Abstract
The global emergence of zoonotic viruses, including poxviruses, poses one of the greatest threats to human and animal health. Forty years after the eradication of smallpox, emerging zoonotic orthopoxviruses, such as monkeypox, cowpox, and vaccinia viruses continue to infect humans as well as wild and domestic animals. Currently, the geographical distribution of poxviruses in a broad range of hosts worldwide raises concerns regarding the possibility of outbreaks or viral dissemination to new geographical regions. Here, we review the global host ranges and current epidemiological understanding of zoonotic orthopoxviruses while focusing on orthopoxviruses with epidemic potential, including monkeypox, cowpox, and vaccinia viruses.
1. Poxvirus and Emerging Diseases
Zoonotic diseases, defined as diseases or infections that are naturally transmissible from vertebrate animals to humans, represent a significant threat to global health [1]. Among the species recognized as pathogenic to humans, more than half originated in animals, and some have been characterized as emerging or re-emerging pathogens [2,3]. Most zoonotic pathogens originated in wild and domesticated mammalian hosts such as bats, rodents, and primates [4]. The analysis of global trends indicates that new zoonotic threats will continue to emerge at an accelerating rate, and are mainly associated with an growthing population, changes in land use, climate changes, increased intercontinental travel, and expanded trade networks [4,5].
Poxviruses are of great veterinary and human importance and infect numerous vertebrate and invertebrate animals, including humans. The Poxviridae family is divided into two subfamilies, namely: Chordopoxvirinae, which infect vertebrates, and Entomopoxvirinae (A–C), which infect invertebrates. The Chordopoxvirinae subfamily is further divided into 18 genera (Avipoxvirus, Capripoxvirus, Centapoxvirus, Cervidpoxvirus, Crocodylidpoxvirus, Leporipoxvirus, Macropopoxvirus, Molluscipoxvirus, Mustelpoxvirus, Orthopoxvirus, Oryzopoxvirus, Parapoxvirus, Pteropopoxvirus, Salmonpoxvirus, Sciuripoxvirus, Suipoxvirus, Vespertilionpoxvirus, and Yatapoxvirus), distinguishable by their serological reactions [6,7].
The family Poxviridae comprises large, brick-shaped or ovoid enveloped viruses containing a linear, double-stranded DNA genome approximately 200 kilobase pairs in length [7,8]. Poxviruses are among mankind’s longest and best-known viruses mainly because of their most feared and lethal representative, Variola virus (VARV), the causative agent of smallpox. Before its remarkable eradication in 1980, VARV represented a centuries-old threat to humans worldwide and killed approximately 300–500 million people during the 20th century [9]. The global eradication of smallpox marked the culmination of an intensive vaccination program and quarantine measures promoted by the World Health Organization (WHO) [8,10,11]. Although VARV was eradicated 40 years ago, many challenges regarding poxvirus infections persist, including the worrisome possibility of VARV reintroduction by accidental release, its use as a biological weapon, or the emergence and re-emergence of zoonotic orthopoxviruses worldwide [12,13].
Orthopoxviruses are remarkable for their wide host spectrum, ranging from humans to domestic and wild animals (Figure 1). Orthopoxvirus is the most important and well-characterized poxvirus genus, mainly due to its impact on human and animal health [7,8]. Here, we review the major aspects related to the dynamics and emergence of zoonotic orthopoxvirus infections worldwide, focusing on the host range and current epidemiological situation relating to monkeypox (MPXV), cowpox (CPXV), vaccinia (VACV), and VACV-like viruses.
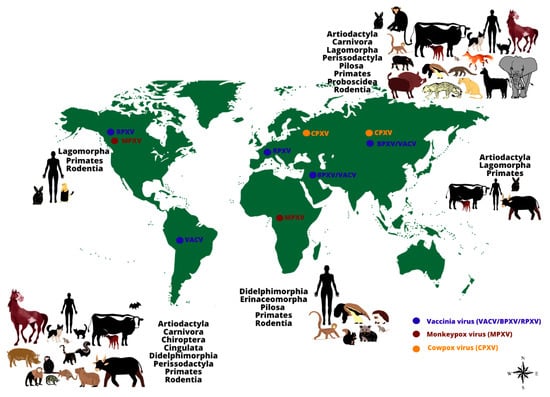
Figure 1.
The worldwide distribution and host range of monkeypox, cowpox and vaccinia viruses.
The image shows the range of animal hosts (represented by orders) that have been demonstrated to be naturally infected by some Orthopoxvirus species, according to different regions of the world (except by Monkeypox virus in the United States of America, represented by imported cases). Orthopoxvirus infections have been demonstrated in animals belonging to different orders, using different methods (virus isolation, molecular detection of viral genomes or serological screening for antibodies against orthopoxviruses). The occurrence of some zoonotic orthopoxviruses has already been confirmed (by virus isolation or molecular detection of the viral genome) in some geographical regions (indicated by colored dots: blue: vaccinia virus (including buffalopox and rabbitpox viruses) in South America, Europe, Asia, and the Middle East; brown: monkeypox virus in Africa and North America; orange: cowpox virus in Europe and Asia).
2. Orthopoxvirus
The Orthopoxvirus genus comprises VARV, VACV, CPXV, MPXV, camelpox virus, Akhmeta virus, and other species with zoonotic potential. All orthopoxviruses share significant DNA sequence similarity and are immunologically cross-reactive and cross-protective. Infection with any orthopoxvirus is considered to generate protection against exposure or re- exposure to any other member of the genus [14,15]. Orthopoxvirus species are named primarily according to the hosts from which they were first isolated and identified; however, the name does not necessarily represents its natural reservoir or complete host range [8,16,17,18,19]. Despite the large number of studies, little is known about the primary hosts and reservoirs of zoonotic orthopoxviruses in nature, or their transmission and maintenance cycles [20]. Regarding the host range, orthopoxviruses can be both highly specialized and host restricted or generalists with a broad host range. For instance, VARV is a highly specialized virus that infects only humans, whereas MPXV, CPXV, and VACV are examples of generalist zoonotic orthopoxviruses that can infect several mammalian host species and also spillover into humans [20].
The evolution of generalists pathogens requires the successful crossing of host transmission barriers [21]. These include geographical, ecological, and behavioral constraints that separate a virus from its possible recipient hosts; virus-host cell incompatibility, such as tissue tropism, differences in receptor binding, genome replication, production, and shedding of infectious particles; and host immunity evasion, which includes cellular barriers or responses that restrict the infection and/or evasion of a virus from the innate immune system of its host [22]. To overcome these barriers, orthopoxviruses have different biological features that can synergistically contribute to the transmission to, and exploitation of, a broad range of new hosts species as observed for CXPV, MPXV, and VACV. Orthopoxviruses can cause both local lesions on the skin and systemic infections, resulting in direct and indirect transmission routes. When accompanied by viral particle stability in the environment, this can increase the likelihood of potential hosts being exposed to the virus independently of direct contact with infected hosts. In addition, orthopoxviruses can infect a variety of mammalian cells in a manner that is mostly independent of species-specific receptors and have large genomes that carry the information essential for viral replication, thereby increasing the possibility of successful infection in a new cell/host. Although the double stranded DNA genomes of orthopoxviruses have low mutation rates when compared with other viruses, such as RNA viruses, orthopoxviruses possess a genetic arsenal comprising several immune-regulatory, virulence, and host range genes [20]. The variety of host-genes among poxviruses enables them to express different viral proteins with important roles in cell tropism, as well as in the modulation of host signaling pathways and immunomodulatory responses, thereby establishing optimal cellular conditions for viral replication [23]. Finally, many of the strategies employed by orthopoxviruses to evade host immune defenses target conserved elements of the immune system in different potential hosts [20]. Combined, these features altogether are crucial for virus-cell and virus-host interactions and can contribute to the success of viral replication and transmission.
