.; Walker, P.J. Secreted Vago Restricts West Nile Virus Infection in Culex Mosquito Cells by Activating the Jak-STAT Pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 18915–18920. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Zhou, J.; Xie, W.; Chen, C.; Wang, Z.; Yang, H.; Cui, J. Zika Virus Evades Interferon-Mediated Antiviral Response through the Co-Operation of Multiple Nonstructural Proteins in Vitro. Cell Discov. 2017, 3, 17006. [Google Scholar] [CrossRef]
- Keller, B.C.; Fredericksen, B.L.; Samuel, M.A.; Mock, R.E.; Mason, P.W.; Diamond, M.S.; Gale, M. Resistance to Alpha/Beta Interferon Is a Determinant of West Nile Virus Replication Fitness and Virulence. J. Virol. 2006, 80, 9424–9434. [Google Scholar] [CrossRef]
- Lin, R.-J.; Liao, C.-L.; Lin, E.; Lin, Y.-L. Blocking of the Alpha Interferon-Induced Jak-Stat Signaling Pathway by Japanese Encephalitis Virus Infection. J. Virol. 2004, 78, 9285–9294. [Google Scholar] [CrossRef]
- Valanne, S.; Wang, J.-H.; Rämet, M. The Drosophila Toll Signaling Pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef]
- Myllymäki, H.; Valanne, S.; Rämet, M. The Drosophila Imd Signaling Pathway. J. Immunol. 2014, 192, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Reichhart, J.M.; Hoffmann, J.A.; Royet, J. Drosophila Toll Is Activated by Gram-Positive Bacteria through a Circulating Peptidoglycan Recognition Protein. Nature 2001, 414, 756–759. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.M.; Hoffmann, J.A. The Dorsoventral Regulatory Gene Cassette Spätzle/Toll/Cactus Controls the Potent Antifungal Response in Drosophila Adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef]
- Leulier, F.; Parquet, C.; Pili-Floury, S.; Ryu, J.-H.; Caroff, M.; Lee, W.-J.; Mengin-Lecreulx, D.; Lemaitre, B. The Drosophila Immune System Detects Bacteria through Specific Peptidoglycan Recognition. Nat. Immunol. 2003, 4, 478–484. [Google Scholar] [CrossRef]
- Kaneko, T.; Goldman, W.E.; Mellroth, P.; Steiner, H.; Fukase, K.; Kusumoto, S.; Harley, W.; Fox, A.; Golenbock, D.; Silverman, N. Monomeric and Polymeric Gram-Negative Peptidoglycan but Not Purified LPS Stimulate the Drosophila IMD Pathway. Immunity 2004, 20, 637–649. [Google Scholar] [CrossRef]
- Horng, T.; Medzhitov, R. Drosophila MyD88 Is an Adapter in the Toll Signaling Pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 12654–12658. [Google Scholar] [CrossRef] [PubMed]
- Tauszig-Delamasure, S.; Bilak, H.; Capovilla, M.; Hoffmann, J.A.; Imler, J.-L. Drosophila MyD88 Is Required for the Response to Fungal and Gram-Positive Bacterial Infections. Nat. Immunol. 2002, 3, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Bristow, B.N.; Qu, G.; Wasserman, S.A. A Heterotrimeric Death Domain Complex in Toll Signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 12871–12876. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.P.; Anderson, K.V. Regulated Nuclear Import of Rel Proteins in the Drosophila Immune Response. Nature 1998, 392, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Choe, K.-M.; Lee, H.; Anderson, K.V. Drosophila Peptidoglycan Recognition Protein LC (PGRP-LC) Acts as a Signal-Transducing Innate Immune Receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 1122–1126. [Google Scholar] [CrossRef]
