The Novel PKC Activator 10-Methyl-Aplog-1 Combined with JQ1 Induced Strong and Synergistic HIV Reactivation with Tolerable Global T Cell Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
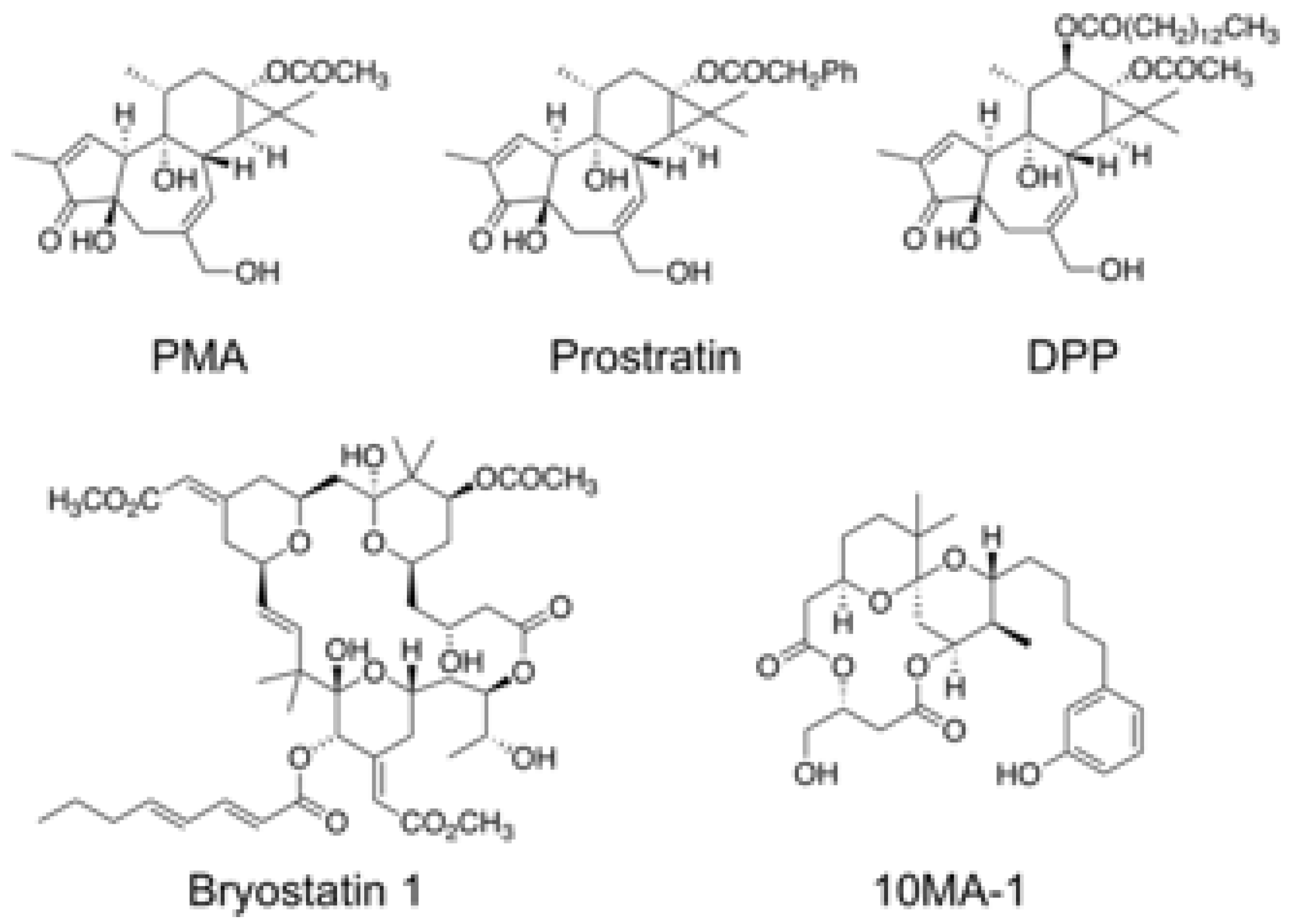
2.2. Reagents
2.3. Evaluation of HIV-1 Reactivation by LRAs
2.4. Cytotoxicity Assay
2.5. Statistical Analysis
2.6. Preparation of Peripheral Blood Mononuclear Cells (PBMCs)
2.7. Evaluation of PBMCs Activation by LRAs
3. Results
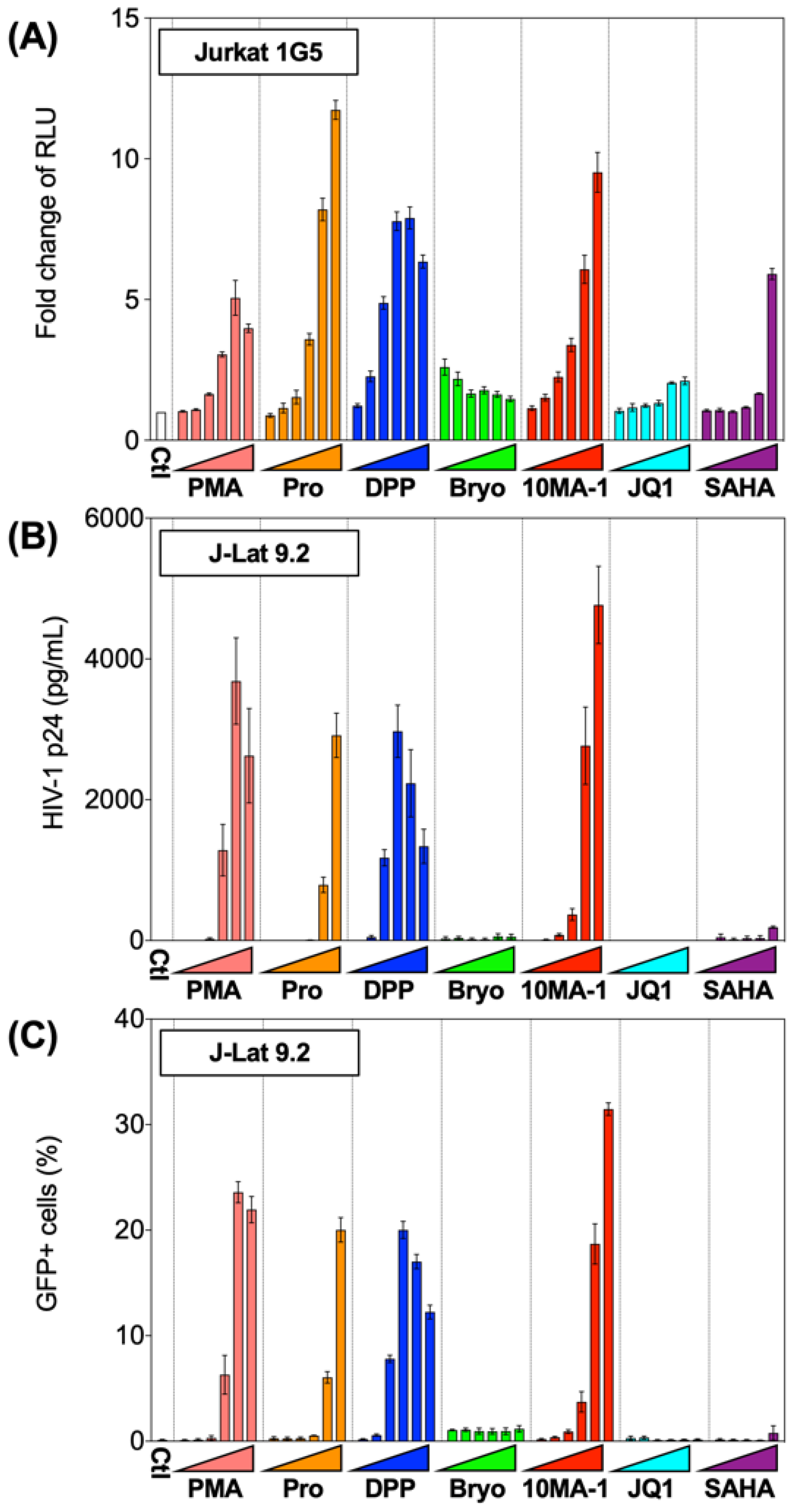
3.1. The Effect of 10MA-1 on HIV Reactivation in HIV Latency Model Cell Lines
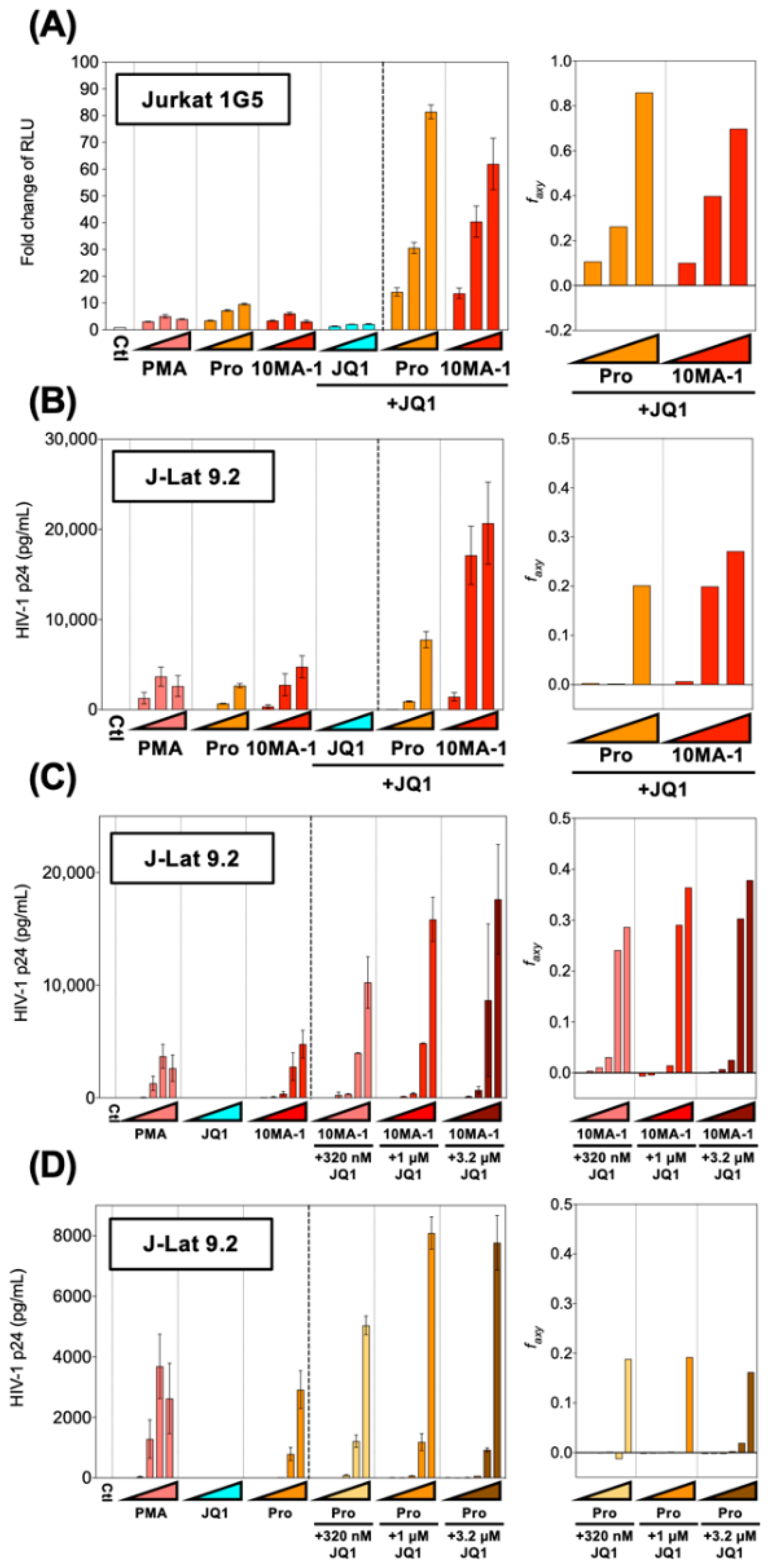
3.2. The Combined Treatment of 10MA-1 with JQ1 Synergistically Reactivated Latent HIV
3.3. The Cytotoxicity of LRAs
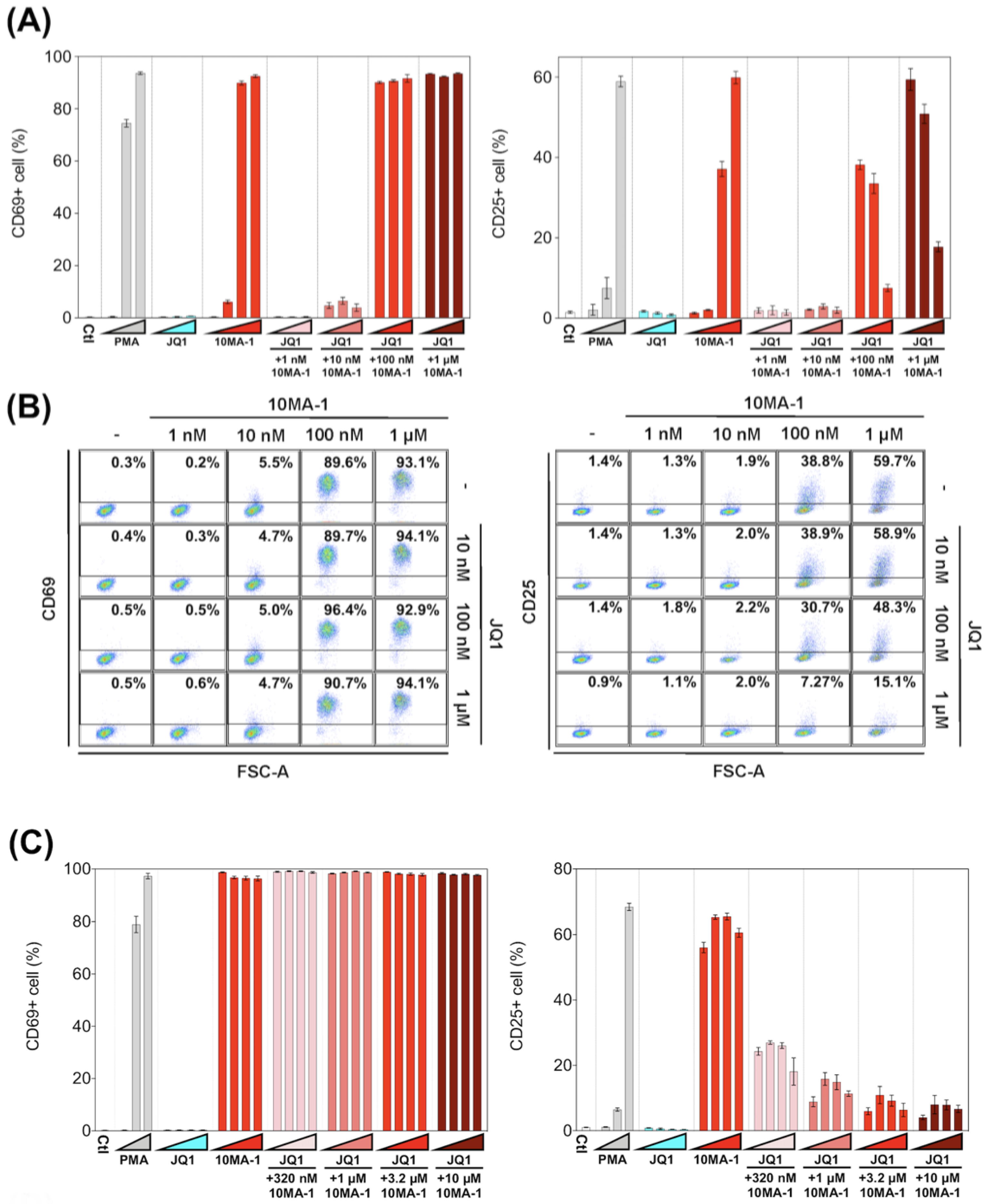
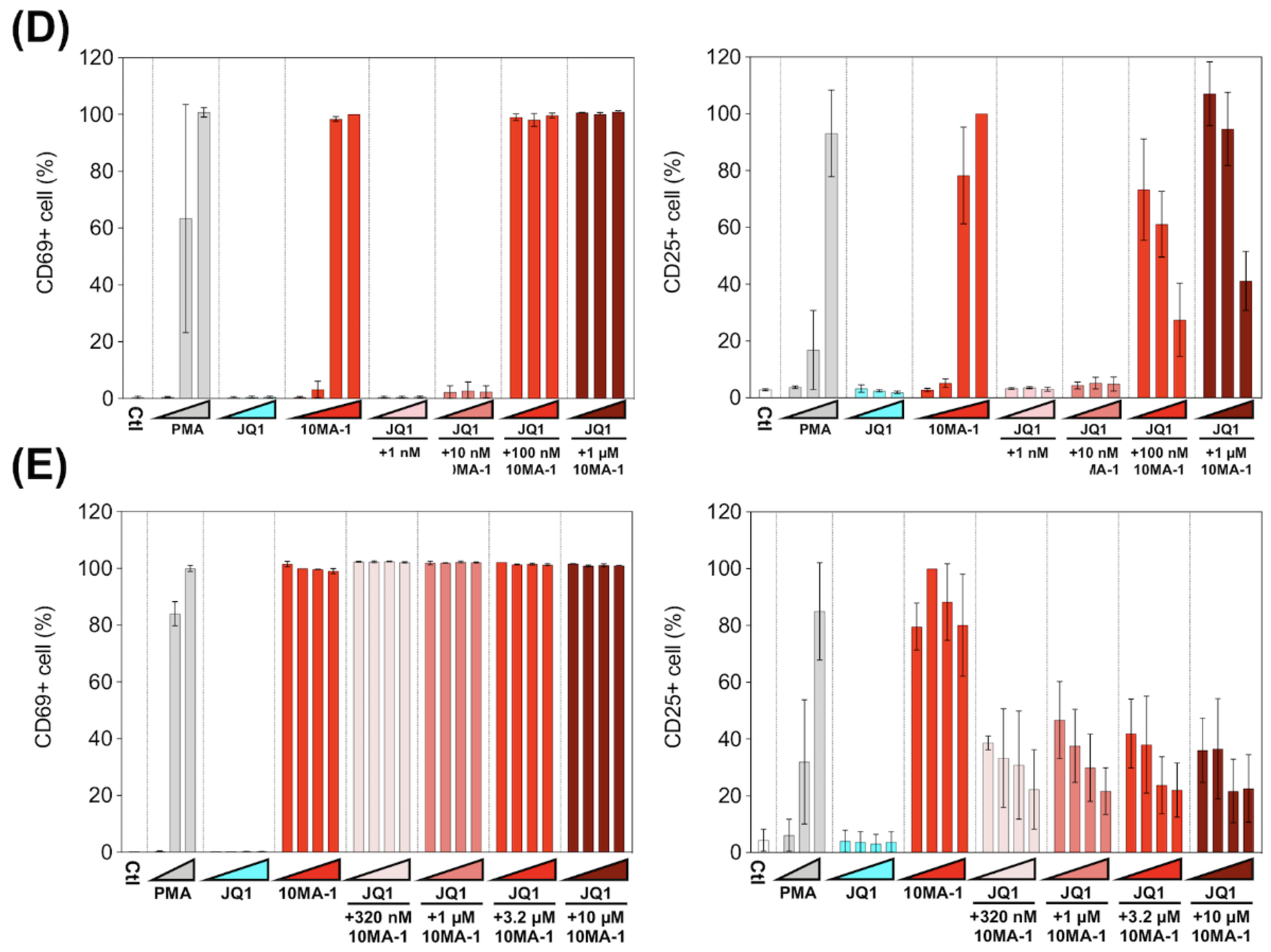
3.4. The Combined Treatment of 10MA-1 with JQ1 Efficiently Suppressed Global T Cell Activation
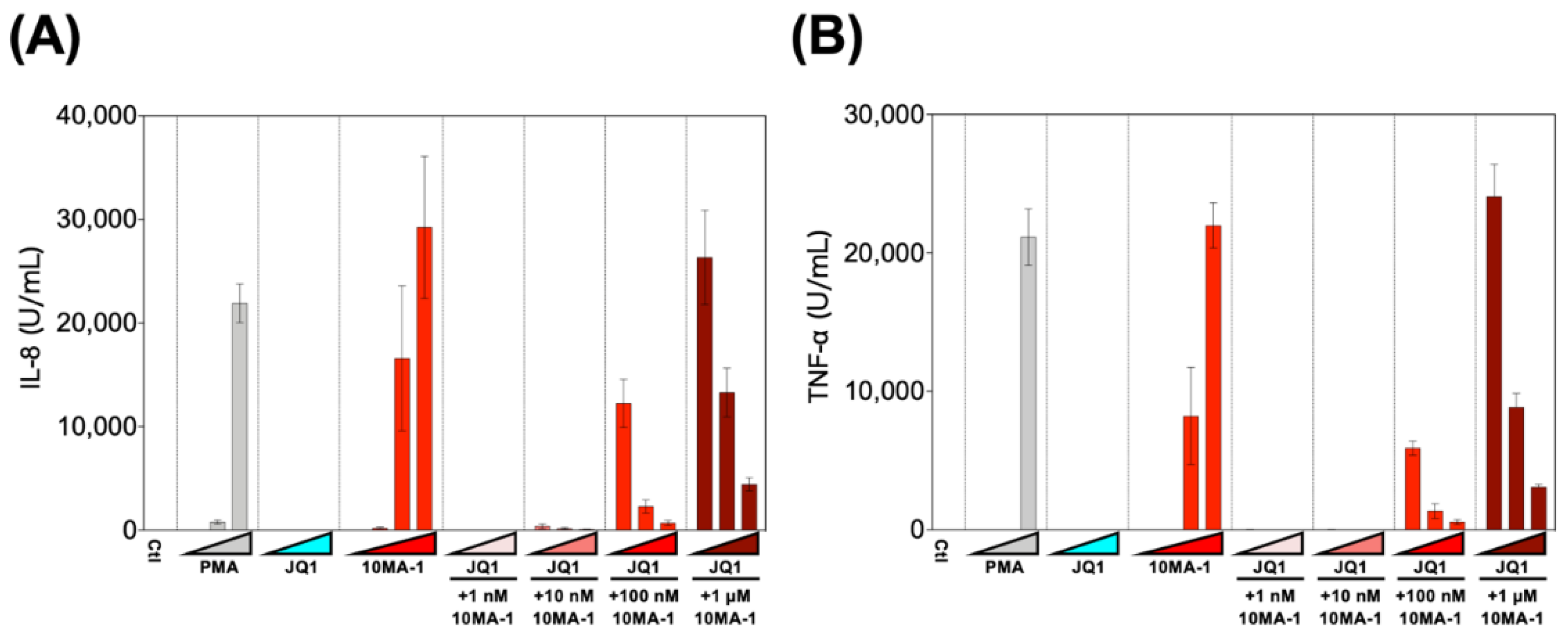
3.5. Combined Treatment of 10MA-1 with JQ1 Efficiently Reduced Pro-Inflammatory Cytokine Production
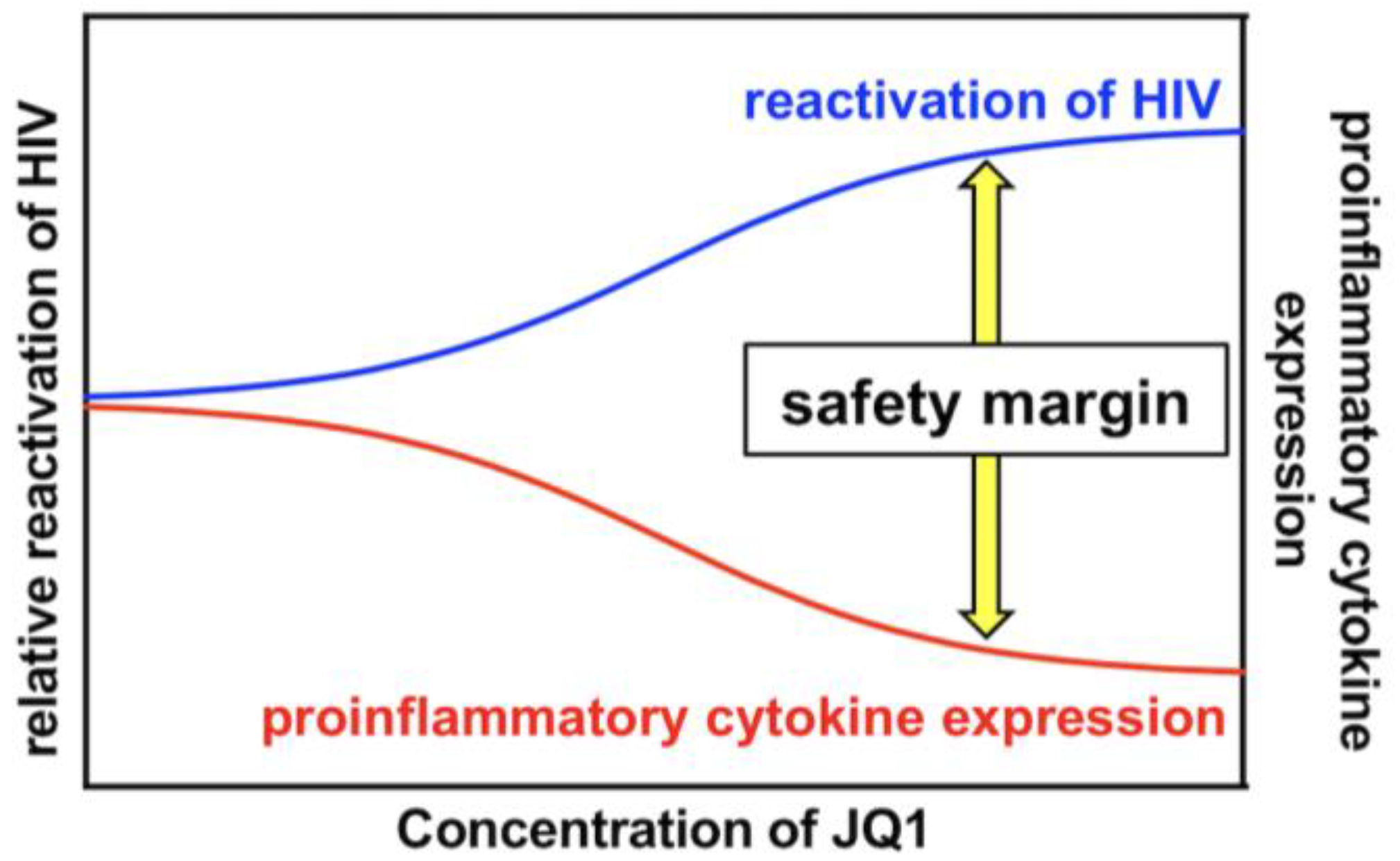
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deeks, S.G. Shock and kill. Nature 2012, 487, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.M.; Garcia, V.J.; Hazuda, D.J.; Haynes, B.F. Latency reversal and viral clearance to cure HIV-1. Science 2016, 353, aaf6517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “kill” into “shock and kill”: Strategies to eliminate latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abner, E.; Jordan, A. HIV “shock and sill” therapy: In need of revision. Antivir. Res. 2019, 166, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Mbonye, U.; Karn, J. The molecular basis for human immunodeficiency virus latency. Annu. Rev. Virol. 