Pathogenicity of H5N8 High Pathogenicity Avian Influenza Virus in Chickens and Ducks from South Korea in 2020–2021
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Animals and Housing
2.3. Experimental Design
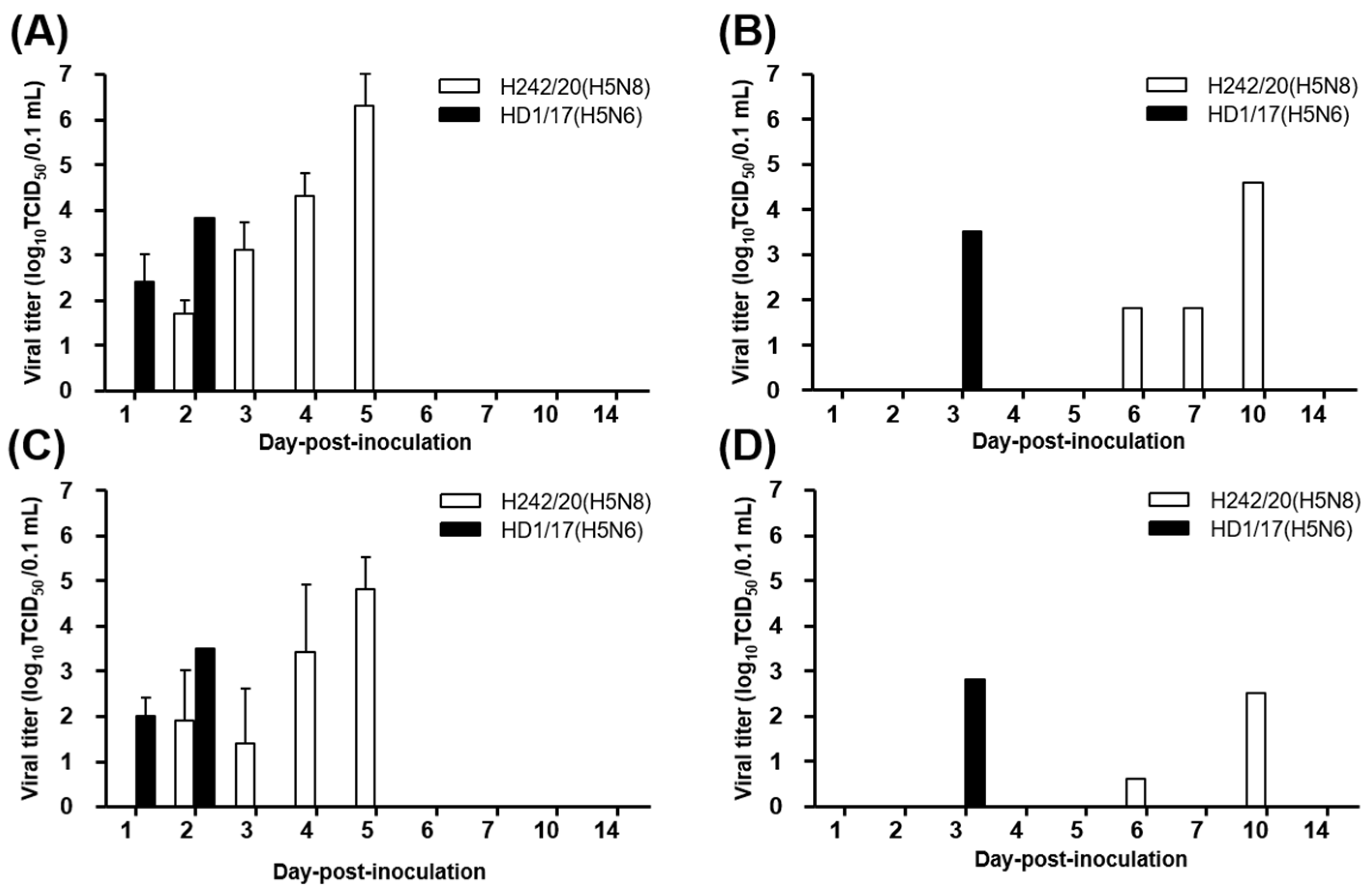
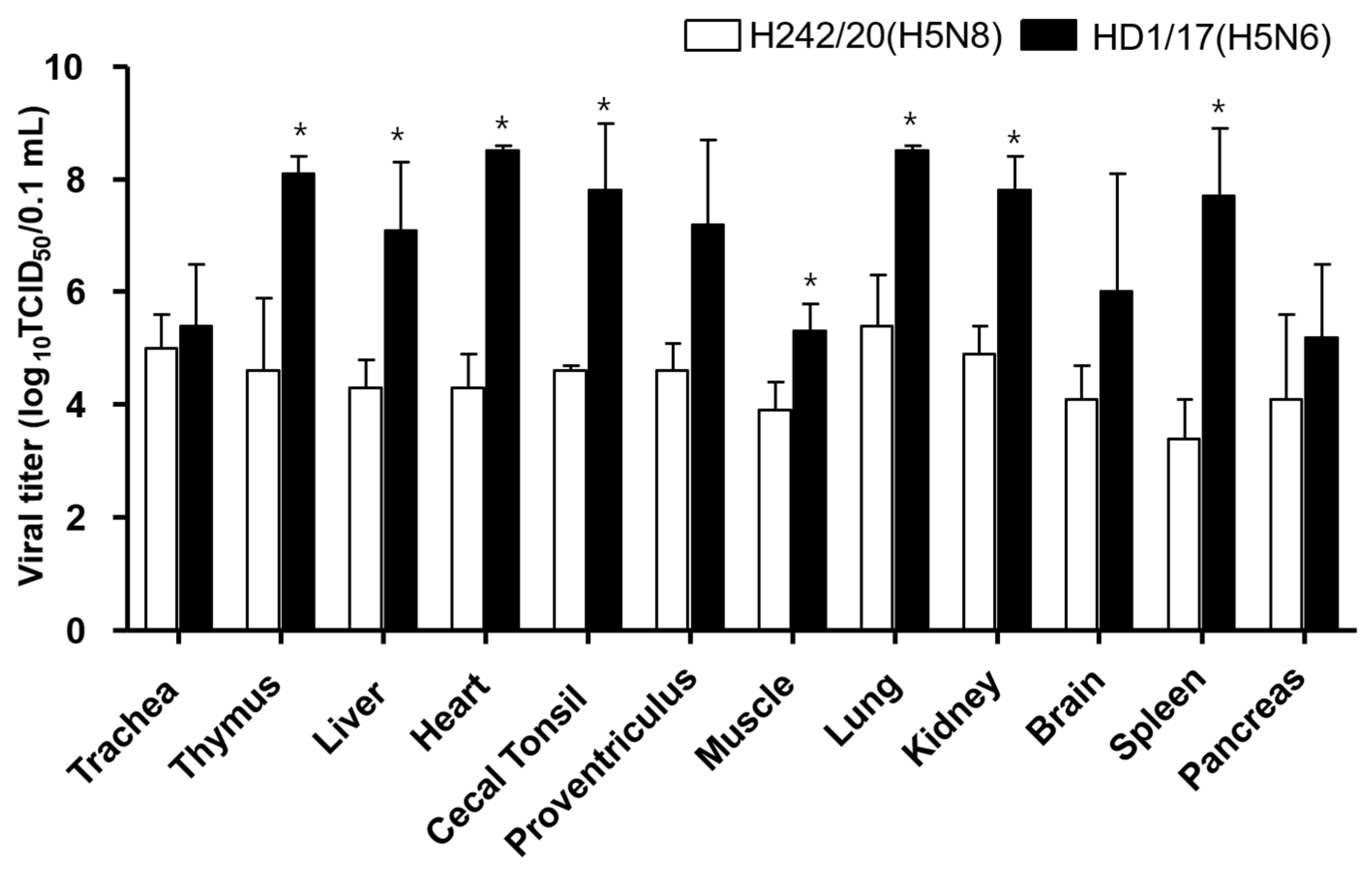
2.4. Viral Shedding and Replication in Internal Organs
2.5. Hemagglutination Inhibition (HI) Test
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Poovorawan, Y.; Pyungporn, S.; Prachayangprecha, S.; Makkoch, J. Global alert to avian influenza virus infection: From H5N1 to H7N9. Pathog. Glob. Health 2013, 107, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-H.; Criado, M.F.; Swayne, D.E. Pathobiological Origins and Evolutionary History of Highly Pathogenic Avian Influenza Viruses. Cold Spring Harb. Perspect. Med. 2021, 11, a038679. [Google Scholar] [CrossRef] [Green Version]
- Song, B.-M.; Lee, E.-K.; Lee, Y.-N.; Heo, G.-B.; Lee, H.-S.; Lee, Y.-J. Phylogeographical characterization of H5N8 viruses isolated from poultry and wild birds during 2014–2016 in South Korea. J. Vet. Sci. 2017, 18, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-H.; Bertran, K.; Kwon, J.-H.; Swayne, D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J. Vet. Sci. 2017, 18, 269–280. [Google Scholar] [CrossRef]
- Lee, E.-K.; Song, B.-M.; Lee, Y.-N.; Heo, G.-B.; Bae, Y.-C.; Joh, S.-J.; Park, S.-C.; Choi, K.-S.; Lee, H.-J.; Jang, I.; et al. Multiple novel H5N6 highly pathogenic avian influenza viruses, South Korea, 2016. Infect. Genet. Evol. 2017, 51, 21–23. [Google Scholar] [CrossRef]
- Park, S.C.; Song, B.M.; Lee, Y.N.; Lee, E.K.; Heo, G.B.; Kye, S.J.; Lee, K.H.; Bae, Y.C.; Lee, Y.J.; Kim, B. Pathogenicity of clade 2.3.4.4 H5N6 highly pathogenic avian influenza virus in three chicken breeds from South Korea in 2016/2017. J. Vet. Sci. 2019, 20, e27. [Google Scholar] [CrossRef]