- Georgel, P.; Naitza, S.; Kappler, C.; Ferrandon, D.; Zachary, D.; Swimmer, C.; Kopczynski, C.; Duyk, G.; Reichhart, J.M.; Hoffmann, J.A. Drosophila Immune Deficiency (IMD) Is a Death Domain Protein That Activates Antibacterial Defense and Can Promote Apoptosis. Dev. Cell 2001, 1, 503–514. [Google Scholar] [CrossRef]
- Stöven, S.; Silverman, N.; Junell, A.; Hedengren-Olcott, M.; Erturk, D.; Engström, Y.; Maniatis, T.; Hultmark, D. Caspase-Mediated Processing of the Drosophila NF-ΚB Factor Relish. Proc. Natl. Acad. Sci. USA 2003, 100, 5991–5996. [Google Scholar] [CrossRef]
- Silverman, N.; Zhou, R.; Stöven, S.; Pandey, N.; Hultmark, D.; Maniatis, T. A Drosophila IκB Kinase Complex Required for Relish Cleavage and Antibacterial Immunity. Genes Dev. 2000, 14, 2461–2471. [Google Scholar] [CrossRef]
- Paquette, N.; Broemer, M.; Aggarwal, K.; Chen, L.; Husson, M.; Ertürk-Hasdemir, D.; Reichhart, J.-M.; Meier, P.; Silverman, N. Caspase Mediated Cleavage, IAP Binding and Ubiquitination: Linking Three Mechanisms Crucial for Drosophila NF-ΚB Signaling. Mol. Cell 2010, 37, 172. [Google Scholar] [CrossRef]
- Dushay, M.S.; Asling, B.; Hultmark, D. Origins of Immunity: Relish, a Compound Rel-like Gene in the Antibacterial Defense of Drosophila. Proc. Natl. Acad. Sci. USA 1996, 93, 10343–10347. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.H.; Lee, S.Y.; Kim, E.; Chung, J. Caspar, a Suppressor of Antibacterial Immunity in Drosophila. Proc. Natl. Acad. Sci. USA 2006, 103, 16358–16363. [Google Scholar] [CrossRef] [PubMed]
- Stöven, S.; Ando, I.; Kadalayil, L.; Engström, Y.; Hultmark, D. Activation of the Drosophila NF-ΚB Factor Relish by Rapid Endoproteolytic Cleavage. EMBO Rep. 2000, 1, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Barletta, A.B.F.; Alves, L.R.; Nascimento Silva, M.C.L.; Sim, S.; Dimopoulos, G.; Liechocki, S.; Maya-Monteiro, C.M.; Sorgine, M.H.F. Emerging Role of Lipid Droplets in Aedes Aegypti Immune Response against Bacteria and Dengue Virus. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Ramirez, J.L.; Dimopoulos, G. The Aedes Aegypti Toll Pathway Controls Dengue Virus Infection. PLoS Pathog. 2008, 4, e1000098. [Google Scholar] [CrossRef] [PubMed]
- Zink, S.D.; Van Slyke, G.A.; Palumbo, M.J.; Kramer, L.D.; Ciota, A.T. Exposure to West Nile Virus Increases Bacterial Diversity and Immune Gene Expression in Culex Pipiens. Viruses 2015, 7, 5619–5631. [Google Scholar] [CrossRef]
- Zambon, R.A.; Nandakumar, M.; Vakharia, V.N.; Wu, L.P. The Toll Pathway Is Important for an Antiviral Response in Drosophila. Proc. Natl. Acad. Sci. USA 2005, 102, 7257–7262. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, D.; Liu, Y.; Xia, X.; Gong, W.; Qiu, Y.; Yang, J.; Zheng, Y.; Li, J.; Wang, Y.F.; et al. Drosophila Dicer-2 Has an RNA Interference–Independent Function That Modulates Toll Immune Signaling. Sci. Adv. 2015, 1, e1500228. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Jan, E.; Sarnow, P.; Schneider, D. The Imd Pathway Is Involved in Antiviral Immune Responses in Drosophila. PLoS ONE 2009, 4, e7436. [Google Scholar] [CrossRef] [PubMed]