2017, 4, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, K.; Rezaei, N.; Nasi, M.; Cossarizza, A. The role of latency reversal agents in the cure of HIV: A review of current data. Immunol. Lett. 2018, 196, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, J.; Wu, Y.; Zhou, Q. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Res. 2013, 41, 277–287. [Google Scholar] [CrossRef]
- Lu, P.; Qu, X.; Shen, Y.; Jiang, Z.; Wang, P.; Zeng, H.; Ji, H.; Deng, J.; Yang, X.; Li, X.; et al. The BET inhibitor OTX015 reactivates latent HIV-1 through P-TEFb. Sci. Rep. 2016, 6, 24100. [Google Scholar] [CrossRef]
- Darcis, G.; Driessche, B.; Lint, C. HIV latency: Should we shock or lock? Trends Immunol. 2017, 38, 217–228. [Google Scholar] [CrossRef]
- Abner, E.; Stoszko, M.; Zeng, L.; Chen, H.-C.; Izquierdo-Bouldstridge, A.; Konuma, T.; Zorita, E.; Fanunza, E.; Zhang, Q.; Mahmoudi, T.; et al. A new quinoline BRD4 inhibitor targets a distinct latent HIV-1 reservoir for reactivation from other “shock” drugs. J. Virol. 2018, 92, e02056-17. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Dandekar, S. Targeting NF-ΚB signaling with protein kinase c agonists as an emerging strategy for combating HIV latency. AIDS Res. Hum. Retrovir. 2015, 31, 4–12. [Google Scholar] [CrossRef]
- Pache, L.; Dutra, M.S.; Spivak, A.M.; Marlett, J.M.; Murry, J.P.; Hwang, Y.; Maestre, A.M.; Manganaro, L.; Vamos, M.; Teriete, P.; et al. BIRC2/CIAP1 is a negative regulator of HIV-1 transcription and can be targeted by smac mimetics to promote reversal of viral latency. Cell Host Microbe 2015, 18, 