- Lee, E.-K.; Lee, Y.-N.; Kye, S.-J.; Lewis, N.S.; Brown, I.H.; Sagong, M.; Heo, G.-B.; Kang, Y.-M.; Cho, H.-K.; Kang, H.-M.; et al. Characterization of a novel reassortant H5N6 highly pathogenic avian influenza virus clade 2.3.4.4 in Korea, 2017. Emerg. Microbes Infect. 2018, 7, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, Y.-G.; Lee, Y.-N.; Lee, D.-H.; Cheon, S.-H.; Kye, S.-J.; Park, Y.-R.; Si, Y.-J.; Lee, M.-H.; Lee, Y.-J. A novel reassortant clade 2.3.4.4 highly pathogenic avian influenza H5N6 virus identified in South Korea in 2018. Infect. Genet. Evol. 2020, 78, 104056. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kang, H.-M.; Lee, E.-K.; Song, B.-M.; Kwon, Y.-K.; Kim, H.-R.; Choi, K.-S.; Kim, J.-Y.; Lee, H.-J.; Moon, O.-K.; et al. Highly pathogenic avian influenza virus (H5N8) in domestic poultry and its relationship with migratory birds in South Korea during 2014. Vet. Microbiol. 2014, 173, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-K.; Song, B.-M.; Kang, H.-M.; Woo, S.-H.; Heo, G.-B.; Jung, S.C.; Park, Y.H.; Lee, Y.-J.; Kim, J.-H. Experimental infection of SPF and Korean native chickens with highly pathogenic avian influenza virus (H5N8). Poult. Sci. 2016, 95, 1015–1019. [Google Scholar] [CrossRef]
- Kang, H.-M.; Lee, E.-K.; Song, B.-M.; Jeong, J.; Choi, J.-G.; Jeong, J.; Moon, O.-K.; Yoon, H.; Cho, Y.; Kang, Y.-M.; et al. Novel Reassortant Influenza A(H5N8) Viruses among Inoculated Domestic and Wild Ducks, South Korea, 2014. Emerg. Infect. Dis. 2015, 21, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Song, B.-M.; Kang, H.-M.; Lee, E.-K.; Youn-Jeong, L.; Kang, Y.; Lee, H.-S.; Lee, Y.-J. Pathogenicity of H5N8 virus in chickens from Korea in 2014. J. Vet. Sci. 2015, 16, 237–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, J.; Bahl, J.; Swayne, D.E.; Lee, Y.; Lee, Y.; Song, C.; Lee, D. Domestic ducks play a major role in the maintenance and spread of H5N8 highly pathogenic avian influenza viruses in South Korea. Transbound. Emerg. Dis. 2019, 67, 844–851. [Google Scholar] [CrossRef]