Despite the eradication of smallpox, the possibility of its re-emergence or the emergence of other orthopoxviruses in human and animal populations is a relevant global health issue. As smallpox vaccination is no longer mandatory, most of the world’s population that is under 40 years of age lacks immunity against orthopoxviruses [24,25]. This scenario is highlighted by numerous reports in recent years of human diseases caused by zoonotic orthopoxviruses such as MPXV [26,27,28,29,30,31,32,33], CPXV [34,35,36,37,38,39,40,41], VACV-like [42,43,44,45,46,47,48,49], and Akhmeta virus [18]. To date, the circulation of orthopoxviruses among wild and domestic animals has been recorded in different regions of the world, including South America, Africa, Europe, the Middle East, and Asia [27,40,42,43,50,51,52,53,54,55,56,57]. These facts raise concerns regarding the host ranges and distribution of orthopoxviruses, as well as their potential to cause outbreaks in animals and human populations, thereby further impacting animal and public health.
2.1. Monkeypox Virus
Monkeypox virus isolates are subdivided into two clades, namely, the West African and the Congo Basin clades, based on genetic and phenotypic (virulence) differences [58]. Notably, several studies have indicated that the clinical signs are similar between infections caused by viruses from either clades [59]. The first observation of MPXV infection was reported in 1958 during an outbreak of pustular rash illness in cynomolgus macaques (Macaca fascicularis) arriving in Copenhagen, Denmark, from Singapore [60]. Despite being named after the first described host, non-human primates are accidental hosts for MPXV [61].
Further insights into the range of taxa susceptible to MPXV infection were obtained by laboratory studies and field surveys. MPXV infections have been reported in a broad range of rodents, such as mice (Mus musculus), rabbits (Oryctolagus cuniculus), hamsters, woodchucks (Marmota monax), jerboas (Jaculus sp.), and porcupines (Atherurus africanus) (Table 1). Similarly, based on methods such as viral isolation, molecular assay, or experimental infection, susceptibility to MPXV infection was reported in ant-eaters (Myrmecophaga tridactyla), black-tailed prairie dogs (Cynomys ludovicianus), southern opossums (Didelphis marsupialis), short-tailed opossums (Monodelphis domestica), African hedgehogs (Atelerix sp.), and several non-human primate species. Additionally, serological surveys have implicated several African rodents, including giant pouched rats (Cricetomys spp.), African dormices (Graphiurus spp.), rope squirrels (Funisciurus spp.), and sun squirrels (Heliosciurus spp.) as primary orthopoxvirus hosts in Africa [61,62,63].

Table 1.
Hosts and susceptible animals to monkeypox virus infection.
Among Old World non-human primates, cynomolgus monkeys (Macaca fascicularis), sooty mangabeys (Cercocebus atys), orangutans (Pongo pygmaeus), and chimpanzees (Pan troglodytes) can be infected with MPXV. Among New World non-human primates [60,64,65,66,67,68,69,70,71,72,73,74,75], the common marmosets (Callithrix jacchus) was shown to be susceptible to MPXV infection through intravenous inoculation [76] (Table 1).
In 2003, a MPXV outbreak occurred in the United States of America (USA). Human infection was associated with direct contact with ill pet prairie dogs that were kept near to infected exotic animals imported from Ghana, West Africa [77]. This episode, as well as the infection of rodents, heightened concerns regarding the introduction of MPXV into the Americas. Meanwhile, the susceptibility of several African rodents to MPXV raised worries about the transmission of the virus to humans, as these animals are sometimes kept as pets [78,79].
Although humans are also accidental hosts [61], MPXV became the most significant pathogenic zoonotic orthopoxvirus for humans since the eradication of smallpox, given its associated morbidity (systemic infection) and lethality. The first human MPXV infection was described in 1970 for a 9-month old child in the Democratic Republic of Congo who had presented smallpox-like skin eruptions [70,80]. Several other human cases were reported in the following years. From 1970 to 1999, the WHO reported at least 404 confirmed and approximately 500 suspected human cases of monkeypox in different African countries (Central African Republic, Cameroon, Nigeria, Côte d’Ivoire, Liberia, Sierra Leone, and Gabon), but mainly in the Democratic Republic of Congo [52,81,82]. From the 2000s, alongside outbreaks in the Democratic Republic of Congo, the Republic of Congo, and South Sudan, the first human cases outside the Africa continent were also described. During May and June of 2003, cases of people with febrile illness and skin eruptions were notified to the Wisconsin Division of Public Health, but no deaths were reported and no person-to-person transmission was proven [78]. The source of this outbreak was traced back to imported infected exotic animals from Ghana [52,62,78]. Fortunately, the multi-state episode of captive rodent infection in the USA was short-lived, and the transmission cycle in the country was broken [83].
Alarmingly, several outbreaks of monkeypox in humans have been reported in African regions in the last decade. In 2010, two confirmed and eight suspected cases were described in the Republic of Congo related to the migration of refugees, regional inter-ethnic conflicts, or autochthonous cases. No deaths were reported among the confirmed cases, although one individual with suspected infection died [84]. In the same year, two cases of MPXV infection associated with hunting and the consumption of wild rodent meat were reported in the Central African Republic, with no deaths [85]. Numerous suspected and confirmed cases were reported in the Democratic Republic of Congo, from 2010 to 2016 [86,87], and in Serra Leone in 2014 [88]. Several suspected and 12 confirmed cases, as well as three deaths were reported in different provinces in the Central African Republic (Mbomou, Basse-Kotto, and Haute-Kotto) [52,89,90]. In 2017, the Republic of Congo reported its largest MPXV outbreak (88 suspected and seven confirmed cases, with six deaths), which affected 18 villages in five districts. This outbreak presented risks of MPXV spreading to neighboring countries given the extent of population mobility and refugee presence in the region [30].
Some African regions have continuously reported human cases of MPXV infection in recent years (2017 to 2020), including the Central African Republican (27 confirmed cases and two deaths) [91,92], Nigeria (181 confirmed cases and seven deaths) [31,93], Sierra Leone (one confirmed case) [94], Liberia (two confirmed cases and two deaths) [95], Cameroon (one confirmed case) [96], and the Democratic Republic of Congo (numerous confirmed cases and 321 deaths) [33,97]. Recently (2018), three cases of monkeypox were reported in the United Kingdom. Two were of people who had traveled to Nigeria, while the third concerned a health care worker who had had contact with one infected patient. One of the patients who had traveled to Nigeria reported having contact with a person with a rash and the possible consumption of bushmeat, raising the possibility that this may have been a case of secondary or even tertiary human-to-human transmission. Meanwhile, the infection contracted by the British care worker confirms human-to-human MPXV transmission [96]. Other cases of MPXV infections outside of Africa were reported in Israel (2018) and Singapore (2019), for travelers who imported the disease from Nigeria [98,99].
The natural source of MPXV and its maintenance cycle in nature remains unknown as the virus has only been isolated twice in nature (wild animals): once from the rope squirrel (Funisciurus anerythrus), Zaire, in 1985 [62], and once from the sooty mangabey (Cercocebus atys), Côte d’Ivoire, in 2012 [100]. To date, naturally occurring MPXV infections remain confined to the forest regions of West and Central Africa [101,102]. Consequently, a higher proportion of human MPXV cases are reported in regions (mainly African villages) where humans and non-human primates live in close proximity. The consumption, hunting, and handling of meat derived from non-human primates, rodents, and other small mammals have also been associated with human cases of MPXV infection [71,85,86,103,104,105]. Close contact with rodents has also been implicated as a source of human infection [67,106].
Human cases of monkeypox have been increasing even though they may have been underreported. Notably, diagnostic capabilities in the affected countries are most often limited, while health care workers worldwide are generally not aware of monkeypox disease. A lack of understanding about monkeypox disease associated with factors such as the increasing encroachment of humans into wild habitats, the inter-continental travel of people from endemic areas to MPXV-free regions, and the importation of animals both as pets and for laboratory studies raises concern regarding MPXV emergence, surveillance, prevention, and control [15]. Additionally, vaccination against smallpox was ceased decades ago, resulting in an increasingly larger number of people that are vulnerable to infection by MPXV or other orthopoxviruses. Although some animal species have been described as being susceptible to MPXV infection, most of what is known about its pathogenesis and clinical characteristics is derived from descriptions of animals in captivity or laboratory facilities. As monkeypox is an emerging zoonotic disease with epidemic potential and much of its host range and maintenance cycle in nature remains obscure, advances are urgently needed to better understand natural cycle of MPXV.