- Nanfack-Minkeu, F.; Mitri, C.; Bischoff, E.; Belda, E.; Casademont, I.; Vernick, K.D. Interaction of RNA Viruses of the Natural Virome with the African Malaria Vector, Anopheles Coluzzii. Sci. Rep. 2019, 9, 6319. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A Human Homologue of the Drosophila Toll Protein Signals Activation of Adaptive Immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Engström, Y.; Kadalayil, L.; Sun, S.C.; Samakovlis, C.; Hultmark, D.; Faye, I. Kappa B-like Motifs Regulate the Induction of Immune Genes in Drosophila. J. Mol. Biol. 1993, 232, 327–333. [Google Scholar] [CrossRef]
- Kumar, M.; Belcaid, M.; Nerurkar, V.R. Identification of Host Genes Leading to West Nile Virus Encephalitis in Mice Brain Using RNA-Seq Analysis. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Daffis, S.; Suthar, M.S.; Michael Gale, J.; Diamond, M.S. Measure and Countermeasure: Type I IFN (IFN-α/β) Antiviral Response against West Nile Virus. J. Innate Immun. 2009, 1, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Szretter, K.J.; Daffis, S.; Patel, J.; Suthar, M.S.; Klein, R.S.; Gale, M.; Diamond, M.S. The Innate Immune Adaptor Molecule MyD88 Restricts West Nile Virus Replication and Spread in Neurons of the Central Nervous System. J. Virol. 2010, 84, 12125–12138. [Google Scholar] [CrossRef]
- Thackray, L.B.; Shrestha, B.; Richner, J.M.; Miner, J.J.; Pinto, A.K.; Lazear, H.M.; Gale, M.; Diamond, M.S. Interferon Regulatory Factor 5-Dependent Immune Responses in the Draining Lymph Node Protect against West Nile Virus Infection. J. Virol. 2014, 88, 11007–11021. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; de Souza Caroci, A.; Ribolla, P.E.M.; de Bianchi, A.G.; Bijovsky, A.T. Functional Morphology of Adult Female Culex Quinquefasciatus Midgut during Blood Digestion. Tissue Cell 2002, 34, 210–219. [Google Scholar] [CrossRef]
- Romoser, W.S.; Wasieloski, L.P.; Pushko, P.; Kondig, J.P.; Lerdthusnee, K.; Neira, M.; Ludwig, G.V. Evidence for Arbovirus Dissemination Conduits from the Mosquito (Diptera: Culicidae) Midgut. J. Med. Entomol. 2004, 41, 467–475. [Google Scholar] [CrossRef]
- Rodriguez-Andres, J.; Rani, S.; Varjak, M.; Chase-Topping, M.E.; Beck, M.H.; Ferguson, M.C.; Schnettler, E.; Fragkoudis, R.; Barry, G.; Merits, A.; et al. Phenoloxidase Activity Acts as a Mosquito Innate Immune Response against Infection with Semliki Forest Virus. PLoS Pathog. 2012, 8, e1002977. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Srivastava, P.; Sirisena, P.; Dubey, S.K.; Kumar, R.; Shrinet, J.; Sunil, S. Mosquito Innate Immunity. Insects 2018, 9, 95. [Google Scholar] [CrossRef]
- Parikh, G.R.; Oliver, J.D.; Bartholomay, L.C. A Haemocyte Tropism for an Arbovirus. J. Gen. Virol. 2009, 90, 292–296. [Google Scholar] [CrossRef]
- Girard, Y.A.; Klingler, K.A.; Higgs, S. West Nile Virus Dissemination and Tissue Tropisms in Orally Infected Culex Pipiens Quinquefasciatus. Vector Borne Zoonotic Dis. Larchmt. N. Y. 2004, 4, 109–122. [Google Scholar] [CrossRef]
