Cross-Antigenicity between EV71 Sub-Genotypes: Implications for Vaccine Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.1.1. Collection of Serum Samples from Phase-Ⅳ Clinical Trial Participants Vaccinated with EV71 Vaccines
2.1.2. Collection of Serum Samples from Rats Inoculated with Different Sub-Genotypes of EV71
2.1.3. Serum Samples from EV71 Vaccine-Immunized Rats
2.2. Cells and Viruses
2.3. Sequence Analyses
2.4. CPE Assays for the Detection of NTAb against Different EV71 Sub-Genotypes
2.5. Statistical Analysis
3. Results
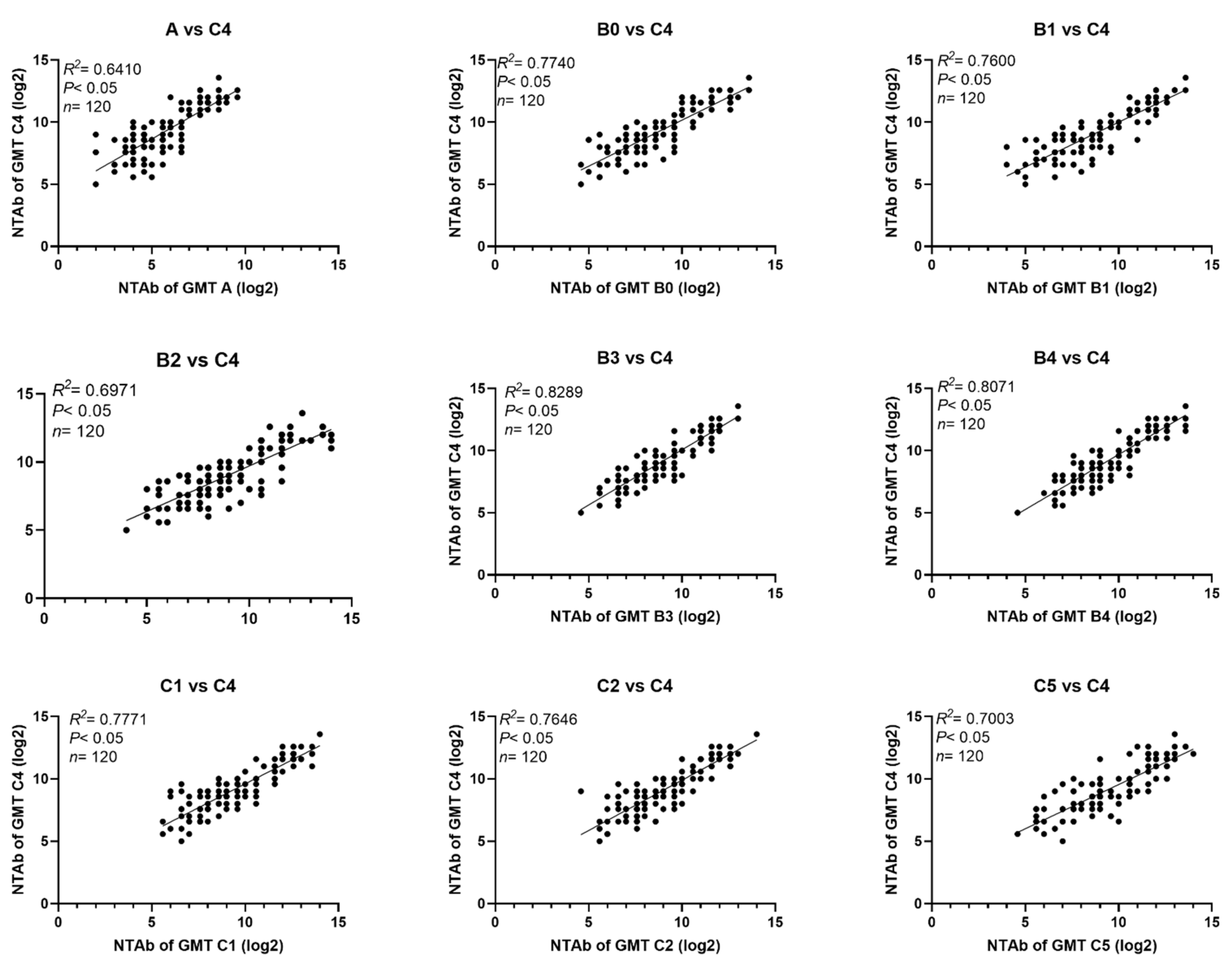
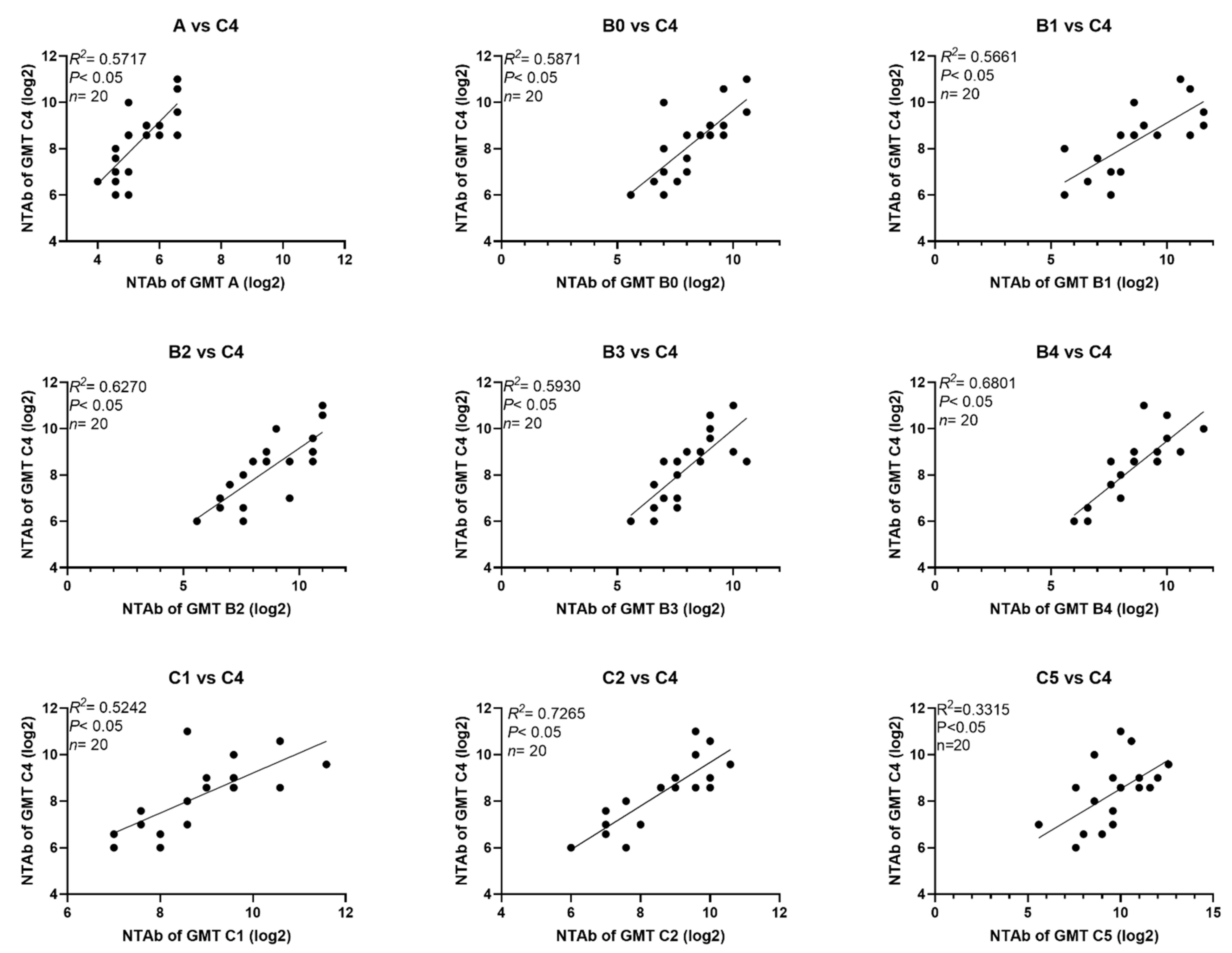
3.1. Cross-Neutralization Activities of Sera from Rats Inoculated with EV71 Virus Strains from 10 Different Genotypes/Sub-Genotypes
3.2. Cross-Neutralizing Activities of Sera from Rats Inoculated with C4 EV71 Inactivated Vaccines
3.3. Cross-Neutralizing Activity of Sera from Infants and Children Immunized with C4 EV71 Inactivated Vaccines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, M.; Chong, P. Is a multivalent hand, foot, and mouth disease vaccine feasible? Hum. Vaccines Immunother. 2015, 11, 2688–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Yip, C.C.; Lau, S.K.; Zhou, B.; Zhang, M.X.; Tsoi, H.W.; Chan, K.H.; Chen, X.C.; Woo, P.C.; Yuen, K.Y. Emergence of enterovirus 71 “double-recombinant” strains belonging to a novel genotype D originating from southern China: First evidence for combination of intratypic and intertypic recombination events in EV71. Arch. Virol. 