345–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spivak, A.M.; Planelles, V. Novel latency reversal agents for HIV-1 cure. Annu. Rev. Med. 2018, 69, 421–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, S.; Matsuda, K.; Tsuchiya, K.; Gatanaga, H.; Oka, S.; Yoshimura, K.; Mitsuya, H.; Maeda, K. Combination of a latency-reversing agent with a smac mimetic minimizes secondary HIV-1 infection in vitro. Front. Microbiol. 2018, 9, 2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, C.C.; Mavigner, M.; Sampey, G.C.; Brooks, A.D.; Spagnuolo, R.; Irlbeck, D.M.; Mattingly, C.; Ho, P.T.; Schoof, N.; Cammon, C.G.; et al. Systemic HIV and SIV latency reversal via non-canonical NF-ΚB signalling in vivo. Nature 2020, 578, 160–165. [Google Scholar] [CrossRef]
- Gschwendt, M.; Fürstenberger, G.; Rose-John, S.; Rogers, M.; Kittstein, W.; Pettit, G.R.; Herald, C.L.; Marks, F. Bryostatin 1, an activator of protein kinase c, mimics as well as inhibits biological effects of the phorbol ester TPA in vivo and in vitro. Carcinogenesis 1988, 9, 555–562. [Google Scholar] [CrossRef]
- McKernan, L.N.; Momjian, D.; Kulkosky, J. Protein kinase C: One pathway towards the eradication of latent HIV-1 reservoirs. Adv. Virol. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Spivak, A.M.; Planelles, V. HIV-1 eradication: Early trials (and tribulations). Trends Mol. Med. 2016, 22, 10–27. [Google Scholar] [CrossRef] [Green Version]
- Spina, C.A.; Anderson, J.; Archin, N.M.; Bosque, A.; Chan, J.; Famiglietti, M.; Greene, W.C.; Kashuba, A.; Lewin, S.R.; Margolis, D.M.; et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 2013, 9, e1003834. [Google Scholar] [CrossRef] [Green Version]
