Three Strains of Tobacco etch virus Distinctly Alter the Transcriptome of Apical Stem Tissue in Capsicum annuum during Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses, Plant Growth Conditions and Experimental Designs
2.2. Virus Infection Evaluations
2.3. Total RNA Isolation and High-Throughput Sequencing
2.4. Illumina mRNA Sequence Data Analysis
2.5. Confirmation of RNA-Sequencing by qPCR
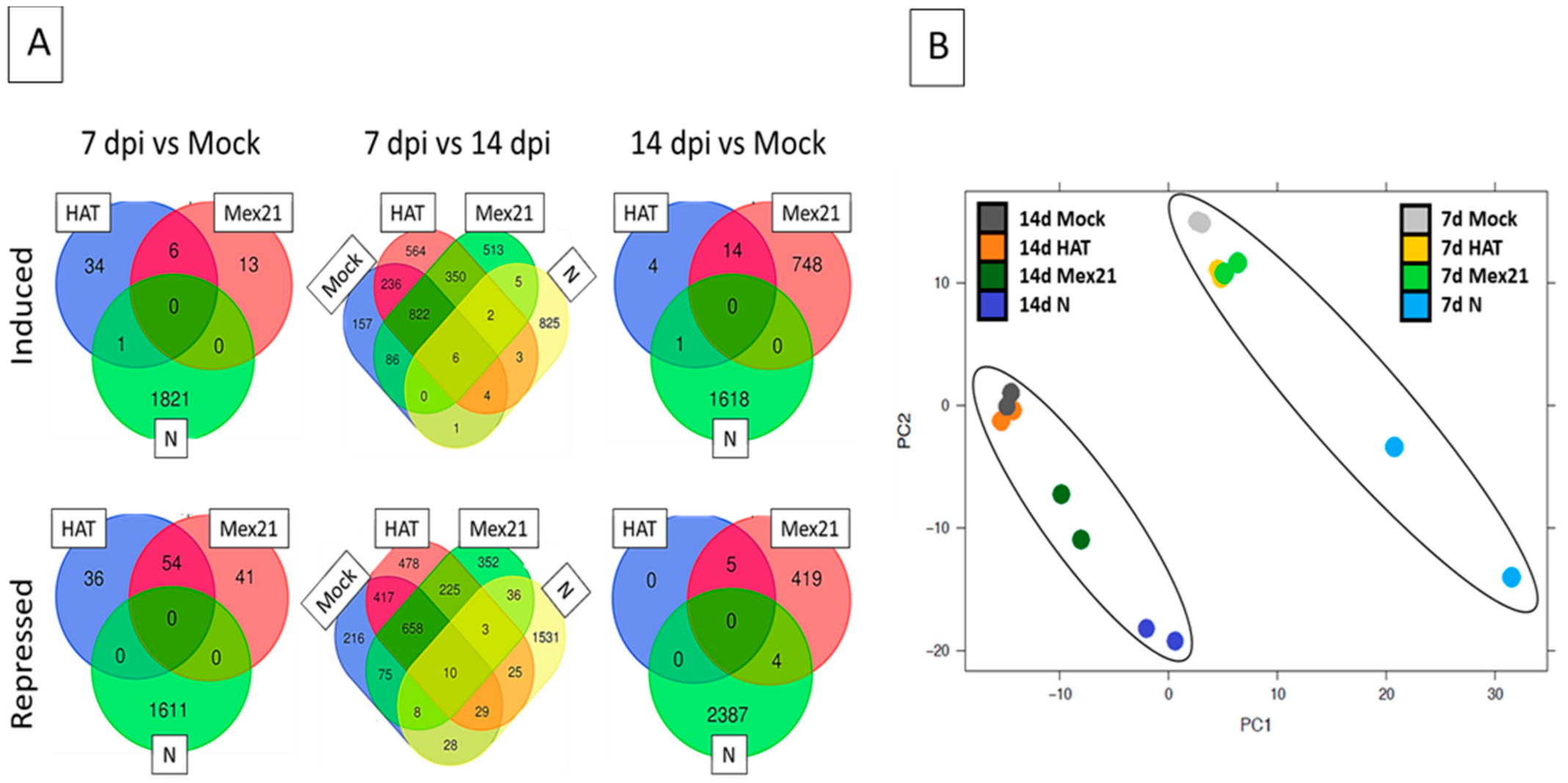
3. Results
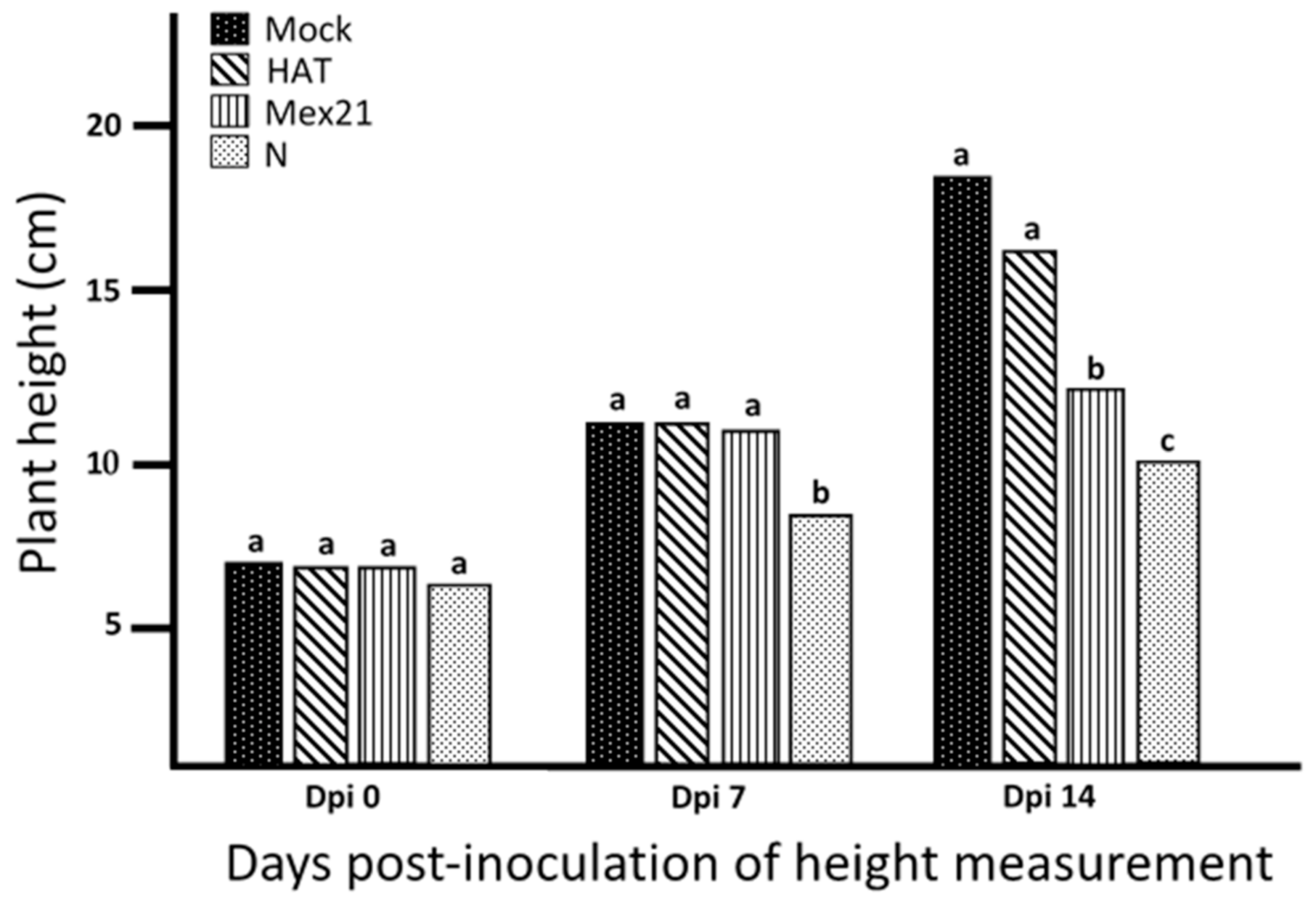
3.1. Virus Infection of RNA Source Plants
3.2. Transcriptome of TEV Strain-Infected and Buffer-Mock Control Plants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, P.H.; Adams, M.J.; Barnett, O.W.; Brunt, A.A.; Hammond, J.; Hill, J.H.; Jordan, R.L.; Kashiwazaki, S.; Rybicki, E.; Spence, N.; et al. Virus Taxonomy. 8th Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2005; pp. 819–884. [Google Scholar]
- Purcifull, D.E.; Hiebert, E. Tobacco Etch Virus; CMI/AAB Description of Plant Viruses, No. 258; Association of Applied Biologists: Warwickshire, UK, 1982. [Google Scholar]
- CABI. Tobacco Etch Virus; Map 1094 in: Distribution Maps of Plant Diseases; CABI: Wallingford, UK, 2010. [Google Scholar]
- Edwardson, J.R.; Christie, R.G. Viruses Infecting Peppers and Other Solanaceous Crops. Volume 1. Viruses Infecting Peppers and Other Solanaceous Crops; University of Florida: Gainesville, FL, USA, 1997. [Google Scholar]
- Shukla, D.D.; Ward, C.W.; Brunt, A.A. Potyviruses: Biology, Molecular Structure and Taxonomy; CAB International: Wallingford, UK, 1994. [Google Scholar]
- Chung, B.Y.; Miller, W.A.; Atkins, J.F.; Firth, A.E. An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. USA 2008, 105, 5897–5902. [Google Scholar] [CrossRef] [PubMed]
- Urcuqui-Inchima, S.; Haenni, A.L.; Bernardi, F. Potyvirus proteins: A wealth of functions. Virus Res. 2001, 74, 157–175. [Google Scholar] [CrossRef]
- Murphy, J.F.; Rhoads, R.E.; Hunt, A.G.; Shaw, J.G. The VPg of tobacco etch virus RNA is the 49-kDa proteinase or the N-terminal 24-kDa part of the proteinase. Virology 1990, 178, 285–288. [Google Scholar] [CrossRef]
- Murphy, J.F.; Wojciech, R.; Rhoads, R.E.; Hunt, A.G.; Shaw, J.G. A tyrosine residue in the small nuclear inclusion protein of tobacco vein mottling virus links the VPg to the viral RNA. J. Virol. 1991, 65, 511–513. [Google Scholar] [CrossRef]