2010, 155, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Bessaud, M.; Razafindratsimandresy, R.; Nougairède, A.; Joffret, M.L.; Deshpande, J.M.; Dubot-Pérès, A.; Héraud, J.M.; de Lamballerie, X.; Delpeyroux, F.; Bailly, J.L. Molecular comparison and evolutionary analyses of VP1 nucleotide sequences of new African human enterovirus 71 isolates reveal a wide genetic diversity. PLoS ONE 2014, 9, e90624. [Google Scholar] [CrossRef]
- Wong, S.S.; Yip, C.C.; Lau, S.K.; Yuen, K.Y. Human enterovirus 71 and hand, foot and mouth disease. Epidemiol. Infect. 2010, 138, 1071–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, P.; Liu, C.C.; Chow, Y.H.; Chou, A.H.; Klein, M. Review of enterovirus 71 vaccines. Clin. Infect. Dis. 2015, 60, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, N.J.; Lennette, E.H.; Ho, H.H. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 1974, 129, 304–309. [Google Scholar] [CrossRef]
- Wu, Y.; Yeo, A.; Phoon, M.C.; Tan, E.L.; Poh, C.L.; Quak, S.H.; Chow, V.T. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: The role of enterovirus 71 and coxsackievirus A strains. Int. J. Infect. Dis. 2010, 14, e1076–e1081. [Google Scholar] [CrossRef] [Green Version]
- Ang, L.W.; Phoon, M.C.; Wu, Y.; Cutter, J.; James, L.; Chow, V.T. The changing seroepidemiology of enterovirus 71 infection among children and adolescents in Singapore. BMC Infect. Dis. 2011, 11, 270. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Lu, S.; Xian, J.; Ye, J.; Xiao, L.; Luo, J.; Zen, K.; Liu, F. Complete genome sequence of a human enterovirus 71 strain isolated in wuhan, china, in 2010. Genome Announc. 2013, 1, e01112-13. [Google Scholar] [CrossRef] [Green Version]