- Albert, B.J.; Niu, A.; Ramani, R.; Marshall, G.R.; Wender, P.A.; Williams, R.M.; Ratner, L.; Barnes, A.B.; Kyei, G.B. Combinations of isoform-targeted histone deacetylase inhibitors and bryostatin analogues display remarkable potency to activate latent HIV without global T-cell activation. Sci. Rep. 2017, 7, 7456. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, C.; Serrano-Villar, S.; Madrid-Elena, N.; Pérez-Elías, M.J.; Martín, M.; Barbas, C.; Ruipérez, J.; Muñoz, E.; Muñoz-Fernández, M.; Castor, T.; et al. Bryostatin-1 for latent virus reactivation in HIV-Infected patients on antiretroviral therapy. AIDS 2016, 30, 1385–1392. [Google Scholar] [CrossRef]
- Philip, P.A.; Rea, D.; Thavasu, P.; Carmichael, J.; Stuart, N.S.A.; Rockett, H.; Talbot, D.C.; Ganesan, T.; Pettit, G.R.; Balkwill, F.; et al. Phase I study of bryostatin 1: Assessment of interleukin 6 and tumor necrosis factor α induction in vivo. J. Natl. Cancer Inst. 1993, 85, 1812–1818. [Google Scholar] [CrossRef]
- Zonder, J.; Shields, A.; Zalupski, M.; Chaplen, R.; Heilbrun, L.; Arlauskas, P.; Philip, P. A phase II trial of bryostatin 1 in the treatment of metastatic colorectal cancer. Clin. Cancer Res. 2001, 7, 38–42. [Google Scholar]
- Jayson, G.; Crowther, D.; Prendiville, J.; McGown, A.; Scheid, C.; Stern, P.; Young, R.; Brenchley, P.; Chang, J.; Owens, S.; et al. A phase I trial of bryostatin 1 in patients with advanced malignancy using a 24 h intravenous infusion. Br. J. Cancer 1995, 72, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Margolis, D.M.; Archin, N.M.; Cohen, M.S.; Eron, J.J.; Ferrari, G.; Garcia, J.V.; Gay, C.L.; Goonetilleke, N.; Joseph, S.B.; Swanstrom, R.; et al. Curing HIV: Seeking to target and clear persistent infection. Cell 2020, 181, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Delagrèverie, H.M.; Delaugerre, C.; Lewin, S.R.; Deeks, S.G.; Li, J.Z. Ongoing clinical trials of human immunodeficiency virus latency-reversing and immunomodulatory agents. Open Forum Infect. Dis. 2016, 3, ofw189. [Google Scholar] [CrossRef]
- Denton, P.W.; Søgaard, O.S.; Tolstrup, M. Impacts of HIV cure interventions on viral reservoirs in tissues. Front. Microbiol. 2019, 10, 1956. [Google Scholar] [CrossRef] [PubMed]
- Darcis, G.; Kula, A.; Bouchat, S.; Fujinaga, K.; Corazza, F.; Ait-Ammar, A.; Delacourt, N.; Melard, A.; Kabeya, K.; Vanhulle, C.; et al. An In-depth comparison of latency-reversing agent combinations in various in vitro and ex vivo HIV-1 latency models identified bryostatin-1+JQ1 and ingenol-B+JQ1 to potently reactivate viral gene expression. PLoS Pathog. 2015, 11, e1005063. [Google Scholar] [CrossRef]
- Matsuda, K.; Kobayakawa, T.; Tsuchiya, K.; Hattori, S.; Nomura, W.; Gatanaga, H.; Yoshimura, K.; Oka, S.; Endo, Y.; Tamamura, H.; et al. Benzolactam-related compounds promote apoptosis of HIV-infected human cells via protein kinase c–induced HIV latency reversal. J. Biol. Chem. 2019, 294, 116–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, G.M.; Bullen, K.C.; Rosenbloom, D.; Martin, A.R.; Hill, A.L.; Durand, C.M.; Siliciano, J.D.; Siliciano, R.F. Ex vivo analysis identifies effective HIV-1 latency–reversing drug combinations. J. Clin. Investig. 2015, 125, 1901–1912. [Google Scholar] [CrossRef]
- Kikumori, M.; Yanagita, R.C.; Tokuda, H.; Suzuki, N.; Nagai, H.; Suenaga, K.; Irie, K. Structure-activity studies on the spiroketal moiety of a simplified analogue of debromoaplysiatoxin with antiproliferative activity. J. Med. Chem. 2012, 55, 5614–5626. [Google Scholar] [CrossRef]