2.2. Cowpox Virus
Edward Jenner was the first to document CPXV infection after observing local lesions on the teats of cows, which he called “cow-pox”. Then, in 1798, Jenner demonstrated the efficacy of “true cow-pox” scarification in inducing immunity against smallpox [8,107]. There were frequent reports of bovine cowpox cases until the early 1970s in Europe, with sporadic transmission to humans, mainly milkers, occurring via contact with infected cows [108]. However, the number of bovine cowpox cases decreased, while reports of “cowpox-like” infections in several animal species, such as cats and elephants, [109] increased. “Cowpox-like” infections were described in a broad range of captive and domestic animals like non-human primates [110,111,112], felines [108,111,113,114,115,116,117], dogs [118], rodents [39,50,111,119,120,121,122,123,124,125], foxes [126,127], rhinoceroses [15,114,128], tapirs [129], okapis [130], horses [131], anteaters [114], mongooses [129], stone martens [132], bearcats [133], and farmed llamas [134,135] (Table 2). The viruses responsible for these infections induced clinical signs similar to those of CPXV infection such as hemorrhagic pocks on the chorioallantoic membrane and A-type inclusions bodies, and were thus considered to be “true cowpox” [136,137]. Most of these animals are thought to be accidental hosts for CPXV, and not reservoirs. Rodents, particularly voles (Microtus spp. and Myodes spp.), are known to be the primary CPXV reservoirs in nature [136,138].

Table 2.
Hosts and susceptible animals to cowpox virus infection.
CPXV is currently mostly found in Europe and northern and central Asia where cases of infections in rodents, cats, and humans continue to be reported. In Great Britain, CPXV is endemic in rodents such as bank voles (Myodes glareolus) and wood mice (Apodemus sylvaticus), while in Turkmenistan and Russia CPXV was isolated in the laboratory as well as in wild rodents [119]. Furthermore, serological surveys have also detected orthopoxvirus infections in France, Austria, and Norway in voles and wood mice [119]. Antibodies against orthopoxviruses were also detected in red foxes (Vulpes vulpes) in Western Europe being possibly related to CPXV infection, halthough red foxes are also known to be susceptible to ectromelia virus [119,139]. These reports of CPXV infection have occurred alongside an increasing number of reported infections in different animal species, leading to the designation of CPXV as an emerging health threat [140]. The first reported case of CPXV in a domestic cat occurred in 1977 in the Netherlands, and the number of CPXV infections in cats has since increased. According to Essbauer and collaborators (2010), more than 400 cases of CPXV infections in domestic cats were described in Western Eurasia until 2004 [15]. In cats, CPXV infection causes multiple skin lesions on the head, neck, forelimb, paws, and eyes (conjunctivitis), and the appearance of vesicles in the oral cavity and tongue. In the most severe cases, the disease can be systemic, affecting inner organs (mainly the lungs), with fatal outcomes being mostly associated with secondary bacterial infection [141]. Cats are the most affected domestic animals, mainly due to their predatory behavior against rodents, which are the CPXV reservoir in domestic and peridomestic environments [15,141,142,143,144]. However, the exact prevalence of feline cowpox is uncertain. CPXV infections in cats are mostly observed after increases in the rat population density [15,144].
The infection of pet rats and domestic cats by CPXV brings a higher risk of exposure to humans in the domestic environment, but rural or wild areas may be important as the source of infection [36]. Cowpox in humans is mainly caused by contact with infected domestic cats or rodents (such as Rattus norvegicus) that are kept as pets [15,34,37,38,121]. Even though human cowpox cases are usually self-limiting and not lethal, most people are susceptible to the disease, particularly children who are more often in close contact with pet animals [37,121]. The zoonotic potential of CPXV and its capacity to cause infection in wild and domestic environments are well established; however, many aspects of its natural maintenance cycle remains unknown. Besides the domestic animals, CPXV has a vast range of hosts and the increase in the breeding and commercialization of exotic animals raises concerns among health authorities regarding the emergence of cowpox, including in new geographical regions.
2.3. Vaccinia Virus and Related Viruses
Although VACV is the most extensively studied orthopoxvirus, its origin remains unknown [145]. Vaccinia virus, the prototype species of the Orthopoxvirus genus, is best known as the live attenuated virus used worldwide by the WHO in the smallpox vaccine [146,147,148]. Despite the successful use of VACV as a vaccine, several vaccine strain-dependent complications have been reported, including progressive vaccinia, eczema vaccinatum, vaccinia gangraenosum, and neurological complications [145,149]. During smallpox eradication campaigns, various VACV strains with different degrees of virulence were used. The highly attenuated and modified VACV Ankara is a well-stablished third generation smallpox vaccine [150,151]. For a long time, VACV vaccine strains were assumed to be incapable of establishing a natural cycle due to their attenuation in the laboratory. However, several VACVs have been isolated from different host species, and in different locations around the world [42,152,153,154]. Similarly, sub-lineages of VACV (as buffalopox virus (BPXV) and rabbitpox virus (RPXV)) have been consistently isolated in different countries and from a wide range of hosts [14,15,16,43,53,155].
In India, BPXV was first described in 1934, and was responsible for infections that mainly affected domestic buffaloes (Bubalus bubalis), but also cows and humans [155]. BPXV resembles VACV in terms of its properties (size, shape, structure, and physicochemical properties) [156], pathogenesis, and pathology. Phylogenetic analyses confirmed the monophyly of the BPXV and its likely origin from the VACV Lister vaccine [155,156,157,158,159]. Since its first description, outbreaks of BPXV have been reported in India, Pakistan, Nepal, Egypt, Bangladesh, Indonesia, Russia, and Italy [15,43,53,160,161,162].
Humans become infected with BPXV through close contact with infected animals and no human-to-human transmission has been reported to date. In 2004–2005, a nosocomial outbreak in humans occurred in Pakistan, and the source of infection was traced to buffalo fat used as a first-aid medication for covering skin burns. This unusual source of infection was indicative of indirect BPXV transmission [14,163]. Additionally, a variety of animal species, such guinea pigs, BALB and white Swiss mice, cows, buffalo calves, rabbits, and chickens have been experimentally demonstrated to be susceptible to BPXV. Nevertheless, the role of these species in BPXV transmission and maintenance in nature remains unknown [44,164] and requires clarification.
RPXV is another VACV described as affecting different animal species worldwide (Table 3). RPXV was first described between 1930–1933 after outbreaks in laboratory rabbits in the USA. Additional outbreaks were later reported in 1941 in the Netherlands, while several other cases were also reported in Europe and the USA [165,166]. To date, no human transmission has been described for RPXV [165,167].

Table 3.
Hosts and susceptible animals to vaccinia and vaccinia-like viruses infection.
Different VACV isolates also circulate in South American countries, including Uruguay, Argentina, Colombia, and Brazil [54,55,56,168]. In the last few decades, several outbreaks of VACV infection have occurred in Brazil where the disease caused by VACV is popularly known as “bovine vaccinia”, due to its association with dairy cattle [42,168]. Bovine vaccinia is characterized by vesiculopustular exanthematous disease in cattle, anddairy workers who have direct contact with infected animals [169,170,171].
Since the detection of VACV in rural areas in Southeast Brazil, in 1999, several Brazilian-VACV (Br-VACV) isolates have been identified in the country (Araçatuba virus, Belo Horizonte virus, Cantagalo virus, Carangola eye virus 1, Carangola eye virus 2, Guarani P1virus, Guarani P2 virus, Mariana virus, Passatempo virus, Pelotas 1 virus, Pelotas 2 virus, and Serro virus) [148,169,172,173,174,175]. One hypothesis for the origin of the Br-VACVs assumes that they are derived from the spillback of a vaccine strain to the sylvatic environment [154,172], while another postulates that they may represent natural genetically and phenotypically diverse VACV populations, circulating in an unknown natural reservoir [148,152,173]. In particular, the presence or absence of an 18 nucleotide sequence within gene A56R gene (viral hemagglutinin) was proposed to be a molecular marker that can separate Br-VACVs into two distinct clades (group 1 and group 2) [176,177,178]. The existence of at least two clades was further confirmed through genetic and evolutionary analyses, of Br-VACVs, causing infection or co-infections in diversity of hosts in Brazil. [47,48,54,55,56,153,169,174,175,179,180,181,182,183,184,185,186,187,188]. In addition to the genetic diversity, some studies have also shown distinct biological profiles between the two Br-VACV groups [189,190]. The biological implications of this diversity in the context of the epidemiology and clinical evolution of the disease in humans should be further investigated.