- Chia, M.Y.; Chiang, P.S.; Chung, W.Y.; Luo, S.T.; Lee, M.S. Epidemiology of enterovirus 71 infections in Taiwan. Pediatr. Neonatol. 2014, 55, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Van Tu, P.; Thao, N.T.T.; Perera, D.; Truong, K.H.; Tien, N.T.K.; Thuong, T.C.; How, O.M.; Cardosa, M.J.; McMinn, P.C. Epidemiologic and virologic investigation of hand, foot, and mouth disease, southern Vietnam, 2005. Emerg. Infect. Dis. 2007, 13, 1733–1741. [Google Scholar] [CrossRef]
- Mizuta, K.; Abiko, C.; Murata, T.; Matsuzaki, Y.; Itagaki, T.; Sanjoh, K.; Sakamoto, M.; Hongo, S.; Murayama, S.; Hayasaka, K. Frequent importation of enterovirus 71 from surrounding countries into the local community of Yamagata, Japan, between 1998 and 2003. J. Clin. Microbiol. 2005, 43, 6171–6175. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H. Enterovirus 71 infection: An experience in Korea, 2009. Korean J. Pediatr. 2010, 53, 616–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, M.; Chen, E.R.; Hsu, K.H.; Twu, S.J.; Chen, K.T.; Tsai, S.F.; Wang, J.R.; Shih, S.R. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N. Engl. J. Med. 1999, 341, 929–935. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission of the People’s Republic of China. Available online: http://www.nhc.gov.cn/jkj/s3578/201802/de926bdb046749abb7b0a8e23d929104.shtml (accessed on 26 February 2018).