- Mourya, D.T.; Mishra, A.C. Antigen Distribution Pattern of Japanese Encephalitis Virus in Culex Tritaeniorhynchus, C. Vishnui & C. Pseudovishnui. Indian J. Med. Res. 2000, 111, 157–161. [Google Scholar]
- Zhang, M.; Zheng, X.; Wu, Y.; Gan, M.; He, A.; Li, Z.; Liu, J.; Zhan, X. Quantitative Analysis of Replication and Tropisms of Dengue Virus Type 2 in Aedes Albopictus. Am. J. Trop. Med. Hyg. 2010, 83, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Girard, Y.A.; Popov, V.; Wen, J.; Han, V.; Higgs, S. Ultrastructural Study of West Nile Virus Pathogenesis in Culex Pipiens Quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 2005, 42, 429–444. [Google Scholar] [CrossRef]
- Weaver, S.C.; Scott, T.W.; Lorenz, L.H.; Lerdthusnee, K.; Romoser, W.S. Togavirus-Associated Pathologic Changes in the Midgut of a Natural Mosquito Vector. J. Virol. 1988, 62, 2083–2090. [Google Scholar] [CrossRef]
- Xu, J.; Hopkins, K.; Sabin, L.; Yasunaga, A.; Subramanian, H.; Lamborn, I.; Gordesky-Gold, B.; Cherry, S. ERK Signaling Couples Nutrient Status to Antiviral Defense in the Insect Gut. Proc. Natl. Acad. Sci. USA 2013, 110, 15025–15030. [Google Scholar] [CrossRef] [PubMed]
- DiAngelo, J.R.; Bland, M.L.; Bambina, S.; Cherry, S.; Birnbaum, M.J. The Immune Response Attenuates Growth and Nutrient Storage in Drosophila by Reducing Insulin Signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20853–20858. [Google Scholar] [CrossRef]
- Drexler, A.L.; Pietri, J.E.; Pakpour, N.; Hauck, E.; Wang, B.; Glennon, E.K.K.; Georgis, M.; Riehle, M.A.; Luckhart, S. Human IGF1 Regulates Midgut Oxidative Stress and Epithelial Homeostasis to Balance Lifespan and Plasmodium Falciparum Resistance in Anopheles Stephensi. PLoS Pathog. 2014, 10, e1004231. [Google Scholar] [CrossRef]
- Haqshenas, G.; Terradas, G.; Paradkar, P.N.; Duchemin, J.-B.; McGraw, E.A.; Doerig, C. A Role for the Insulin Receptor in the Suppression of Dengue Virus and Zika Virus in Wolbachia-Infected Mosquito Cells. Cell Rep. 2019, 26, 529–535.e3. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and Virus Protection in Insects. Science 2008, 322, 702. [Google Scholar] [CrossRef] [PubMed]
- Luckhart, S.; Giulivi, C.; Drexler, A.L.; Antonova-Koch, Y.; Sakaguchi, D.; Napoli, E.; Wong, S.; Price, M.S.; Eigenheer, R.; Phinney, B.S.; et al. Sustained Activation of Akt Elicits Mitochondrial Dysfunction to Block Plasmodium Falciparum Infection in the Mosquito Host. PLoS Pathog. 2013, 9, e1003180. [Google Scholar] [CrossRef]
- Altindis, E.; Cai, W.; Sakaguchi, M.; Zhang, F.; GuoXiao, W.; Liu, F.; De Meyts, P.; Gelfanov, V.; Pan, H.; DiMarchi, R.; et al. Viral Insulin-like Peptides Activate Human Insulin and IGF-1 Receptor Signaling: A Paradigm Shift for Host–Microbe Interactions. Proc. Natl. Acad. Sci. USA 2018, 115, 2461–2466. [Google Scholar] [CrossRef]
- Liang, Q.; Luo, Z.; Zeng, J.; Chen, W.; Foo, S.-S.; Lee, S.-A.; Ge, J.; Wang, S.; Goldman, S.A.; Zlokovic, B.V.; et al. Zika Virus NS4A and NS4B Proteins Deregulate Akt-MTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell 2016, 19, 663–671. [Google Scholar] [CrossRef]