- Arcoleo, J.P.; Weinstein, I.B. Activation of protein kinase C by tumor promoting phorbol esters, teleocidin and aplysiatoxin in the absence of added calcium. Carcinogenesis 1985, 6, 213–217. [Google Scholar] [CrossRef]
- Kikumori, M.; Yanagita, R.C.; Irie, K. Improved and large-scale synthesis of 10-methyl-aplog-1, a potential lead for an anticancer drug. Tetrahedron 2014, 70, 9776–9782. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Cordova, E.; Chinen, J.; Donehower, L.; Lewis, D.E.; Belmont, J.W. A sensitive reporter cell line for HIV-1 Tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res. Hum. Retrovir. 1994, 10, 295–301. [Google Scholar] [CrossRef]
- Jordan, A.; Bisgrove, D.; Verdin, E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003, 22, 1868–1877. [Google Scholar] [CrossRef] [Green Version]
- Shibata, R.; Kawamura, M.; Sakai, H.; Hayami, M.; Ishimoto, A.; Adachi, A. Generation of a chimeric human and simian immunodeficiency virus infectious to monkey peripheral blood mononuclear cells. J. Virol. 1991, 65, 3514–3520. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Scheuer, P.J. Aplysiatoxin and debromoaplysiatoxin, constituents of the marine mollusk stylocheilus longicauda. J. Am. Chem. Soc. 1974, 96, 2245–2246. [Google Scholar] [CrossRef]
- Jiang, G.; Mendes, E.A.; Kaiser, P.; Wong, D.P.; Tang, Y.; Cai, I.; Fenton, A.; Melcher, G.P.; Hildreth, J.E.; Thompson, G.R.; et al. Synergistic reactivation of latent HIV expression by ingenol-3-angelate, PEP005, targeted NF-KB signaling in combination with JQ1 induced p-TEFb activation. PLoS Pathog. 