- Mao, Q.Y.; Gao, F.; He, P.F.; Liang, Z.L.; Xu, M. Background and key-point for WHO issued recommendations for the quality, safety and efficacy of enterovirus 71 vaccines (inactivated). Chin. J. Biol. 2021, 34, 121–126. [Google Scholar]
- Mao, Q.Y.; Wang, Y.; Bian, L.; Xu, M.; Liang, Z. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Rev. Vaccines 2016, 15, 599–606. [Google Scholar] [CrossRef]
- Zhu, F.C.; Meng, F.Y.; Li, J.X.; Li, X.L.; Mao, Q.Y.; Tao, H.; Zhang, Y.T.; Yao, X.; Chu, K.; Chen, Q.H.; et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013, 381, 2024–2032. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, W.; Xia, J.; Liang, Z.; Liu, Y.; Zhang, X.; Tan, X.; Wang, L.; Mao, Q.; Wu, J.; et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N. Engl. J. Med. 2014, 370, 818–828. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Mo, Z.; Wang, X.; Xia, J.; Liang, Z.; Zhang, Y.; Li, Y.; Mao, Q.; Wang, J.; et al. An inactivated enterovirus 71 vaccine in healthy children. N. Engl. J. Med. 2014, 370, 829–837. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China. National Notifiable Infectious Diseases. Available online: http://www.nhc.gov.cn/jkj/s3578/202103/f1a448b7df7d4760976fea6d55834966.shtml (accessed on 12 March 2021).
- Zhifang, L.; Juanjuan, G.; Qihang, H. Molecular epidemiology and evolution of human enterovirus 71 and hand, foot and mouth disease. Hereditas 2015, 37, 426–435. [Google Scholar]
- Van der Sanden, S.; Koopmans, M.; Uslu, G.; van der Avoort, H.; Dutch Working Group for Clinical Virology. Epidemiology of enterovirus 71 in the Netherlands, 1963 to 2008. J. Clin. Microbiol. 2009, 47, 2826–2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.A.; Oberste, M.S.; Alexander, J.-J.; Kennett, M.L.; Pallansch, M.A. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. J. Virol. 1999, 73, 9969–9975. [Google Scholar] [CrossRef] [Green Version]
- Kapusinszky, B.; Szomor, K.N.; Farkas, A.; Takács, M.; Berencsi, G. Detection of non-polio enteroviruses in Hungary 2000-2008 and molecular epidemiology of enterovirus 71, coxsackievirus A16, and echovirus 30. Virus Genes 2010, 40, 163–173. [Google Scholar] [CrossRef]
- Hosoya, M.; Kawasaki, Y.; Sato, M.; Honzumi, K.; Kato, A.; Hiroshima, T.; Ishiko, H.; Suzuki, H. Genetic diversity of enterovirus 71 associated with hand, foot and mouth disease epidemics in Japan from 1983 to 2003. Pediatr. Infect. Dis. J. 2006, 25, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Utama, A.; Onnimala, N.; Li, C.; Li-Bi, Z.; Yu-Jie, M.; Pongsuwanna, Y.; Miyamura, T. Molecular epidemiology of enterovirus 71 infection in the Western Pacific Region. Pediatr. Int. 2004, 46, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.A.; Herrero, L.J.; McPhie, K.; Chow, S.S.; Craig, M.E.; Dwyer, D.E.; Rawlinson, W.; McMinn, P.C. Molecular epidemiology of enterovirus 71 over two decades in an Australian urban community. Arch. Virol. 