- Hari, V.; Siegel, A.; Rozek, C.; Timberlake, W.E. The RNA of tobacco etch virus contains poly(A). Virology 1979, 92, 568–571. [Google Scholar] [CrossRef]
- Allison, R.; Johnston, R.E.; Dougherty, W.G. The nucleotide sequence of the coding region of tobacco etch virus genomic RNA: Evidence for the synthesis of a single polyprotein. Virology 1986, 154, 9–20. [Google Scholar] [CrossRef]
- Velasquez, N.; Hossain, M.J.; Murphy, J.F. Differential disease symptoms and full-length genome sequence analysis for three strains of Tobacco etch virus. Virus Genes 2015, 50, 442–449. [Google Scholar] [CrossRef]
- Murphy, J.F.; Morawo, T. Comparative evaluation of disease induced by three strains of Tobacco etch virus in Capsicum annuum L. Plant Dis. 2017, 101, 217–223. [Google Scholar] [CrossRef]
- Qin, C.; Yu, C.; Shen, Y.; Fang, X.; Chen, L.; Min, J.; Cheng, J.; Zhao, S.; Xu, M.; Luo, Y.; et al. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Kang, J.; Gan, Y.; Yu, J.; Calderón-Urrea, A.; Lyu, J.; Zhang, G.; Feng, Z.; Xie, J. Transcriptome analysis of pepper (Capsicum annuum) revealed a role of 24-epibrassinolide in response to chilling. Front. Plant Sci. 2016, 7, 1281. [Google Scholar] [CrossRef]
- Martínez-López, L.A.; Ochoa-Alejo, N.; Martínez, O. Dynamics of the chili pepper transcriptome during fruit development. BMC Genom. 2014, 15, 143. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhao, S.N.; Liu, G.F.; Huang, Z.M.; Cao, Z.M.; Cheng, S.H.; Lin, S.S. Discovery of putative capsaicin biosynthetic genes by RNA-Seq and digital gene expression analysis of pepper. Sci. Rep. 2016, 6, 34121. [Google Scholar] [CrossRef]
- Zanardo, L.G.; de Souza, G.B.; Alves, M.S. Transcriptomics of plant–virus interactions: A review. Theor. Exp. Plant Phys. 2019, 31, 103–125. [Google Scholar] [CrossRef]
- Agudelo-Romero, P.; Carbonell, P.; de al Iglesia, F.; Carrera, J.; Rodrigo, G.; Jaramillo, A.; Perez-Amador, M.A.; Elena, S.F. Changes in the gene expression profile of Arabidopsis thaliana after infection with Tobacco etch virus. Virol. J. 2008, 5, 92. [Google Scholar] [CrossRef]
- Hillung, J.; Cuevas, J.M.; Elena, S.F. Transcript profiling of different Arabidopsis thaliana ecotypes in response to Tobacco etch potyvirus infection. Front. Microbiol. 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Andrianifahanana, M.; Lovins, K.; Dute, R.; Sikora, E.; Murphy, J.F. Pathway for phloem-dependent movement of pepper mottle potyvirus in the stem of Capsicum annuum. Phytopathology 1997, 87, 892–898. [Google Scholar] [CrossRef]