2015, 11, e1005066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brogdon, J.; Ziani, W.; Wang, X.; Veazey, R.S.; Xu, H. In vitro effects of the small-molecule protein kinase C agonists on HIV latency reactivation. Sci. Rep. 2016, 6, 39032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajmirza, A.; Emadali, A.; Gauthier, A.; Casasnovas, O.; Gressin, R.; Callanan, M.B. BET family protein BRD4: An emerging actor in NFκB signaling in inflammation and cancer. Biomedicines 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W.B.; Fedorov, O.; Morse, E.M.; Keates, T.; Hickman, T.T.; Felletar, I.; et al. Selective inhibition of BET bromodomains. Nature 2010, 468, 1067–1073. [Google Scholar] [CrossRef] [Green Version]
- Wadhwa, E.; Nicolaides, T. Bromodomain inhibitor review: Bromodomain and extra-terminal family protein inhibitors as a potential new therapy in central nervous system tumors. Cureus 2016, 8, e620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqahtani, A.; Choucair, K.; Ashraf, M.; Hammouda, D.M.; Alloghbi, A.; Khan, T.; Senzer, N.; Nemunaitis, J. Bromodomain and extra-terminal motif inhibitors: A review of preclinical and clinical advances in cancer therapy. Futur Sci. OA 2019, 5, FSO372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiki, H.; Sugimura, T. New classes of tumor promoters: Teleocidin, aplysiatoxin, and palytoxin. Adv. Cancer Res. 1987, 49, 223–264. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Yanagita, R.C.; Hamada, N.; Murakami, A.; Takahashi, H.; Saito, N.; Nagai, H.; Irie, K. A Simple analogue of tumor-promoting aplysiatoxin is an antineoplastic agent rather than a tumor promoter: Development of a synthetically accessible protein kinase C activator with bryostatin-like activity. J. Am. Chem. Soc. 2009, 131, 7573–7579. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Chen, H.; Chang, N.; Xu, Y.; Jiao, J.; Zhang, H. Unlocking the drug potential of the bryostatin family: Recent advances in product synthesis and biomedical applications. Chem. Eur. J. 2020, 26, 1166–1195. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Washizaki, A.; Murata, M.; Seki, Y.; Kikumori, M.; Tang, Y.; Tan, W.; Wardani, N.P.; Irie, K.; Akari, H. The Novel PKC Activator 10-Methyl-Aplog-1 Combined with JQ1 Induced Strong and Synergistic HIV Reactivation with Tolerable Global T Cell Activation. Viruses 2021, 13, 2037. https://doi.org/10.3390/v13102037
Washizaki A, Murata M, Seki Y, Kikumori M, Tang Y, Tan W, Wardani NP, Irie K, Akari H. The Novel PKC Activator 10-Methyl-Aplog-1 Combined with JQ1 Induced Strong and Synergistic HIV Reactivation with Tolerable Global T Cell Activation. Viruses. 2021; 13(10):2037. https://doi.org/10.3390/v13102037
Chicago/Turabian StyleWashizaki, Ayaka, Megumi Murata, Yohei Seki, Masayuki Kikumori, Yinpui Tang, Weikeat Tan, Nadita P. Wardani, Kazuhiro Irie, and Hirofumi Akari. 2021. "The Novel PKC Activator 10-Methyl-Aplog-1 Combined with JQ1 Induced Strong and Synergistic HIV Reactivation with Tolerable Global T Cell Activation" Viruses 13, no. 10: 2037. https://doi.org/10.3390/v13102037
APA StyleWashizaki, A., Murata, M., Seki, Y., Kikumori, M., Tang, Y., Tan, W., Wardani, N. P., Irie, K., & Akari, H. (2021). The Novel PKC Activator 10-Methyl-Aplog-1 Combined with JQ1 Induced Strong and Synergistic HIV Reactivation with Tolerable Global T Cell Activation. Viruses, 13(10), 2037. https://doi.org/10.3390/v13102037