2006, 151, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.F.; Wee, K.L.; Chiam, C.W.; Khor, C.S.; Chan, S.Y.; Amalina, W.M.Z.; Sam, I.C. Comparative genetic analysis of VP4, VP1 and 3D gene regions of enterovirus 71 and coxsackievirus A16 circulating in Malaysia between 1997–2008. Trop. Biomed. 2012, 29, 451–466. [Google Scholar] [PubMed]
- Zhu, J.; Luo, Z.; Wang, J.; Xu, Z.; Chen, H.; Fan, D.; Gao, N.; Ping, G.; Zhou, Z.; Zhang, Y.; et al. Phylogenetic analysis of Enterovirus 71 circulating in Beijing, China from 2007 to 2009. PLoS ONE 2013, 8, e56318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, X.J.; Wang, H.Y.; Yan, D.M.; Zhu, S.L.; Wang, D.Y.; Ji, F.; Wang, X.J.; Gao, Y.J.; Chen, L.; et al. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J. Clin. Virol. 2009, 44, 262–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Z.; Yang, W.; Ren, J.; Tan, X.; Wang, Y.; Mao, N.; Xu, S.; Zhu, S.; Cui, A.; et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol. J. 2010, 7, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardosa, M.J.; Perera, D.; Brown, B.A.; Cheon, D.; Chan, H.M.; Chan, K.P.; Cho, H.; McMinn, P. Molecular epidemiology of human enterovirus 71 strains and recent outbreaks in the Asia-Pacific region: Comparative analysis of the VP1 and VP4 genes. Emerg. Infect. Dis. 2003, 9, 461–468. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; He, D.; Cheng, T.; Ge, S.; Shih, J.W.; Zhao, Q.; Chen, P.J.; Zhang, J.; Xia, N. Antigenic analysis of divergent genotypes human Enterovirus 71 viruses by a panel of neutralizing monoclonal antibodies: Current genotyping of EV71 does not reflect their antigenicity. Vaccine 2013, 31, 425–430. [Google Scholar] [CrossRef]
- Mao, Q.Y.; Guo, Z.B.; Hao, C.S.; Yu, D.; Gao, F.; Xie, Z.P.; Li, X.L.; Liang, Z.L. Screening and preparation of candidate standard strain for determination of neutralizing antibody against enterovirus 71. Chin. J. Biol. 2012, 25, 725–729. [Google Scholar]
- Mizuta, K.; Aoki, Y.; Suto, A.; Ootani, K.; Katsushima, N.; Itagaki, T.; Ohmi, A.; Okamoto, M.; Nishimura, H.; Matsuzaki, Y.; et al. Cross-antigenicity among EV71 strains from different genogroups isolated in Yamagata, Japan, between 1990 and 2007. Vaccine 2009, 27, 3153–3158. [Google Scholar] [CrossRef]