Initially, VACV outbreaks were described as affecting dairy cattle and humans in rural environments. Consequently, the epidemiology of bovine vaccine in Brazil is associated with economic losses resulting from compromised milking herds [42,171,191,192]. In Brazil, bovine vaccinia have been mainly reported in the Southeast (Minas Gerais State), which has the largest dairy cattle herds in the country [42,148]. Nevertheless, VACV circulation in Brazil has already been documented for all the regions, affecting farm animals other than cattle, as well as wild animals [42]. Consistent with its wide geographical occurrence in Brazil, VACV has been detected in different biomes and related fauna. VACV genomes and antibodies against orthopoxviruses have been detected in a broad range of animalsincluding non-human primates (Sapajus apella and Alouatta caraya) [193]; procynoides (Didelphis aurita, Didelphis albiventris, and, Nasua nasua) [188,194]; cingulates (Euphractus sexcintus) [185]; marsupials (Didelphis sp. and Caluromys philander) [153,194]; bats (Molossus rufus and Eumops perotis) [185]; and wild rodents (Oligoryzomys nigripes, Oligoryzomys flavescens, Sooretamys angouya, Calomys sp., Akodon sp., Necromys lasiurus, Necromys squamipes, Trinomys setosu, Cerradomys subflavus, Mus musculus, Rattus rattus, and Hydrochoerus hydrochaeris) [153,180,185,195,196]. Furthermore, VACV has been detected in diverse peridomestic and domestic animals, including buffaloes [183,197,198], horses, donkeys [174,181,182,195], pigs [195], cows [195], dogs [188], cats [179], and mice [184] (Table 2).
Although direct VACV transmission between wild and domestic animals and between wild animals and humans has not been documented to date, these possibilities cannot be excluded. Several studies have indicated that cattle have a role as amplifiers in the bovine vaccinia cycle and have also demonstrated that VACV excretion in feces may favor viral transmission and its maintenance in the environment [170,199,200,201]. Subsequently, it was proposed that other farm animals could also be implicated in the VACV transmission chain, although direct transmission to humans has yet to be documented. Lastly, wild rodents could be VACV reservoirs, while peridomestic rodents could act as the link for VACV spread between wild and rural environments, promoting the transmission among wild mammals and farm animals [183,184].
Although VACV is known to have a broad range of hosts, many aspects of its natural history remain unknown. Bovine vaccinia is mainly caused by contact with infected cattle and is associated with economic losses to the dairy industry in Asia and South America [42,43,45,53,148]; however, the epidemic potential of VACV is a reality. Although VACV infection is usually self-limiting and not lethal, the disease profile in immunocompromised individuals may be differentially affected, presenting with severe and generalized manifestation [202], similar to that observed for cowpox. As currently documented for VACV, until the 1970s, CPXV mainly infected cattle and milkers. However, when cattle were replaced by cats and other animals as the primary hosts of CPXV infection, the number of human cases of CPXV infection increased. Given the similarities with CPXV, its plausible that VACV could follow similar path. Although farm animals are important sources of infection, the commercialization and consumption of dairy products could be alternative routes of zoonotic VACV transmission. In addition, VACV circulation in domestic animals such as cats and dogs bring the risk of viral transmission to humans in the domestic environment. The urban emergence of VACV could be an important health burden due to the unpreparedness of healthcare professionals to correctly identify and handle emerging cases [203]. Moreover, VACV infection presents a high attack rate, and VACV emerging cases in an urban area, where agglomeration of people is more frequent, could favor transmission, and trigger a public health emergency [148,204].
3. What Is Next for Monkeypox, Cowpox, and Vaccinia Viruses?
The history of poxviruses and orthopoxviruses has frequently been related to human cultural behavior. The establishment of agricultural settlements is considered one of the factors that favored the emergence of smallpox approximately 10,000 years ago. Orthopoxviruses continue to emerge and re-emerge due to the increasing proximity of humans to wild and rural habitats. Following smallpox eradication, the global scenario is marked by a vast naïve human population and the wide circulation of different orthopoxviruses. These facts raise concerns on the possible epidemic potential of these viruses in animals and humans. In fact, zoonotic orthopoxviruses already represent an important issue for animal health and economics. An example is the case of VACV and BPXV that have been associated with significant economic losses resulting from dairy cattle and livestock infection in several Asian and South American countries [42,43,53,191,192].
Currently, MPXV is mostly observed in Africa; CPXV in Europe and Asia; BPXV in Asia and the Middle East; and VACV in South America [53,54,55,56,119,140,160,162,168,205]. Although the factors that restrict the geographic distribution of some zoonotic orthopoxviruses are still unknown, their distribution range has been increasing as MPXV has been exported to parts of the USA [77], United Kingdom [96], Israel [98], and Singapore [99]. Legal or illegal trade of animals or animal-derived products, migration of animal populations, and traveling and migration of people are some factors that can contribute to the geographic dissemination of orthopoxviruses on local or global scales. Indeed, animal trade leading to the MPXV importation into the USA illustrated how globalization can favor the spatial spread of viruses [77]. On a local scale, migration of refugees within Africa is another example related to MPXV dissemination [84].
Because orthopoxviruses such as VACV, CPXV, and MPXV have genetic and phenotypic traits that allow them to possess a variety of mammals hosts [23], one cannot exclude the possibility of the virus infecting susceptible hosts in new geographical areas. These viruses are more prevalent in certain animal species, such as VACV in cattle and BPXV in buffalos. Molecular and immunological factors may be associated with productive infections in these animals while ecological factors may be linked to transmission between individuals of the same species. On the other hand, to cross host barriers and infect a new host, a virus must be able to infect and replicate in the new host, evade the immune system, and be efficiently transmitted [22]. Regardless of the remote possibility of an orthopoxvirus infecting new hosts, the current host plasticity is already notable, especially for MPXV, CPXV, and VACV. In addition, the emergence of a virus in a new host does not necessarily require evolutionary changes (mutations, rearrangements, etc.). One example of this process is the canine distemper virus, which has a very wide host range in mammals and its emergence in these species appears to be limited primarily by contact [22].
Orthopoxvirus outbreaks are usually related to populations living in rural areas or small villages. However, factors such as a high population density, increased urbanization, agriculture activities, deforestation, approximation to wild habitats, and inter-continental travel of people from endemic to pox-free regions may introduce poxviruses into different zones including urban environments [3,206]. A primary concern related to infected animals in periurban and urbanized environments is associated with a possible increase in orthopoxvirus transmission in a naïve population and even human-to-human transmission.
The epidemic potential of a virus is related to several factors, including geographical distribution, route of transmission, pathogenicity, and host range, among others. The epidemic potential may be lower for orthopoxviruses than for other RNA viruses or viruses transmitted by airway routes. Nevertheless, orthopoxviruses are remarkable regarding their transmission and dissemination among several hosts and environments. Wild and domestic animals could act as intermediate hosts for the emergence or re-emergence of orthopoxviruses in the human population. For instance, CPXV is transmitted from wild to domestic animals and then to humans, MPXV can be transmitted directly from wild animals to humans, and VACV is transmitted from domestic animals to humans [15,42,85,86,104,144]. Zoonotic orthopoxviruses may be transmitted either directly or indirectly and new forms of viral transmission have been described, which is a concern for public health. Milk and dairy products might be a potential source of VACV exposure or transmission [191]. Even under a low transmission rate, human-to-human transmission has already been demonstrated for zoonotic orthopoxviruses (MPXV and VACV) [96,207]. These are significant findings that should be further evaluated and closely monitored.