- Jeong, O.-M.; Kim, M.-J.; Kang, H.-M.; Kim, H.-R.; Kim, Y.-J.; Joh, S.-J.; Kwon, J.-H.; Lee, Y.-J. Experimental infection of chickens, ducks and quails with the highly pathogenic H5N1 avian influenza virus. J. Vet. Sci. 2009, 10, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, Y.-G.; Lee, Y.-N.; Lee, D.-H.; Shin, J.-I.; Lee, J.-H.; Chung, D.; Lee, E.-K.; Heo, G.-B.; Sagong, M.; Kye, S.-J.; et al. Multiple Reassortants of H5N8 Clade 2.3.4.4b Highly Pathogenic Avian Influenza Viruses Detected in South Korea during the Winter of 2020–2021. Viruses 2021, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Schulze, C.; Engelhardt, A.; Hlinak, A.; Lennermann, S.-L.; Rigbers, K.; Skuballa, J.; Staubach, C.; Mettenleiter, T.C.; Harder, T.; et al. Novel HPAIV H5N8 Reassortant (Clade 2.3.4.4b) Detected in Germany. Viruses 2020, 12, 281. [Google Scholar] [CrossRef] [Green Version]
- Świętoń, E.; Fusaro, A.; Shittu, I.; Niemczuk, K.; Zecchin, B.; Joannis, T.; Bonfante, F.; Śmietanka, K.; Terregino, C. Sub-Saharan Africa and Eurasia Ancestry of Reassortant Highly Pathogenic Avian Influenza A(H5N8) Virus, Europe, December 2019. Emerg. Infect. Dis. 2020, 26, 1557–1561. [Google Scholar] [CrossRef]
- Lewis, N.S.; Banyard, A.C.; Whittard, E.; Karibayev, T.; Al Kafagi, T.; Chvala, I.; Byrne, A.; Akberovna, S.M.; King, J.; Harder, T.; et al. Emergence and spread of novel H5N8, H5N5 and H5N1 clade 2.3.4.4 highly pathogenic avian influenza in 2020. Emerg. Microbes Infect. 2021, 10, 148–151. [Google Scholar] [CrossRef]
- OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.03.04_AI.pdf (accessed on 20 April 2021).
- Reed, L.J.; Muench, H. A simpe method of estimating fifity percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kwon, J.-H.; Noh, J.-Y.; Park, J.-K.; Yuk, S.-S.; Erdene-Ochir, T.-O.; Lee, J.-B.; Park, S.-Y.; Choi, I.-S.; Lee, S.-W.; et al. Pathogenicity of the Korean H5N8 highly pathogenic avian influenza virus in commercial domestic poultry species. Avian Pathol. 2016, 45, 208–211. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority; European Centre for Disease Prevention and Control and European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Kuiken, T.; Niqueux, É.; Staubach, C.; Terregino, C.; Muñoz Guajardo, I.; Baldinelli, F. Avian influenza overview May–August 2020. EFSA J. 2020, 18, e06270. [Google Scholar]