- Samy, A.M.; Elaagip, A.H.; Kenawy, M.A.; Ayres, C.F.J.; Peterson, A.T.; Soliman, D.E. Climate Change Influences on the Global Potential Distribution of the Mosquito Culex Quinquefasciatus, Vector of West Nile Virus and Lymphatic Filariasis. PLoS ONE 2016, 11, e0163863. [Google Scholar] [CrossRef] [PubMed]
- Alaniz, A.J.; Carvajal, M.A.; Bacigalupo, A.; Cattan, P.E. Global Spatial Assessment of Aedes Aegypti and Culex Quinquefasciatus: A Scenario of Zika Virus Exposure. Epidemiol. Infect. 2018, 147. [Google Scholar] [CrossRef] [PubMed]
- Muttis, E.; Balsalobre, A.; Chuchuy, A.; Mangudo, C.; Ciota, A.T.; Kramer, L.D.; Micieli, M.V. Factors Related to Aedes Aegypti (Diptera: Culicidae) Populations and Temperature Determine Differences on Life-History Traits with Regional Implications in Disease Transmission. J. Med. Entomol. 2018, 55, 1105–1112. [Google Scholar] [CrossRef]
- Miller, M.J.; Loaiza, J.R. Geographic Expansion of the Invasive Mosquito Aedes Albopictus across Panama—Implications for Control of Dengue and Chikungunya Viruses. PLoS Negl. Trop. Dis. 2015, 9, e0003383. [Google Scholar] [CrossRef]
- Weger-Lucarelli, J.; Rückert, C.; Chotiwan, N.; Nguyen, C.; Luna, S.M.G.; Fauver, J.R.; Foy, B.D.; Perera, R.; Black, W.C.; Kading, R.C.; et al. Vector Competence of American Mosquitoes for Three Strains of Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e0005101. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Quiroga, D.; Medina-Sánchez, A.; Sánchez-González, J.M.; Eckert, K.A.; Villalobos-Sánchez, E.; Navarro-Zúñiga, A.R.; Sánchez-Tejeda, G.; Correa-Morales, F.; González-Acosta, C.; Arias, C.F.; et al. Zika Virus in Salivary Glands of Five Different Species of Wild-Caught Mosquitoes from Mexico. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Jupp, P.G.; Kemp, A.; Grobbelaar, A.; Lema, P.; Burt, F.J.; Alahmed, A.M.; Al Mujalli, D.; Al Khamees, M.; Swanepoel, R. The 2000 Epidemic of Rift Valley Fever in Saudi Arabia: Mosquito Vector Studies. Med. Vet. Entomol. 2002, 16, 245–252. [Google Scholar] [CrossRef]
- Moser, L.A.; Lim, P.-Y.; Styer, L.M.; Kramer, L.D.; Bernard, K.A. Parameters of Mosquito-Enhanced West Nile Virus Infection. J. Virol. 2016, 90, 292–299. [Google Scholar] [CrossRef]
- Sun, P.; Nie, K.; Zhu, Y.; Liu, Y.; Wu, P.; Liu, Z.; Du, S.; Fan, H.; Chen, C.-H.; Zhang, R.; et al. A Mosquito Salivary Protein Promotes Flavivirus Transmission by Activation of Autophagy. Nat. Commun. 2020, 11, 260. [Google Scholar] [CrossRef]
- Styer, L.M.; Lim, P.-Y.; Louie, K.L.; Albright, R.G.; Kramer, L.D.; Bernard, K.A. Mosquito Saliva Causes Enhancement of West Nile Virus Infection in Mice. J. Virol. 2011, 85, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.R.; James, A.A. Salivary Gland Anticoagulants in Culicine and Anopheline Mosquitoes (Diptera:Culicidae). J. Med. Entomol. 1996, 33, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.S.; Soong, L.; Coffey, L.L.; Stevenson, H.L.; McGee, C.E.; Higgs, S. Aedes Aegypti Saliva Alters Leukocyte Recruitment and Cytokine Signaling by Antigen-Presenting Cells during West Nile Virus Infection. PLoS ONE 2010, 5, e11704. [Google Scholar] [CrossRef]