- Guerini, M.N.; Murphy, J.F. Resistance of Capsicum annuum ‘Avelar’ to pepper mottle potyvirus and alleviation of this resistance by co-infection with cucumber mosaic cucumovirus are associated with virus movement. J. Gen. Virol. 1999, 80, 2785–2792. [Google Scholar] [CrossRef]
- Kim, S.; Park, M.; Yeom, S.I.; Kim, Y.M.; Lee, J.M.; Lee, H.A.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.T.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Gen. 2014, 46, 270–278. [Google Scholar] [CrossRef]
- Wu, T.D.; Watanabe, C.K. GMAP: A genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 2005, 21, 1859–1875. [Google Scholar] [CrossRef]
- Miller, N.A.; Kingsmore, S.F.; Farmer, A.; Langley, R.J.; Mudge, J.; Crow, J.A.; Gonzalez, A.J.; Schilkey, F.D.; Kim, R.J.; Van Velkinburgh, J.; et al. Management of high-throughput DNA sequencing projects: Alpheus. J. Comp. Sci. Sys. Biol. 2008, 1, 132. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical, Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Anders, S.; Huber, W. Differential Expression of RNA-Seq Data at the Gene Level–The DESeq Package; European Molecular Biology Laboratory (EMBL): Heidelberg, Germany, 2012. [Google Scholar]
- Shi, X.; Gupta, S.; Lindquist, I.E.; Cameron, C.T.; Mudge, J.; Rashotte, A.M. Transcriptome analysis of cytokinin response in tomato leaves. PLoS ONE 2013, 8, e55090. [Google Scholar]
- Bin, W.S.; Wei, L.K.; Ping, D.W.; Li, Z.; Wei, G.; Bing, L.J.; Gui, P.B.; Jian, W.H.; Feng, C.J. Evaluation of appropriate reference genes for gene expression studies in pepper by quantitative real-time PCR. Mol. Breed. 2012, 30, 1393–1400. [Google Scholar] [CrossRef]
- Shin, R.; Lee, G.J.; Park, C.J.; Kim, T.Y.; You, J.S.; Nam, Y.W.; Paek, K.H. Isolation of pepper mRNAs differentially expressed during the hypersensitive response to tobacco mosaic virus and characterization of a proteinase inhibitor gene. Plant Sci. 2001, 161, 727–737. [Google Scholar] [CrossRef]
- Shin, R.; An, J.M.; Park, C.J.; Kim, Y.J.; Joo, S.; Kim, W.T.; Paek, K.H. Capsicum annuum tobacco mosaic virus-induced clone 1 expression perturbation alters the plant’s response to ethylene and interferes with the redox homeostasis. Plant Physiol. 2004, 135, 561–573. [Google Scholar] [CrossRef]