- Verhagen, J.H.; Fouchier, R.A.; Lewis, N. Highly Pathogenic Avian Influenza Viruses at the Wild–Domestic Bird Interface in Europe: Future Directions for Research and Surveillance. Viruses 2021, 13, 212. [Google Scholar] [CrossRef]
- Sakuma, S.; Uchida, Y.; Kajita, M.; Tanikawa, T.; Mine, J.; Tsunekuni, R.; Saito, T. First Outbreak of an H5N8 Highly Pathogenic Avian Influenza Virus on a Chicken Farm in Japan in 2020. Viruses 2021, 13, 489. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Fujimoto, Y.; Kojima, I.; Esaki, M.; Ri, K.; Masatani, T.; Matsui, T.; Ozawa, M. Genetic Characterization of H5N8 Highly Pathogenic Avian Influenza Viruses Isolated from Falcated Ducks and Environmental Water in Japan in November 2020. Pathogens 2021, 10, 171. [Google Scholar] [CrossRef]
- Isoda, N.; Twabela, A.T.; Bazarragchaa, E.; Ogasawara, K.; Hayashi, H.; Wang, Z.-J.; Kobayashi, D.; Watanabe, Y.; Saito, K.; Kida, H.; et al. Re-Invasion of H5N8 High Pathogenicity Avian Influenza Virus Clade 2.3.4.4b in Hokkaido, Japan, 2020. Viruses 2020, 12, 1439. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.C.; Lee, Y.-J.; Song, B.-M.; Kang, H.-M.; Lee, E.-K.; Hanna, A.; Gilbert, M.; Brown, I.H.; Pybus, O.G. Wild waterfowl migration and domestic duck density shape the epidemiology of highly pathogenic H5N8 influenza in the Republic of Korea. Infect. Genet. Evol. 2015, 34, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Beerens, N.; Germeraad, E.A.; Venema, S.; Verheij, E.; Pritz-Verschuren, S.B.; Gonzales, J.L. Comparative pathogenicity and environmental transmission of recent highly pathogenic avian influenza H5 viruses. Emerg. Microbes Infect. 2021, 10, 97–108. [Google Scholar] [CrossRef] [PubMed]


| Virus | IVPI | cLD50 (EID50) | Virus Dose (EID50/0.1 mL) | Mortality (%) | MDT (Day) | HI Titer a (log2, Mean ± SD) |
|---|---|---|---|---|---|---|
| A/mandarin duck/ Korea/H242/2020 (H5N8) | 2.88 | 104.5 | 106 | 5/5 (100) | 4.3 | NT |
| 105 | 4/5 (80) | 5.2 | -(0/1) | |||
| 104 | 1/4 (1) (25) | 7.4 | -(0/3) | |||