- Bai, F.; Kong, K.-F.; Dai, J.; Qian, F.; Zhang, L.; Brown, C.R.; Fikrig, E.; Montgomery, R.R. A Paradoxical Role for Neutrophils in the Pathogenesis of West Nile Virus. J. Infect. Dis. 2010, 202, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nathan, D.; Huitinga, I.; Lustig, S.; van Rooijen, N.; Kobiler, D. West Nile Virus Neuroinvasion and Encephalitis Induced by Macrophage Depletion in Mice. Arch. Virol. 1996, 141, 459–469. [Google Scholar] [CrossRef]
- Vogt, M.B.; Lahon, A.; Arya, R.P.; Kneubehl, A.R.; Clinton, J.L.S.; Paust, S.; Rico-Hesse, R. Mosquito Saliva Alone Has Profound Effects on the Human Immune System. PLoS Negl. Trop. Dis. 2018, 12, e0006439. [Google Scholar] [CrossRef]
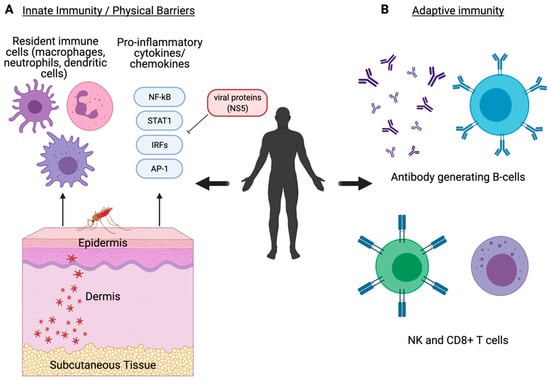
- Pingen, M.; Bryden, S.R.; Pondeville, E.; Schnettler, E.; Kohl, A.; Merits, A.; Fazakerley, J.K.; Graham, G.J.; McKimmie, C.S. Host Inflammatory Response to Mosquito Bites Enhances the Severity of Arbovirus Infection. Immunity 2016, 44, 1455–1469. [Google Scholar] [CrossRef]
- Lim, P.-Y.; Behr, M.J.; Chadwick, C.M.; Shi, P.-Y.; Bernard, K.A. Keratinocytes Are Cell Targets of West Nile Virus In Vivo. J. Virol. 2011, 85, 5197–5201. [Google Scholar] [CrossRef]
- Johnston, L.J.; King, N.J.C.; Halliday, G.M. Langerhans Cells Migrate to Local Lymph Nodes Following Cutaneous Infection with an Arbovirus. J. Investig. Dermatol. 2000, 114, 560–568. [Google Scholar] [CrossRef]
- Samuel, M.A.; Diamond, M.S. Alpha/Beta Interferon Protects against Lethal West Nile Virus Infection by Restricting Cellular Tropism and Enhancing Neuronal Survival. J. Virol. 2005, 79, 13350–13361. [Google Scholar] [CrossRef]
- Hershkovitz, O.; Rosental, B.; Rosenberg, L.A.; Navarro-Sanchez, M.E.; Jivov, S.; Zilka, A.; Gershoni-Yahalom, O.; Brient-Litzler, E.; Bedouelle, H.; Ho, J.W.; et al. NKp44 Receptor Mediates Interaction of the Envelope Glycoproteins from the West Nile and Dengue Viruses with NK Cells. J. Immunol. Baltim. Md 1950 2009, 183, 2610–2621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Daniel, S.; Huang, Y.; Chancey, C.; Huang, Q.; Lei, Y.F.; Grinev, A.; Mostowski, H.; Rios, M.; Dayton, A. Anti-West Nile Virus Activity of in Vitro Expanded Human Primary Natural Killer Cells. BMC Immunol. 2010, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Vargas-Inchaustegui, D.A.; Raimer, S.S.; Kelly, B.C.; Hu, J.; Zhu, L.; Sun, J.; Soong, L. Type I IFN Receptor Regulates Neutrophil Functions and Innate Immunity to Leishmania Parasites. J. Immunol. Baltim. Md 1950 2010, 184, 7047–7056. [Google Scholar] [CrossRef]