- Van der Sanden, S.; van der Avoort, H.; Lemey, P.; Uslu, G.; Koopmans, M. Evolutionary trajectory of the VP1 gene of human enterovirus 71 genogroup B and C viruses. J. Gen. Virol. 2010, 91, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Chia, M.Y.; Chung, W.Y.; Chiang, P.S.; Chien, Y.S.; Ho, M.S.; Lee, M.S. Monitoring antigenic variations of enterovirus 71: Implications for virus surveillance and vaccine development. PLoS Negl. Trop. Dis. 2014, 8, e3044. [Google Scholar] [CrossRef] [PubMed]
- Salk, J.E. Considerations in the preparation and use of poliomyelitis virus vaccine. JAMA 1984, 158, 1239–1248. [Google Scholar] [CrossRef]
- Krugman, S.; Warren, J.; Eiger, M.S.; Berman, P.H.; Michaels, R.M.; Sabin, A.B. Immunization with live attenuated poliovirus vaccine. Am. J. Dis. Child. 1961, 101, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Innis, B.L.; Snitbhan, R.; Kunasol, P.; Laorakpongse, T.; Poopatanakool, W.; Kozik, C.A.; Suntayakorn, S.; Suknuntapong, T.; Safary, A.; Tang, D.B. Protection against hepatitis A by an inactivated vaccine. JAMA 1994, 271, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.H.; Liu, C.C.; Chang, J.Y.; Jiang, R.; Hsieh, Y.C.; Tsao, A.; Wu, C.L.; Huang, J.L.; Fung, C.P.; Hsieh, S.M.; et al. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody against subgenotypes B1, B4, B5 and C4A in adult volunteers. PLoS ONE 2013, 8, e79783. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Cheng, T.; Zhu, F.; Li, J.; Wang, Y.; Li, Y.; Gao, F.; Yang, L.; Yao, X.; Shao, J.; et al. The cross-neutralizing activity of enterovirus 71 subgenotype c4 vaccines in healthy chinese infants and children. PLoS ONE 2013, 8, e79599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sub-Genotype | Name | Year | Country | Genbank No. | Mean of Virus Titer (Lg CCID/mL) |
|---|---|---|---|---|---|
| A | BrCr/A | 1970 | USA | U22521.1 | 7.69 |
| B0 | 66-10857/B0 | 1966 | Netherlands | AB524084 | 9.53 |
| B1 | 71-17000/B1 | 1971 | Netherlands | AB524110 | 9.13 |
| B2 | 86-11316/B2 | 1986 | Netherlands | AB524132 | 8.84 |
| B3 | MAL-97-B3/B3 | 1997 | Malaysia | JN874550 | 8.53 |
| B4 | JPN-97-B4/B4 | 1997 | Japan | LC375765 | 8.84 |
| C1 | 91-480/C1 | 1991 | Netherlands | AB524200 | 9.5 |
| C2 | 36-92/C2 | 2007 | Netherlands | AB524268 | 9.25 |
| C4 | 523-07T/C4 | China | EU753398.2 | 8.88 | |
| C5 | E200525-TW/C5 | 2006 | Japan | / | 8.72 |
| Sub-Genotype | Serum Sample | GMTs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-A | Anti-B0 | Anti-B1 | Anti-B2 | Anti-B3 | Anti-B4 | Anti-C1 | Anti-C2 | Anti-C4 | Anti-C5 | ||