| 103 | 0/5 (0) | - | -(0/5) | |||
| Contact | 1/3 (33) | 10 | -(0/2) | |||
| A/duck/Korea/ HD1/2017 (H5N6) | 2.98 | 103.6 | 106 | 5/5 (100) | 2.2 | NT |
| 105 | 5/5 (100) | 4.8 | NT | |||
| 104 | 4/5 (80) | 5.7 | -(0/1) | |||
| 103 | 0/5 (0) | - | -(0/5) | |||
| Contact | 1/2 (2) (50) | 7 | -(0/1) |
| Virus | Virus Dose (EID50/0.1 mL) | Mortality (%) | HI Titer a (log2, Mean ± SD) | BID50 (EID50) |
|---|---|---|---|---|
| A/mandarin duck/ Korea/H242/2020 (H5N8) | 106 | 0/5 (0) | 3.0 ± 1.4 (4/5) | 105.3 |
| 104 | 0/5 (0) | -(0/5) | ||
| 102 | 0/5 (0) | -(0/5) | ||
| Contact | 0/3 (0) | 2.7 ± 0.6 (3/3) | ||
| A/duck/Korea/ HD1/2017 (H5N6) | 106 | 0/5 (0) | 2.3 ± 1.3 (5/5) | 105.0 |
| 104 | 0/5 (0) | -(0/5) | ||
| 102 | 0/5 (0) | -(0/5) | ||
| Contact | 0/3 (0) | 3.0 ± 1.7 (3/3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.-J.; Cha, R.M.; Kye, S.-J.; Lee, Y.-N.; Kim, N.-Y.; Baek, Y.-G.; Heo, G.-B.; Sagong, M.; Lee, K.-N.; Lee, Y.-J.; et al. Pathogenicity of H5N8 High Pathogenicity Avian Influenza Virus in Chickens and Ducks from South Korea in 2020–2021. Viruses 2021, 13, 1903. https://doi.org/10.3390/v13101903
Park M-J, Cha RM, Kye S-J, Lee Y-N, Kim N-Y, Baek Y-G, Heo G-B, Sagong M, Lee K-N, Lee Y-J, et al. Pathogenicity of H5N8 High Pathogenicity Avian Influenza Virus in Chickens and Ducks from South Korea in 2020–2021. Viruses. 2021; 13(10):1903. https://doi.org/10.3390/v13101903
Chicago/Turabian StylePark, Min-Ji, Ra Mi Cha, Soo-Jeong Kye, Yu-Na Lee, Na-Yeong Kim, Yoon-Gi Baek, Gyeong-Beom Heo, Mingeun Sagong, Kwang-Nyeong Lee, Youn-Jeong Lee, and et al. 2021. "Pathogenicity of H5N8 High Pathogenicity Avian Influenza Virus in Chickens and Ducks from South Korea in 2020–2021" Viruses 13, no. 10: 1903. https://doi.org/10.3390/v13101903
APA StylePark, M.-J., Cha, R. M., Kye, S.-J., Lee, Y.-N., Kim, N.-Y., Baek, Y.-G., Heo, G.-B., Sagong, M., Lee, K.-N., Lee, Y.-J., & Lee, E.-K. (2021). Pathogenicity of H5N8 High Pathogenicity Avian Influenza Virus in Chickens and Ducks from South Korea in 2020–2021. Viruses, 13(10), 1903. https://doi.org/10.3390/v13101903