The cessation of routine vaccination against smallpox decades ago has resulted in a large contingent of people that are susceptible to orthopoxvirus infections, which have high morbidity rates. Moreover, in immunosuppressed individuals, exposure to orthopoxvirus infection can result in severe forms of the disease, or even death [208]. To date, there have been no reports of fatalities resulting from CXPV or VACV infection; however, MPXV infection in humans can progress to a lethality of up to 10% [209]. These facts indicate that VACV, MPXV, and CPXV pose a potential threat not only for humans, but for animals in different regions of the world. Together, these factors highlight the need for continuous epidemiological surveillance and the need to better understand the natural cycles and evolution of orthopoxviruses, their host range and reservoirs, the burden of outbreaks and dissemination of orthopoxvirus-associated diseases. This information is crucial for the development and application of control measures such as sanitary barriers and public policies aimed at controlling these viruses.
Author Contributions
Conceptualization, N.I.O.S. and B.P.D.; writing—original draft preparation, N.I.O.S. and J.S.d.O.; writing—review and editing, N.I.O.S., J.S.d.O., E.G.K., G.d.S.T. and B.P.D.; visualization, N.I.O.S. and J.S.d.O.; supervision, G.d.S.T. and B.P.D.; project administration, G.d.S.T. and B.P.D.; funding acquisition, E.G.K., G.d.S.T. and B.P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was developed in the context of projects supported by different agencies. B.P.D., E.G.K., and G.d.S.T. were supported by the National Council for Scientific and Technological Development (CNPq) (grant: Research fellowship). The authors acknowledge Fundação de Amparo à Pesquisa no Estado de Minas Gerais (FAPEMIG)—Finance codes CDS-APQ-01574-17 and APQ-04039-17, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and CNPq. G.D.S.T. acknowledges Centers for Disease, Control and Prevention (USA)—Finance Zoonotic Poxviruses. N.I.O.S. acknowledges CNPq (grant graduate scholarship). J.S.d.O. acknowledges CAPES—Finance code 001 (grant graduate scholarship). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We thank the support of our colleagues from Laboratório de Virus-UFMG.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- World Health Organization (WHO). Zoonoses. Available online: https://www.who.int/topics/zoonoses/en/#:~:text=Azoonosisisanydisease,zoonoticinfectionsinnature (accessed on 24 October 2020).
- Woolhouse, M.E.J.; Gowtage-Sequeria, S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005. [Google Scholar] [CrossRef] [PubMed]
- Bird, B.H.; Mazet, J.A.K. Detection of Emerging Zoonotic Pathogens: An Integrated One Health Approach. Annu. Rev. Anim. Biosci. 2018. [Google Scholar] [CrossRef]
- Woolhouse, M.; Gaunt, E. Ecological origins of novel human pathogens. Crit. Rev. Microbiol. 2007, 33, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J. Risk factors for human disease emergence. Philos. Trans. R. Soc. B Biol. Sci. 2001. [Google Scholar] [CrossRef]
- Fenner, F. Adventures with poxviruses of vertebrates. FEMS Microbiol. Rev. 2000, 24, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Poxviridae: The viruses and their replication. In Fields Virology; Fields, B.N., Knipe, D.M., Howley, P.M., Griffin, D.E., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 2, pp. 2126–2159. ISBN 9781451105636. [Google Scholar]
- Fenner, F. The Poxviruses. In Portraits of Viruses—A History of Virology; Gibbs, A., Ed.; Karger: Basil, Switzerland, 1988; pp. 1–23. [Google Scholar]
- Thèves, C.; Biagini, P.; Crubézy, E. The rediscovery of smallpox. Clin. Microbiol. Infect. 2014, 20, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Ladnyi, I.D.; Breman, J.G. Smallpox eradication: Progress and problems. Dev. Biol. Stand. 1978, 41, 281–290. [Google Scholar]
- Damon, I.K. Poxviruses. In Manual of Clinical Microbiology, 10th ed.; Versalovic, J., Carroll, K., Funke, G., Jorgensen, J., Landry, M., Warnock, D., Eds.; American Society of Microbiology: Washington, DC, USA, 2011; pp. 1647–1658. [Google Scholar]
- Lefkowitz, E.J.; Wang, C.; Upton, C. Poxviruses: Past, present and future. Virus Res. 2006, 117, 105–118. [Google Scholar] [CrossRef]
- Mahy, B.W.J. An overview on the use of a viral pathogen as a bioterrorism agent: Why smallpox? Antiviral Res. 2003, 57, 1–5. [Google Scholar] [CrossRef]
- Gubser, C.; Hué, S.; Kellam, P.; Smith, G.L. Poxvirus genomes: A phylogenetic analysis. J. Gen. Virol. 2004, 85, 105–117. [Google Scholar] [CrossRef]
- Essbauer, S.; Pfeffer, M.; Meyer, H. Zoonotic poxviruses. Vet. Microbiol. 2010, 140, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N. An Increasing Danger of Zoonotic Orthopoxvirus Infections. PLoS Pathog. 2013, 9, e1003756. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, A.I.; Abdelazim, F. Human and Dromedary Camel Infection. Vector-Borne Zoonotic Dis. 2017, 17, 281–284. [Google Scholar] [CrossRef]
- Vora, N.M.; Li, Y.; Ph, D.; Geleishvili, M.; Emerson, G.L.; Ph, D.; Khmaladze, E.; Maghlakelidze, G.; Navdarashvili, A. Human Infection with a Zoonotic Orthopoxvirus in the Country of Georgia. N. Engl. J. Med. 2016, 372, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Doty, J.B.; Maghlakelidze, G.; Sikharulidze, I.; Tu, S.-L.; Morgan, C.N.; Mauldin, M.R.; Parkadze, O.; Kartskhia, N.; Turmanidze, M.; Matheny, A.M.; et al. Isolation and Characterization of Akhmeta Virus from Wild-Caught Rodents (Apodemus spp.) in Georgia. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.G.; Guagliardo, S.A.J.; Nakazawa, Y.J.; Doty, J.B.; Mauldin, M.R. Understanding orthopoxvirus host range and evolution: From the enigmatic to the usual suspects. Curr. Opin. Virol. 2018, 28, 108–115. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Taylor, L.H.; Haydon, D.T. Population biology of multihost pathogens. Science 2001, 292, 1109–1112. [Google Scholar] [CrossRef]
- Parrish, C.R.; Holmes, E.C.; Morens, D.M.; Park, E.-C.; Burke, D.S.; Calisher, C.H.; Laughlin, C.A.; Saif, L.J.; Daszak, P. Cross-Species Virus Transmission and the Emergence of New Epidemic Diseases. Microbiol. Mol. Biol. Rev. 2008. [Google Scholar] [CrossRef]
- Werden, S.J.; Rahman, M.M.; McFadden, G. Chapter 3 Poxvirus Host Range Genes. Adv. Virus Res. 2008, 71, 135–171. [Google Scholar] [CrossRef]
- Gallwitz, S.; Schutzbank, T.; Heberling, R.L.; Kalter, S.S.; Galpin, J.E. Smallpox: Residual antibody after vaccination. J. Clin. Microbiol. 2003, 41, 4068–4070. [Google Scholar] [CrossRef]
- Shchelkunov, S.N. Emergence and reemergence of smallpox: The need for development of a new generation smallpox vaccine. Vaccine 2011, 29, D49–D53. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.E.; Slifka, M.K. Retrospective analysis of monkeypox infection. Emerg. Infect. Dis. 2008, 14, 592. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.H.; Guagliardo, S.A.J.; Doty, J.B.; Babeaux, A.D.; Matheny, A.; Burgado, J.; Townsend, M.B.; Morgan, C.N.; Satheshkumar, P.S.; Ndakala, N. Epidemiologic and ecologic investigations of monkeypox, Likouala Department, Republic of the Congo, 2017. Emerg. Infect. Dis. 2019, 25, 273. [Google Scholar] [CrossRef] [PubMed]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Nakoune, E.; Lampaert, E.; Ndjapou, S.G.; Janssens, C.; Zuniga, I.; Van Herp, M.; Fongbia, J.P.; Koyazegbe, T.D.; Selekon, B.; Komoyo, G.F. A nosocomial outbreak of human monkeypox in the Central African Republic. Open forum Infect. Dis. 2017, 4, ofx168. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Weekly Bulletin on Outbreaks and Other Emergencies, Week 48. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/259557/OEW482504122017.pdf?sequence=1 (accessed on 25 September 2020).