- Hallmark, H.T.; Rashotte, A.M. Review–Cytokinin Response Factors: Responding to More than Cytokinin. Plant Sci. 2019, 110251. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef]
- Collum, T.D.; Padmanabhan, M.S.; Hsieh, Y.C.; Culver, J.N. Tobacco mosaic virus-directed reprogramming of auxin/indole acetic acid protein transcriptional responses enhances virus phloem loading. Proc. Natl. Acad. Sci. USA 2016, 113, E2740–E2749. [Google Scholar] [CrossRef]
- Góngora-Castillo, E.; Ibarra-Laclette, E.; Trejo-Saavedra, D.L.; Rivera-Bustamante, R.F. Transcriptome analysis of symptomatic and recovered leaves of geminivirus-infected pepper (Capsicum annuum). Virol. J. 2012, 9, 295. [Google Scholar] [CrossRef]
- Zhu, C.; Li, X.; Zheng, J. Transcriptome profiling using Illumina-and SMRT-based RNA-seq of hot pepper for in-depth understanding of genes involved in CMV infection. Gene 2018, 666, 123–133. [Google Scholar] [CrossRef]

| Samples a | Reads Per | % Reads Uniquely | Reads | % Reads Uniquely | Reads |
|---|---|---|---|---|---|
| Sample b | Aligned | Aligned b | Aligned b | Aligned | |
| 7 dpi Mock (1) | 18,974,511 | 16,346,906 | 86.20 | 15,529,610 | 81.80 |
| 7 dpi Mock (2) | 20,301,364 | 17,521,361 | 86.30 | 16,644,069 | 82.00 |
| 7 dpi HAT (1) | 20,919,036 | 17,346,241 | 82.90 | 16,473,194 | 78.70 |
| 7 dpi HAT (2) | 21,866,966 | 18,241,859 | 83.40 | 17,324,729 | 79.20 |
| 7 dpi Mex21 (1) | 21,902,531 | 18,325,050 | 83.70 | 17,366,834 | 79.30 |
| 7 dpi Mex21 (2) | 22,172,613 | 18,505,468 | 83.50 | 17,558,247 | 79.20 |
| 7 dpi N (1) | 19,018,389 | 15,053,277 | 79.20 | 14,224,515 | 74.80 |
| 7 dpi N (2) | 21,561,491 | 17,528,288 | 81.30 | 16,547,524 | 76.70 |
| 14 dpi Mock (1) | 20,439,241 | 17,553,946 | 85.90 | 16,649,649 | 81.50 |
| 14 dpi Mock (2) | 19,268,355 | 16,568,352 | 86.00 | 15,696,565 | 81.50 |
| 14 dpi HAT (1) | 21,163,737 | 17,695,058 | 83.60 | 16,761,885 | 79.20 |
| 14 dpi HAT (2) | 20,397,503 | 17,195,270 | 84.30 | 16,288,450 | 79.90 |
| 14 dpi Mex21 (1) | 19,084,583 | 13,684,022 | 71.70 | 12,955,283 | 67.90 |
| 14 dpi Mex21 (2) | 22,247,287 | 16,450,047 | 73.90 | 15,541,636 | 69.90 |
| 14 dpi N (1) | 23,209,601 | 11,424,127 | 49.20 | 10,768,080 | 46.40 |
| 14 dpi N (2) | 21,062,652 | 12,048,713 | 57.20 | 11,358,940 | 53.90 |
| Samples a | % Reads | % Reads | Samples a | % Reads | % Reads | Samples a | % Reads | % Reads |