- Purtha, W.E.; Chachu, K.A.; Virgin, H.W.; Diamond, M.S. Early B-Cell Activation after West Nile Virus Infection Requires Alpha/Beta Interferon but Not Antigen Receptor Signaling. J. Virol. 2008, 82, 10964–10974. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.K.; Daffis, S.; Brien, J.D.; Gainey, M.D.; Yokoyama, W.M.; Sheehan, K.C.F.; Murphy, K.M.; Schreiber, R.D.; Diamond, M.S. A Temporal Role of Type I Interferon Signaling in CD8+ T Cell Maturation during Acute West Nile Virus Infection. PLoS Pathog. 2011, 7, e1002407. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.P.; McCarthy, M.K.; Davenport, B.J.; Morrison, T.E.; Diamond, M.S. Clearance of Chikungunya Virus Infection in Lymphoid Tissues Is Promoted by Treatment with an Agonistic Anti-CD137 Antibody. J. Virol. 2019, 93, e01231-19. [Google Scholar] [CrossRef]
- Lazear, H.M.; Pinto, A.K.; Ramos, H.J.; Vick, S.C.; Shrestha, B.; Suthar, M.S.; Gale, M.; Diamond, M.S. Pattern Recognition Receptor MDA5 Modulates CD8+ T Cell-Dependent Clearance of West Nile Virus from the Central Nervous System. J. Virol. 2013, 87, 11401–11415. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Shresta, S.; Kyle, J.L.; Snider, H.M.; Basavapatna, M.; Beatty, P.R.; Harris, E. Interferon-Dependent Immunity Is Essential for Resistance to Primary Dengue Virus Infection in Mice, Whereas T- and B-Cell-Dependent Immunity Are Less Critical. J. Virol. 2004, 78, 2701–2710. [Google Scholar] [CrossRef]
- Lubick, K.J.; Robertson, S.J.; McNally, K.L.; Freedman, B.A.; Rasmussen, A.L.; Taylor, R.T.; Walts, A.D.; Tsuruda, S.; Sakai, M.; Ishizuka, M.; et al. Flavivirus Antagonism of Type I Interferon Signaling Reveals Prolidase as a Regulator of IFNAR1 Surface Expression. Cell Host Microbe 2015, 18, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Laurent-Rolle, M.; Maestre, A.M.; Rajsbaum, R.; Pisanelli, G.; Simon, V.; Mulder, L.C.F.; Fernandez-Sesma, A.; García-Sastre, A. Dengue Virus Co-Opts UBR4 to Degrade STAT2 and Antagonize Type I Interferon Signaling. PLoS Pathog. 2013, 9, e1003265. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Rolle, M.; Morrison, J.; Rajsbaum, R.; Macleod, J.M.L.; Pisanelli, G.; Pham, A.; Ayllon, J.; Miorin, L.; Martinez, C.; tenOever, B.R.; et al. The Interferon Signaling Antagonist Function of Yellow Fever Virus NS5 Protein Is Activated by Type I Interferon. Cell Host Microbe 2014, 16, 314–327. [Google Scholar] [CrossRef]
- Fros, J.J.; Liu, W.J.; Prow, N.A.; Geertsema, C.; Ligtenberg, M.; Vanlandingham, D.L.; Schnettler, E.; Vlak, J.M.; Suhrbier, A.; Khromykh, A.A.; et al. Chikungunya Virus Nonstructural Protein 2 Inhibits Type I/II Interferon-Stimulated JAK-STAT Signaling. J. Virol. 2010, 84, 10877–10887. [Google Scholar] [CrossRef]
- Tirado, S.M.C.; Yoon, K.-J. Antibody-Dependent Enhancement of Virus Infection and Disease. Viral Immunol. 2003, 16, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Gollins, S.W.; Porterfield, J.S. Flavivirus Infection Enhancement in Macrophages: Radioactive and Biological Studies on the Effect of Antibody on Viral Fate. J. Gen. Virol. 1984, 65 Pt 8, 1261–1272. [Google Scholar] [CrossRef]
- Gollins, S.W.; Porterfield, J.S. Flavivirus Infection Enhancement in Macrophages: An Electron Microscopic Study of Viral Cellular Entry. J. Gen. Virol. 1985, 66 Pt 9, 1969–1982. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-Reacting Antibodies Enhance Dengue Virus Infection in Humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef]