- World Health Organization (WHO). Weekly Bulletin on Outbreaks and Other Emergencies, Week 52. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/259794/OEW52-2329122017.pdf?sequence=1 (accessed on 25 September 2020).
- World Health Organization (WHO). Weekly Bulletin on Outbreaks and Other Emergencies, Week 01. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/330353/OEW01-05012020.pdf (accessed on 26 September 2020).
- World Health Organization (WHO). Weekly Bulletin on Outbreaks and Other Emergencies, Week 37. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/334303/OEW37-0713092020.pdf (accessed on 26 September 2020).
- Coras, B.; Eßbauer, S.; Pfeffer, M.; Meyer, H.; Schröder, J.; Stolz, W.; Landthaler, M.; Vogt, T. Cowpox and a cat. Lancet 2005, 365, 446. [Google Scholar] [CrossRef]
- Ninove, L.; Domart, Y.; Vervel, C.; Voinot, C.; Salez, N.; Raoult, D.; Meyer, H.; Capek, I.; Zandotti, C.; Charrel, R.N. Cowpox virus transmission from pet rats to humans, France. Emerg. Infect. Dis. 2009, 15, 781–784. [Google Scholar] [CrossRef]
- Popova, A.Y.; Maksyutov, R.A.; Taranov, O.S.; Tregubchak, T.V.; Zaikovskaya, A.V.; Sergeev, A.A.; Vlashchenko, I.V.; Bodnev, S.A.; Ternovoi, V.A.; Alexandrova, N.S.; et al. Cowpox in a human, Russia, 2015. Epidemiol. Infect. 2017, 145, 755–759. [Google Scholar] [CrossRef]
- Nardin, C.; Dupond, A.S.; Pelletier, F.; Puzenat, E.; Aubin, F. Skin Lesions in a Child after Contact with a Domestic Rat. Clin. Infect. Dis. 2019, 68, 1063–1064. [Google Scholar] [CrossRef]
- Haddadeen, C.; Van Ouwerkerk, M.; Vicek, T.; Fityan, A. A case of cowpox virus infection in the UK occurring in a domestic cat and transmitted to the adult male owner. Br. J. Dermatol. 2020, 19319, 19319. [Google Scholar] [CrossRef]
- Wolfs, T.F.W.; Wagenaar, J.A.; Niesters, H.G.M.; Osterhaus, A.D.M.E. Rat-to-human transmission of cowpox infection. Emerg. Infect. Dis. 2002, 8, 1495. [Google Scholar] [CrossRef]
- Ducournau, C.; Ferrier-Rembert, A.; Ferraris, O.; Joffre, A.; Favier, A.-L.; Flusin, O.; Van Cauteren, D.; Kecir, K.; Auburtin, B.; Védy, S. Concomitant human infections with 2 cowpox virus strains in related cases, France, 2011. Emerg. Infect. Dis. 2013, 19, 1996. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Sárdy, M.; Glos, K.; Korting, H.C.; Ruzicka, T.; Wollenberg, A. The Munich outbreak of cutaneous cowpox infection: Transmission by infected pet rats. Acta Derm. Venereol. 2012, 92, 126–131. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.S.; Figueiredo, P.d.O.; Costa, G.B.; De Assis, F.L.; Drumond, B.P.; Da Fonseca, F.G.; Nogueira, M.L.; Kroon, E.G.; de Souza Trindade, G. Vaccinia virus natural infections in Brazil: The good, the bad, and the ugly. Viruses 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, G.; Balamurugan, V.; Prabhu, M.; Yogisharadhya, R.; Bora, D.P.; Gandhale, P.N.; Sankar, M.S.; Kulkarni, A.M.; Singh, R.K.; Bhanuprakash, V. Emerging and re-emerging zoonotic buffalopox infection: A severe outbreak in Kolhapur (Maharashtra), India. Vet Ital 2010, 46, 439–448. [Google Scholar] [PubMed]
- Singh, R.K.; Hosamani, M.; Balamurugan, V.; Bhanuprakash, V.; Rasool, T.J.; Yadav, M.P. Buffalopox: An emerging and re-emerging zoonosis. Anim. Heal. Res. Rev. 2007, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Gurav, Y.K.; Raut, C.G.; Yadav, P.D.; Tandale, B.V.; Sivaram, A.; Pore, M.D.; Basu, A.; Mourya, D.T.; Mishra, A.C. Buffalopox outbreak in humans and animals in Western Maharashtra, India. Prev. Vet. Med. 2011, 100, 242–247. [Google Scholar] [CrossRef]
- Silva-Fernandes, A.T.; Travassos, C.E.P.F.; Ferreira, J.M.S.; Abrahão, J.S.; de Oliveira Rocha, E.S.; Viana-Ferreira, F.; dos Santos, J.R.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Natural human infections with Vaccinia virus during bovine vaccinia outbreaks. J. Clin. Virol. 2009, 44, 308–313. [Google Scholar] [CrossRef]
- Nagasse-Sugahara, T.K.; Kisielius, J.J.; Ueda-Ito, M.; Curti, S.P.; Figueiredo, C.A.; Cruz, Á.S.; Silva, M.M.J.; Ramos, C.H.; Silva, M.C.C.; Sakurai, T. Human vaccinia-like virus outbreaks in Sao Paulo and Goias States, Brazil: Virus detection, isolation and identification. Rev. Inst. Med. Trop. Sao Paulo 2004, 46, 315–322. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Campos, R.K.; Trindade, G.S.; da Fonseca, F.G.; Ferreira, P.C.P.; Kroon, E.G. Outbreak of severe zoonotic vaccinia virus infection, Southeastern Brazil. Emerg. Infect. Dis. 2015, 21, 695–698. [Google Scholar] [CrossRef]
- Oliveira, D.B.; Assis, F.L.; Ferreira, P.C.P.; Bonjardim, C.A.; de Souza Trindade, G.; Kroon, E.G.; Abrahão, J.S. Group 1 vaccinia virus zoonotic outbreak in Maranhao State, Brazil. Am. J. Trop. Med. Hyg. 2013, 89, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Kurth, A.; Hessler, F.; Kramp, H.; Gokel, M.; Hoffmann, R.; Kuczka, A.; Nitsche, A. Cowpox virus infection in pet rat owners: Not always immediately recognized. Dtsch. Arztebl. Int. 2009, 106, 329. [Google Scholar] [CrossRef]
- Lu, B.; Cui, L.-B.; Gu, M.-H.; Shi, C.; Sun, C.-W.; Zhao, K.-C.; Bi, J.; Tan, Z.-M.; Guo, X.-L.; Huo, X. Outbreak of Vaccinia Virus Infection from Occupational Exposure, China, 2017. Emerg. Infect. Dis. 2019, 25, 1192. [Google Scholar] [CrossRef] [PubMed]
- Sklenovská, N.; Van Ranst, M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front. Public Heal. 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Balamurugan, V.; Bhanuprakash, V.; Venkatesan, G.; Hosamani, M. Emergence and reemergence of vaccinia-like viruses: Global scenario and perspectives. Indian J. Virol. 2012, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Franco-Luiz, A.P.M.; Fagundes-Pereira, A.; Costa, G.B.; Alves, P.A.; Oliveira, D.B.; Bonjardim, C.A.; Ferreira, P.C.P.; de Souza Trindade, G.; Panei, C.J.; Galosi, C.M. Spread of vaccinia virus to cattle herds, Argentina, 2011. Emerg. Infect. Dis. 2014, 20, 1576. [Google Scholar] [CrossRef] [PubMed]
- Franco-Luiz, A.P.M.; Oliveira, D.B.; Pereira, A.F.; Gasparini, M.C.S.; Bonjardim, C.A.; Ferreira, P.C.P.; de Souza Trindade, G.; Puentes, R.; Furtado, A.; Abrahão, J.S. Detection of vaccinia virus in dairy cattle serum samples from 2009, Uruguay. Emerg. Infect. Dis. 2016, 22, 2174. [Google Scholar] [CrossRef]
- Usme-Ciro, J.A.; Paredes, A.; Walteros, D.M.; Tolosa-Pérez, E.N.; Laiton-Donato, K.; del Carmen Pinzón, M.; Petersen, B.W.; Gallardo-Romero, N.F.; Li, Y.; Wilkins, K. Detection and molecular characterization of zoonotic poxviruses circulating in the Amazon region of Colombia, 2014. Emerg. Infect. Dis. 2017, 23, 649. [Google Scholar] [CrossRef]