|---|---|---|---|---|---|---|---|---|
| Aligned to C. annuum b | Aligned to TEV-HAT b | Aligned to C. annuum b | Aligned to TEV-Mex21 b | Aligned to C. annuum b | Aligned to TEV-N b | |||
| 7 dpi HAT (1) | 82.90 | 3.79 | 7 dpi Mex (1) | 83.70 | 3.08 | 7 dpi N (1) | 79.20 | 4.75 |
| 7 dpi HAT (2) | 83.40 | 3.42 | 7 dpi Mex (2) | 83.50 | 3.16 | 7 dpi N (2) | 81.30 | 3.45 |
| 14 dpi HAT (1) | 83.60 | 2.89 | 14 dpi Mex (1) | 71.70 | 16.67 | 14 dpi N (1) | 49.20 | 15.71 |
| 14 dpi HAT (2) | 84.30 | 2.19 | 14 dpi Mex (2) | 73.90 | 14.28 | 14 dpi N (2) | 57.20 | 11.79 |
| Comparison 7 dpi vs. 14 dpi Buffer-Mock Control a | |||
|---|---|---|---|
| Gene ID b | Gene Description | log2 FC | p adj |
| CA11g18010 | Late blight resistance protein homolog R1B-16-like | 2.16 | 2.32 × 10−6 |
| CA09g10460 | Late blight resistance protein homolog R1B-16-like | 1.85 | 2.62 × 10−7 |
| CA06g15650 | Nematode resistance-like protein | 1.64 | 0.02524 |
| CA10g12800 | Disease resistance protein | 0.93 | 0.0059 |
| CA12g02350 | Disease resistance protein A19 (Fragment) | 0.91 | 0.04575 |
| CA11g05830 | Potyviral capsid protein interacting protein 1 | 0.84 | 8.39 × 10−6 |
| CA07g12630 | NBS-LRR type disease resistance protein | 0.67 | 0.00018 |
| CA06g01130 | NBS-LRR resistance protein-like protein | 0.6 | 0.0273 |
| CA06g19920 | TMV resistance protein N-like | 0.55 | 0.00155 |
| CA02g19570 | Nematode resistance-like protein | 0.5 | 0.02059 |
| CA03g03390 | Late blight resistance protein homolog R1B-23-like | 0.4 | 0.0327 |
| CA02g24750 | Root-knot nematode resistance protein | −0.39 | 0.04397 |
| CA12g20500 | Disease resistance protein | −0.53 | 0.00814 |
| CA04g18210 | Natural resistance-associated macrophage protein | −0.74 | 0.00032 |
| CA09g17400 | Disease resistance protein BS2 | −0.82 | 0.0006 |
| CA06g10690 | Disease resistance response protein 206-like | −0.96 | 0.00966 |
| CA04g17660 | Disease resistance protein RPP13-like | −0.96 | 0.01513 |
| CA06g02450 | NBS-LRR resistance protein-like protein | −1.04 | 2.99 × 10−5 |
| CA09g12200 | Verticillium wilt disease resistance protein | −1.41 | 9.25 × 10−7 |
| Comparison 7 dpi vs. 14 dpi HAT a | |||
| Gene ID b | Gene Description | log2 FC | p adj |
| CA11g18010 | Late blight resistance protein homolog R1B-16-like | 1.84 | 0.0044 |
| CA09g10460 | Late blight resistance protein homolog R1B-16-like | 1.56 | 7.49 × 10−11 |