- Charles, A.S.; Christofferson, R.C. Utility of a Dengue-Derived Monoclonal Antibody to Enhance Zika Infection In Vitro. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- Kliks, S.C.; Nimmanitya, S.; Nisalak, A.; Burke, D.S. Evidence That Maternal Dengue Antibodies Are Important in the Development of Dengue Hemorrhagic Fever in Infants. Am. J. Trop. Med. Hyg. 1988, 38, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Kliks, S.C.; Nisalak, A.; Brandt, W.E.; Wahl, L.; Burke, D.S. Antibody-Dependent Enhancement of Dengue Virus Growth in Human Monocytes as a Risk Factor for Dengue Hemorrhagic Fever. Am. J. Trop. Med. Hyg. 1989, 40, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.G.; Kourí, G.; Valdés, L.; Bravo, J.; Vázquez, S.; Halstead, S.B. Enhanced Severity of Secondary Dengue-2 Infections: Death Rates in 1981 and 1997 Cuban Outbreaks. Rev. Panam. Salud Publica Pan Am. J. Public Health 2002, 11, 223–227. [Google Scholar] [CrossRef]
- de Silva, A.M.; Harris, E. Which Dengue Vaccine Approach Is the Most Promising, and Should We Be Concerned about Enhanced Disease after Vaccination? Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.K.; Bygbjerg, I.C. Zika Virus and Hyperglycaemia in Pregnancy. Lancet 2016, 387, 1812. [Google Scholar] [CrossRef][Green Version]
- Kumar, M.; Roe, K.; Nerurkar, P.V.; Orillo, B.; Thompson, K.S.; Verma, S.; Nerurkar, V.R. Reduced Immune Cell Infiltration and Increased Pro-Inflammatory Mediators in the Brain of Type 2 Diabetic Mouse Model Infected with West Nile Virus. J. Neuroinflammation 2014, 11, 80. [Google Scholar] [CrossRef]
- Kumar, M.; Roe, K.; Nerurkar, P.V.; Namekar, M.; Orillo, B.; Verma, S.; Nerurkar, V.R. Impaired Virus Clearance, Compromised Immune Response and Increased Mortality in Type 2 Diabetic Mice Infected with West Nile Virus. PLoS ONE 2012, 7, e44682. [Google Scholar] [CrossRef]
- Lee, I.-K.; Hsieh, C.-J.; Lee, C.-T.; Liu, J.-W. Diabetic Patients Suffering Dengue Are at Risk for Development of Dengue Shock Syndrome/Severe Dengue: Emphasizing the Impacts of Co-Existing Comorbidity(Ies) and Glycemic Control on Dengue Severity. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi 2020, 53, 69–78. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Rückert, C.; Cavany, S.M.; Perkins, T.A.; Ebel, G.D.; Grubaugh, N.D. Arbovirus Coinfection and Co-Transmission: A Neglected Public Health Concern? PLoS Biol. 2019, 17, e3000130. [Google Scholar] [CrossRef] [PubMed]
- Chaves, B.A.; Orfano, A.S.; Nogueira, P.M.; Rodrigues, N.B.; Campolina, T.B.; Nacif-Pimenta, R.; Pires, A.C.A.M.; Júnior, A.B.V.; da Costa Paz, A.; da Costa Vaz, E.B.; et al. Coinfection with Zika Virus (ZIKV) and Dengue Virus Results in Preferential ZIKV Transmission by Vector Bite to Vertebrate Host. J. Infect. Dis. 2018, 218, 563–571. [Google Scholar] [CrossRef]
- Dodson, B.L.; Hughes, G.L.; Paul, O.; Matacchiero, A.C.; Kramer, L.D.; Rasgon, J.L. Wolbachia Enhances West Nile Virus (WNV) Infection in the Mosquito Culex Tarsalis. PLoS Negl. Trop. Dis. 2014, 8, e2965. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).