- Eder, I.; Vollmar, P.; Pfeffer, M.; Naether, P.; Rodloff, A.C.; Meyer, H. Two distinct clinical courses of human cowpox, Germany, 2015. Viruses 2017, 9, 375. [Google Scholar] [CrossRef]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J.; et al. Outbreak of human monkeypox in Nigeria in 2017–18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- von Magnus, P.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A pox-like disease in cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Parker, S.; Nuara, A.; Schultz, D.A. Human monkeypox: An emerging zoonotic disease. Future Microbiol. 2007, 2, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Khodakevich, L.; Ježek, Z.; Kinzanzka, K. Isolation of monkeypox virus from wild squirrel infected in nature. Isol. Monkeypox Virus Wild Squirrel Infected Nat. 1986, 98–99. [Google Scholar] [CrossRef]
- Doty, J.B.; Malekani, J.M.; Kalemba, L.N.; Stanley, W.T.; Monroe, B.P.; Nakazawa, Y.U.; Id, M.R.M.; Bakambana, L.; Liyandja, T.; Liyandja, D.; et al. Assessing Monkeypox Virus Prevalence in Small Mammals at the Human—Animal Interface in the Democratic Republic of the Congo. Viruses 2017, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wachtman, L.; Mansfield, K. Viral diseases of nonhuman primates. Nonhum. Primates Biomed. Res. 2012, 1. [Google Scholar] [CrossRef]
- Ellis, C.K.; Carroll, D.S.; Lash, R.R.; Townsend Peterson, A.; Damon, I.K.; Malekani, J.; Formenty, P. Ecology and geography of human monkeypox case occurrences across Africa. J. Wildl. Dis. 2012, 48, 335–347. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Carroll, D.S.; Olson, V.A.; Hughes, C.; Galley, J.; Likos, A.; Montgomery, J.M.; Suu-Ire, R.; Kwasi, M.O.; Root, J.J.; et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: Evidence for multi-species involvement in the absence of widespread human disease. Am. J. Trop. Med. Hyg. 2010. [Google Scholar] [CrossRef]
- Fuller, T.; Thomassen, H.A.; Mulembakani, P.M.; Johnston, S.C.; Lloyd-Smith, J.O.; Kisalu, N.K.; Lutete, T.K.; Blumberg, S.; Fair, J.N.; Wolfe, N.D.; et al. Using remote sensing to map the risk of human monkeypox virus in the Congo basin. Ecohealth 2011, 8, 14–25. [Google Scholar] [CrossRef]
- Peters, J.C. An epizootic of monkey pox at Rotterdam Zoo. Int. Zoo Yearb. 1966, 6, 274–275. [Google Scholar] [CrossRef]
- Hutin, Y.J.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Arita, I.; Henderson, D.A. Smallpox and monkeypox in non-human primates. Bull. World Health Organ. 1968, 39, 277. [Google Scholar] [PubMed]
- Marennikova, S.S.; Seluhina, E.M.; Mal’ceva, N.N.; Ladnyj, I.D. Poxviruses isolated from clinically ill and asymptomatically infected monkeys and a chimpanzee. Bull. World Health Organ. 1972, 46, 613–620. [Google Scholar]
- Gispen, R.; Brand Saathof, B.; Hekker, A.C. Monkeypox specific antibodies in human and simian sera from the Ivory Coast and Nigeria. Bull. World Health Organ. 1976, 53, 355–360. [Google Scholar] [PubMed]
- Marennikova, S.S.; Seluhina, E.M. Susceptibility of some rodent species to monkeypox virus, and course of the infection. Bull. World Health Organ. 1976, 53, 13–20. [Google Scholar] [PubMed]
- Falendysz, E.A.; Londoño-Navas, A.M.; Meteyer, C.U.; Pussini, N.; Lopera, J.G.; Osorio, J.E.; Rocke, T.E. Evaluation of monkeypox virus infection of black-tailed prairie dogs (Cynomys ludovicianus) using in vivo bioluminescent imaging. J. Wildl. Dis. 2014, 50, 524–536. [Google Scholar] [CrossRef]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- Mucker, E.M.; Chapman, J.; Huzella, L.M.; Huggins, J.W.; Shamblin, J.; Robinson, C.G.; Hensley, L.E. Susceptibility of marmosets (Callithrix jacchus) to monkeypox virus: A low dose prospective model for monkeypox and smallpox disease. PLoS ONE 2015, 10, e0131742. [Google Scholar] [CrossRef]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Update: Multistate Outbreak of Monkeypox—Illinois, Indiana, Missouri, Ohio, and Wisconsin. 2003. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5227a5.htm (accessed on 26 September 2020).
- Sklenovská, N. Monkeypox Virus. In Animal-Origin Viral Zoonoses—Livestock Diseases and Management; Malik, Y.S., Singh, R.K., Kuldeep, D., Eds.; Springer: Singapore, 2020; pp. 39–68. [Google Scholar]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593. [Google Scholar]
- Heymann, D.L.; Szczeniowski, M.; Esteves, K. Re-emergence of monkeypox in Africa: A review of the past six years. Br. Med. Bull. 1998, 54, 693–702. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human monkeypox in Kasai Oriental, Democratic Republic of the Congo (former Zaire): Preliminary report of October, 1997 investigation. Wkly Epidemiol Rec. 1997, 72, 369–372. [Google Scholar]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.G.; Emerson, G.L.; Pukuta, E.; Karhemere, S.; Muyembe, J.J.; Bikindou, A.; McCollum, A.M.; Moses, C.; Wilkins, K.; Zhao, H.; et al. Short report: Detection of human monkeypox in the Republic of the Congo following intensive community education. Am. J. Trop. Med. Hyg. 2013, 88, 982–985. [Google Scholar] [CrossRef]
- Berthet, N.; Nakouné, E.; Whist, E.; Selekon, B.; Burguire, A.M.; Manuguerra, J.C.; Gessain, A.; Kazanji, M. Maculopapular lesions in the Central African Republic. Lancet 2011, 378, 1354. [Google Scholar] [CrossRef]
- McCollum, A.M.; Nakazawa, Y.; Ndongala, G.M.; Pukuta, E.; Karhemere, S.; Lushima, R.S.; Ilunga, B.K.; Kabamba, J.; Wilkins, K.; Gao, J.; et al. Case report: Human monkeypox in the kivus, a conflict region of the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2015, 93, 718–721. [Google Scholar] [CrossRef]
- Mwamba, D.K.; Kebela, B.I.; Shongo, R.L.; Pukuta, E.; Kayembe, N.J.M. Profil épidemiologique du monkeypox en RDC, 2010–2014. Ann. African Med. 2014, 8, 1855–1860. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). About Monkeypox. Available online: https://www.cdc.gov/poxvirus/monkeypox/about.html (accessed on 24 September 2020).
- Kalthan, E.; Dondo-Fongbia, J.P.; Yambele, S.; Dieu-Creer, L.R.; Zepio, R.; Pamatika, C.M. Twelve cases of monkeypox virus outbreak in Bangassou District (Central African Republic) in December 2015. Bull. Soc. Pathol. Exot. 2016, 109, 358–363. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Monkeypox in Central African Republic. Available online: https://www.who.int/csr/don/13-october-2016-monkeypox-caf/en/ (accessed on 25 September 2020).
- World Health Organization (WHO). Weekly Bulletin on Outbreaks and Other Emergencies, Week 35. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/258888/OEW35-268192017.pdf?sequence=1 (accessed on 25 September 2020).
- World Health Organization (WHO). Weekly Bulletin on Outbreaks and Other Emergencies, Week 22. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/325086/OEW22-270502062019.pdf (accessed on 25 September 2020).
- World Health Organization (WHO). Weekly Bulletin on Outbreaks and Other Emergencies, Week 11. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/331451/OEW11-0915032020.pdf (accessed on 25 September 2020).