| CA09g00920 | Disease resistance protein BS2 | 1.17 | 0.00069 |
| CA10g19890 | Disease resistance RPP13-like protein 4-like | 1.07 | 0.04508 |
| CA01g01370 | Grave disease carrier protein, putative | 0.92 | 0.00255 |
| CA10g12800 | Disease resistance protein 1 | 0.7 | 0.0422 |
| CA11g05830 | Potyviral capsid protein interacting protein | 0.63 | 0.00039 |
| CA07g12630 | NBS-LRR type disease resistance protein | 0.48 | 0.00069 |
| CA01g08330 | Blight resistance protein | 0.43 | 0.03268 |
| CA11g02410 | Late blight resistance protein homolog R1C-3-like | 0.3 | 0.0387 |
| CA02g18130 | Tobamovirus multiplication 1 | −0.43 | 0.01041 |
| CA04g20220 | Xenotropic and polytropic retrovirus receptor | −0.47 | 0.00935 |
| CA02g11830 | Nbs-lrr resistance protein | −0.56 | 0.01068 |
| CA09g17400 | Disease resistance protein BS2 | −0.57 | 0.02554 |
| CA01g17390 | Nbs-lrr resistance protein | −0.59 | 0.00276 |
| CA05g00030 | Cc-nbs-lrr resistance protein | −0.69 | 0.01179 |
| CA04g18210 | Natural resistance-associated macrophage protein | −0.78 | 4.73 × 10−5 |
| CA06g01230 | Late blight resistance protein Rpi-blb2 | −0.81 | 0.01808 |
| CA07g01130 | Disease resistance protein BS2 | −0.92 | 0.00587 |
| CA06g03690 | Root-knot nematode resistance protein | −1.38 | 0.02771 |
| CA12g20430 | Disease resistance protein | −1.55 | 0.00506 |
| CA06g03680 | Late blight resistance protein homolog R1B-14-like | −2.2 | 9.98 × 10−5 |
| CA07g01000 | NBS-coding resistance gene analog (Fragment) | −2.23 | 0.01325 |
| CA09g18620 | Nematode resistance-like protein | −3.68 | 0.04036 |
| Comparison 7 dpi vs. 14 dpi Mex21 a | |||
| Gene ID b | Gene Description | log2 FC | p adj |
| CA11g18010 | Late blight resistance protein homolog R1B-16-like | 1.66 | 0.00027 |
| CA09g10460 | Late blight resistance protein homolog R1B-16-like | 1.29 | 3.39 × 10−6 |
| CA06g05010 | Antiviral helicase SKI2-like | 1.23 | 0.02099 |
| CA10g19760 | NBS resistance protein | 0.87 | 0.00401 |
| CA06g12190 | Late blight resistance protein homolog R1C-3-like | 0.86 | 0.02072 |
| CA07g12630 | NBS-LRR type disease resistance protein | 0.62 | 0.00026 |
| CA11g05830 | Potyviral capsid protein interacting protein 1 | 0.54 | 0.01863 |
| CA03g03390 | Late blight resistance protein homolog R1B-23-like | 0.5 | 0.00761 |
| CA11g02410 | Late blight resistance protein homolog R1C-3-like | 0.49 | 0.00338 |