| A | 64 | 4 | 96 | 16 | 4 | 48 | 4 | 4 | 12 | 24 | 14.3 * |
| B0 | 64 | 128 | 384 | 512 | 8 | 24 | 24 | 24 | 48 | 1024 | 78.4 ** |
| B1 | 384 | 32 | 512 | 384 | 16 | 32 | 16 | 16 | 48 | 96 | 65.5 *** |
| B2 | 1536 | 24 | 128 | 1536 | 96 | 32 | 16 | 48 | 48 | 512 | 115.4 |
| B3 | 8192 | 96 | 512 | 1024 | 192 | 24 | 16 | 32 | 256 | 128 | 178 |
| B4 | 16,384 | 256 | 768 | 6144 | 384 | 8 | 48 | 64 | 256 | 192 | 313.5 # |
| C1 | 256 | 48 | 1536 | 768 | 128 | 24 | 24 | 64 | 64 | 1024 | 146.3 |
| C2 | 128 | 64 | 1536 | 1024 | 48 | 32 | 24 | 96 | 96 | 1024 | 146.3 |
| C4 | 1024 | 48 | 192 | 768 | 48 | 32 | 12 | 24 | 192 | 256 | 104.7 |
| C5 | 192 | 32 | 1024 | 512 | 64 | 32 | 16 | 32 | 64 | 1024 | 108.3 ## |
| MAX/MIN (with A) | 256 | 64 | 16 | 384 | 96 | 6 | 12 | 24 | 21.3 | 42.7 | 22 |
| MAX/MIN (without A) | 256 | 10.7 | 12 | 16 | 48 | 4 | 4 | 6 | 5.3 | 10.7 | 4.8 |
| Sub-Genotypes | Vaccines | GMTs | ||
|---|---|---|---|---|
| A | B | C | ||
| A | 37.4 (13.8–101.0) | 172.9 (36.8–811.8) | 805.7 (486.3–1334.9) | 173.4 * |
| B0 | 176.0 (34.4–899.2) | 1627.0 (199.2–13,288.9) | 2390.9 (829.4–6892.1) | 881.4 |
| B1 | 156.8 (41.0–598.8) | 1204.3 (144.4–10,043.6) | 2390.9 (943.0–6061.8) | 767.2 |
| B2 | 237.2 (38.9–1446.3) | 1137.0 (97.4–13,269.4) | 3478.3 (962.3–12,572.4) | 978.9 |
| B3 | 284.9 (76.0–1068.3) | 1137.0 (132.0–9794.6) | 717.8 (324.0–1590.3) | 614.9 |
| B4 | 395.0 (93.3–1672.3) | 1137.0 (132.0–9794.6) | 1315.7 (513.2–3373.4) | 839.1 |
| C1 | 627.1 (138.2–2846.2) | 2273.9 (332.4–15,553.6) | 2088.6 (906.0–4815.0) | 1438.8 ** |
| C2 | 313.5 (50.4–1950.9) | 1416.4 (132.7–15,121.1) | 1690.6 (681.9–4191.3) | 908.8 |
| C4 | 88.0 (22.5–344.4) | 841.6 (60.5–11,708.5) | 1219.1 (528.9–2809.9) | 448.6 *** |
| C5 | 522.2 (98.9–2756.8) | 5325.2 (380.0–74,634.8) | 3649.1 (1243.7–10,707.0) | 2165 |
| Max/Min (with A) | 16.8 | 30.8 | 5.1 | - |
| Max/Min (without A) | 7.1 | 6.3 | 5.1 | - |
| Sub-Genotype | Neutralizing Antibody Titer Induced by | Naturally Infected Serum | |||||
|---|---|---|---|---|---|---|---|
| Vaccine A | Vaccine B | Vaccine C | |||||
| Prevaccination Sero-Negative | Prevaccination Sero-Positive | Prevaccination Sero-Negative | Prevaccination Sero-Positive | Prevaccination Sero-Negative | Prevaccination Sero-Positive | ||
| A | 15.3 (11.1–21.1) | 113.1 (88.0–145.4) | 32.0 (24.0–42.8) | 183.8 (122.8–275.1) | 42.8 (33.8–54.1) | 205.1 (152.2–276.5) | 40.8 (31.9–52.2) |
| B0 | 118.5 (82.5–170.2) | 1306.0 (1005.0–1697.2) | 199.8 (146.5–272.5) | 2268.0 (1257.1–4092.0) | 328.4 (237.4–454.4) | 2681.3 (1890.9–3802.0) | 318.1 (210.3–481.2) |