| CA02g24750 | Root-knot nematode resistance protein | −0.53 | 0.00412 |
| CA09g17010 | BED finger-nbs-lrr resistance protein | −0.56 | 0.04580 |
| CA02g11830 | Nbs-lrr resistance protein | −0.57 | 0.04129 |
| CA04g18210 | Natural resistance-associated macrophage protein | −0.71 | 0.00133 |
| CA05g00030 | Cc-nbs-lrr resistance protein | −0.72 | 0.02653 |
| CA05g17790 | Late blight resistance protein homolog R1C-3-like | −0.74 | 0.03569 |
| CA10g19860 | Late blight resistance protein homolog R1B-17-like | −0.78 | 0.01313 |
| CA06g02730 | Root-knot nematode resistance protein | −0.84 | 0.0245 |
| CA04g02910 | Cc-nbs-lrr resistance protein, isoform 1 | −0.84 | 0.04803 |
| CA01g17390 | Nbs-lrr resistance protein | −0.85 | 0.00013 |
| CA11g06220 | Tir-nbs-lrr resistance protein | −1.06 | 0.00748 |
| CA07g00840 | Disease resistance protein BS2 | −1.16 | 0.04134 |
| CA01g32390 | Disease resistance protein At4g27190-like | −1.21 | 0.02812 |
| CA09g17400 | Disease resistance protein BS2 | −1.24 | 5.25 × 10−7 |
| CA06g02450 | NBS-LRR resistance protein-like protein | −1.28 | 0.00087 |
| CA12g20430 | Disease resistance protein | −2.27 | 7.54 × 10−5 |
| CA05g04310 | Late blight resistance protein homolog R1A-10-like | −2.34 | 7.64 × 10−5 |
| CA02g19860 | Disease resistance response protein 206-like | −2.63 | 1.61 × 10−6 |
| CA08g01440 | Late blight resistance protein homolog R1A-10-like | −2.70 | 0.0118 |
| Comparison 7 dpi vs. 14 dpi N a | |||
| Gene ID b | Gene Description | log2 FC | p adj |
| CA09g16190 | Disease resistance protein | 1.64 | 0.02537 |
| CA07g00840 | Disease resistance protein BS2 | 1.62 | 0.00905 |
| CA06g02060 | Late blight resistance protein homolog R1B-14-like | 1.60 | 0.00124 |
| CA06g01750 | Root-knot nematode resistance protein | 1.49 | 0.01234 |
| CA07g00860 | Disease resistance protein BS2 | 1.33 | 0.02024 |
| CA06g15080 | Nematode resistance-like protein | 1.29 | 0.04563 |
| CA04g01190 | Disease resistance protein Cf-2.1-like | 1.14 | 0.02752 |
| CA07g00880 | Resistance protein PSH-RGH6 | 1.13 | 0.00427 |
| CA11g06220 | Tir-nbs-lrr resistance protein | 1.09 | 0.02214 |
| CA09g17400 | Disease resistance protein BS2 | 0.76 | 0.04073 |
| CA04g18210 | Natural resistance-associated macrophage protein | 0.71 | 0.04064 |
| CA10g20630 | Disease resistance protein At4g27220-like isoform X1 | −0.71 | 0.02130 |