| B1 | 120.5 (78.8–184.4) | 1379.8 (1081.7–2219.7) | 235.6 (158.9–349.4) | 2544.6 (1561.5–4146.7) | 432.0 (322.3–579.0) | 3151.3 (2416.3–4109.9) | 382.7 (217.1–674.8) |
| B2 | 170.0 (100.1–288.8) | 956.1 (748.2–1221.8) | 292.5 (202.3–423.0) | 3044.0 (1611.7–5749.0) | 549.1 (352.3–855.9) | 3715.8 (2515.2–5489.6) | 427.2 (258.2–706.9) |
| B3 | 195.3 (135.3–281.8) | 701.7 (523.2–941.2) | 242.5 (164.4–357.7) | 1862.8 (1219.1–2846.3) | 352.0 (236.0–525.0) | 2923.0 (2232.3–3827.4) | 256.2 (171.6–382.5) |
| B4 | 237.6 (164.9–342.4) | 772.0 (586.4–1016.4) | 355.0 (251.5–501.1) | 2388.2 (1480.2–3853.4) | 475.2 (323.4–698.4) | 3747.5 (2646.3–5307.1) | 369.7 (235.6–580.2) |
| C1 | 227.6 (167.7–308.8) | 1091.8 (816.4–1460.1) | 331.2 (214.4–511.8) | 3589.3 (2233.1–5769.0) | 485.0 (322.5–729.3) | 4874.3 (3646.6–6515.3) | 487.8 (337.7–704.6) |
| C2 | 179.6 (121.2–266.2) | 1287.4 (965.2–1717.1) | 292.5 (196.4–435.7) | 2923.0 (1716.7–4977.0) | 422.2 (314.9–566.0) | 2799.5 (2166.1–3618.2) | 370.7 (249.1–551.6) |
| C4 | 296.8 (213.3–413.0) | 1513.0 (1110.0–2062.4) | 284.2 (201.1–401.7) | 2354.1 (1377.5–4023.2) | 304.6 (212.1–437.6) | 2743.4 (2116.3–3556.2) | 313.5 (202.5–485.5) |
| C5 | 400.7 (265.3–605.2) | 1688.7 (1251.2–2279.3) | 290.1 (171.7–490.1) | 3000.5 (1559.4–5773.2) | 391.6 (246.8–621.3) | 4613.8 (3773.4–5641.3) | 724.6 (428.0–1226.7) |
| MAX/ MIN (with A) | 26.2 | 14.9 | 11.1 | 19.5 | 12.8 | 23.8 | 17.8 |
| MAX/MIN (without A) | 3.4 | 2.4 | 1.8 | 1.9 | 1.8 | 1.8 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Yuan, Y.; Cui, B.; Huo, Y.; Bian, L.; Chen, L.; Liu, S.; Wang, C.; Xu, Y.; Tedcastle, A.; et al. Cross-Antigenicity between EV71 Sub-Genotypes: Implications for Vaccine Efficacy. Viruses 2021, 13, 720. https://doi.org/10.3390/v13050720
Liu P, Yuan Y, Cui B, Huo Y, Bian L, Chen L, Liu S, Wang C, Xu Y, Tedcastle A, et al. Cross-Antigenicity between EV71 Sub-Genotypes: Implications for Vaccine Efficacy. Viruses. 2021; 13(5):720. https://doi.org/10.3390/v13050720
Chicago/Turabian StyleLiu, Pei, Yadi Yuan, Bopei Cui, Yaqian Huo, Lianlian Bian, Lei Chen, Siyuan Liu, Chenfei Wang, Yingzhi Xu, Alison Tedcastle, and et al. 2021. "Cross-Antigenicity between EV71 Sub-Genotypes: Implications for Vaccine Efficacy" Viruses 13, no. 5: 720. https://doi.org/10.3390/v13050720
APA StyleLiu, P., Yuan, Y., Cui, B., Huo, Y., Bian, L., Chen, L., Liu, S., Wang, C., Xu, Y., Tedcastle, A., Gao, F., Mao, Q., Martin, J., & Liang, Z. (2021). Cross-Antigenicity between EV71 Sub-Genotypes: Implications for Vaccine Efficacy. Viruses, 13(5), 720. https://doi.org/10.3390/v13050720