| CA07g12630 | NBS-LRR type disease resistance protein | −0.79 | 0.00043 |
| CA10g19760 | NBS resistance protein | −1.07 | 0.00772 |
| CA09g10460 | Late blight resistance protein homolog R1B-16-like | −1.13 | 0.00188 |
| CA11g18690 | Disease resistance RPP13-like protein 1-like isoform X2 | −1.25 | 0.03543 |
| CA12g06200 | Resistance gene-like | −1.40 | 0.00075 |
| CA02g19860 | Disease resistance response protein 206-like | −1.56 | 0.00521 |
| CA11g18010 | Late blight resistance protein homolog R1B-16-like | −2.09 | 0.02585 |
| CA12g16200 | TMV resistance protein N-like | −2.40 | 0.03846 |
| Samples a | TIN1 b | WRKY26 b | FAD b | CaCRF1 b | AIP b | PIP1 b |
|---|---|---|---|---|---|---|
| CA10g03990 | CA02g14640 | CA08g04180 | CA06g25980 | CA04g13560 | Ca02g11250 | |
| N-RNAseq (7–14d) | −2.68 | −2.08 | −13.05 | −3.16 | −2.82 | −5.46 |
| N-qPCR (7–14d) | −2.74 ± 1.93 | −1.47 ± 0.52 | −9.73 ± 5.28 | −2.31 ± 0.97 | −1.74 ± 0.15 | −4.15 ± 3.20 |
| N-qPCR (7d) | 5.27 ± 3.31 | 2.26 ± 0.90 | 11.67 ± 9.15 | 5.43 ± 1.68 | 2.24 ± 0.06 | 11.25 ± 5.43 |
| N-qPCR (14d) | 1.92 ± 0.43 | 1.54 ± 0.06 | 1.20 ± 0.09 | 2.35 ± 0.03 | 1.29 ± 0.25 | 2.71 ± 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, J.F.; Hallmark, H.T.; Ramaraj, T.; Sundararajan, A.; Schilkey, F.; Rashotte, A.M. Three Strains of Tobacco etch virus Distinctly Alter the Transcriptome of Apical Stem Tissue in Capsicum annuum during Infection. Viruses 2021, 13, 741. https://doi.org/10.3390/v13050741
Murphy JF, Hallmark HT, Ramaraj T, Sundararajan A, Schilkey F, Rashotte AM. Three Strains of Tobacco etch virus Distinctly Alter the Transcriptome of Apical Stem Tissue in Capsicum annuum during Infection. Viruses. 2021; 13(5):741. https://doi.org/10.3390/v13050741
Chicago/Turabian StyleMurphy, John F., H. Tucker Hallmark, Thiruvarangan Ramaraj, Anitha Sundararajan, Faye Schilkey, and Aaron M. Rashotte. 2021. "Three Strains of Tobacco etch virus Distinctly Alter the Transcriptome of Apical Stem Tissue in Capsicum annuum during Infection" Viruses 13, no. 5: 741. https://doi.org/10.3390/v13050741
APA StyleMurphy, J. F., Hallmark, H. T., Ramaraj, T., Sundararajan, A., Schilkey, F., & Rashotte, A. M. (2021). Three Strains of Tobacco etch virus Distinctly Alter the Transcriptome of Apical Stem Tissue in Capsicum annuum during Infection. Viruses, 13(5), 741. https://doi.org/10.3390/v13050741