- World Health Organization (WHO). Weekly Bulletin on Outbreaks and Other Emergencies, Week 21. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/255579/OEW21-202652017.pdf?sequence=1 (accessed on 25 September 2020).
- World Health Organization (WHO). Weekly Bulletin on Outbreaks and Other Emergencies, Week 08. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/260335/OEW8-1723022018.pdf?sequence=1 (accessed on 26 September 2020).
- World Health Organization (WHO). Weekly Bulletin on Outbreaks and Other Emergencies, Week 39. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/275136/OEW39-2228092018.pdf (accessed on 26 September 2020).
- World Health Organization (WHO). Weekly Bulletin on Outbreaks and Other Emergencies, Week 01. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/278952/OEW01-29122018-04012019.pdf?sequence=1&isAllowed=y (accessed on 26 September 2020).
- Ministry of Health, State of Isreal—Monkeypox Patient Diagnosed. Available online: https://www.health.gov.il/English/News_and_Events/Spokespersons_Messages/Pages/12102018_1.aspx (accessed on 29 September 2020).
- Mauldin, M.R.; Mccollum, A.M.; Nakazawa, Y.J.; Mandra, A.; Whitehouse, E.R.; Davidson, W.; Zhao, H.; Gao, J.; Li, Y.; Doty, J.; et al. Exportation of Monkeypox Virus From the African Continent. J. Infect. Dis. 2020, 1–10. [Google Scholar] [CrossRef]
- Radonić, A.; Metzger, S.; Dabrowski, P.W.; Couacy-Hymann, E.; Schuenadel, L.; Kurth, A.; Mätz-Rensing, K.; Boesch, C.; Leendertz, F.H.; Nitsche, A. Fatal monkeypox in wild-living sooty mangabey, Côte d’Ivoire, 2012. Emerg. Infect. Dis. 2014, 20, 1009–1011. [Google Scholar] [CrossRef]
- Ligon, B.L. Monkeypox: A review of the history and emergence in the Western hemisphere. Semin. Pediatr. Infect. Dis. 2004, 15, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Lee, K.N.; Abel, J.; Carroll, D.S.; Montgomery, J.M.; Olson, V.A.; Li, Y.; Davidson, W.; Hughes, C.; Dillon, M.; et al. Monkeypox zoonotic associations: Insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am. J. Trop. Med. Hyg. 2007, 76, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Ježek, Z.; Grab, B.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull. World Health Organ. 1988, 66, 459. [Google Scholar] [PubMed]
- Kalthan, E.; Tenguere, J.; Ndjapou, S.G.; Koyazengbe, T.A.; Mbomba, J.; Marada, R.M.; Rombebe, P.; Yangueme, P.; Babamingui, M.; Sambella, A.; et al. Investigation of an outbreak of monkeypox in an area occupied by armed groups, Central African Republic. Med. Mal. Infect. 2018, 48, 263–268. [Google Scholar] [CrossRef]
- Laudisoit, A.; Baelo, P.; Mussaw Awazi, M.; VanHoutte, N.; VanHees, M.; Amundala, N.; Leirs, H. Biodiversity, Bushmeat and Monkeypox in the Democratic Republic of the Congo: Another viral threat upon larger cities? Trop. Med. Int. Heal. 2015, 1, 30–31. [Google Scholar]
- Kabuga, A.I.; El Zowalaty, M.E. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J. Med. Virol. 2019, 91, 533–540. [Google Scholar] [CrossRef]
- Mauldin, M.R.; Antwerpen, M.; Emerson, G.L.; Li, Y.; Zoeller, G.; Carroll, D.S.; Meyer, H. Cowpox virus: What’s in a name? Viruses 2017, 9, 1–15. [Google Scholar] [CrossRef]
- Baxby, D.; Bennett, M. Cowpox: A re-evaluation of the risks of human cowpox based on new epidemiological information. Arch. Virol. Suppl. 1997, 13, 1–12. [Google Scholar] [CrossRef]
- Gehring, H.; Mahnel, H.; Mayer, H. Kurze Mitteilungen: Elefantenpocken. Zent. Veterinärmedizin Reihe B 1972. [Google Scholar] [CrossRef]
- Mätz-Rensing, K.; Ellerbrok, H.; Ehlers, B.; Pauli, G.; Floto, A.; Alex, M.; Czerny, C.P.; Kaup, F.J. Fatal poxvirus outbreak in a colony of New World Monkeys. Vet. Pathol. 2006, 43, 212–218. [Google Scholar] [CrossRef]
- Martina, B.E.E.; Van Doornum, G.; Dorrestein, G.M.; Niesters, H.G.M.; Stittelaar, K.J.; Wolters, M.A.B.I.; Van Bolhuis, H.G.H.; Osterhaus, A.D.M.E. Cowpox virus transmission from rats to monkeys, the Netherlands. Emerg. Infect. Dis. 2006, 12, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Girling, S.J.; Pizzi, R.; Cox, A.; Beard, P.M. Fatal cowpox virus infection in two squirrel monkeys (Saimiri sciureus). Vet. Rec. 2011, 169, 156. [Google Scholar] [CrossRef] [PubMed]
- Baxby, D.; Jones, D.M.; Ashton, D.G.; Thomsett, L.R. An outbreak of cowpox in captive cheetahs: Virological and epidemiological studies. J. Hyg. 1982, 89, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Marennikova, S.S.; Maltseva, N.N.; Korneeva, V.I.; Garanina, N.M. Outbreak of pox disease among carnivora (Felidae) and edentata. J. Infect. Dis. 1977, 135, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Willemse, A.; Egberink, H.F. Transmission of cowpox virus infection from domestic cat to man. Lancet 1985, 325, 1515. [Google Scholar] [CrossRef]
- Tryland, M.; Sandvik, T.; Hansen, H.; Haukenes, G.; Holtet, L.; Bennett, M.; Mehl, R.; Moens, U.; Olsvik, Ø.; Traavik, T. Characteristics of four cowpox virus isolates from Norway and Sweden. APMIS 1998. [Google Scholar] [CrossRef]
- Tryland, M.; Okeke, M.I.; af Segerstad, C.H.; Mörner, T.; Traavik, T.; Ryser-Degiorgis, M.P. Orthopoxvirus DNA in Eurasian Lynx, Sweden. Emerg. Infect. Dis. 2011, 17, 626–632. [Google Scholar] [CrossRef]
- Smith, K.C.; Bennett, M.; Garrett, D.C. Skin lesions caused by orthopoxvirus infection in a dog. J. Small Anim. Pract. 1999. [Google Scholar] [CrossRef]
- Chantrey, J.; Meyer, H.; Baxby, D.; Begon, M.; Bown, K.J.; Hazel, S.M.; Jones, T.; Montgomery, W.I.; Bennett, M. Cowpox: Reservoir hosts and geographic range. Epidemiol. Infect. 1999, 122, 455–460. [Google Scholar] [CrossRef]
- Hazel, S.M.; Bennett, M.; Chantrey, J.; Bown, K.; Cavanagh, R.; Jones, T.R.; Baxby, D.; Begon, M. A longitudinal study of an endemic disease in its wildlife reservoir: Cowpox and wild rodents. Epidemiol. Infect. 2000, 124, 551–562. [Google Scholar] [CrossRef]
- Campe, H.; Zimmermann, P.; Glos, K.; Bayer, M.; Bergemann, H.; Dreweck, C.; Graf, P.; Weber, B.K.; Meyer, H.; Büttner, M.; et al. Cowpox virus transmission from Pet Rats to humans, Germany. Emerg. Infect. Dis. 2009, 15, 777–780. [Google Scholar] [CrossRef]
- Marennikova, S.S.; Ladnyj, I.D.; Ogorodnikova, Z.I.; Shelukhina, E.M.; Maltseva, N.N. Identification and study of a poxvirus isolated from wild rodents in Turkmenia. Arch. Virol. 1978, 56, 7–14. [Google Scholar] [CrossRef]
- Essbauer, S.; Hartnack, S.; Misztela, K.; Kießling-tsalos, J. Patterns of Orthopox Virus Wild Rodent Host in South Germany. Vector-Borne Zoonotic Dis. 2009, 9, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Sandvik, T.; Tryland, M.; Hansen, H.; Mehl, R.; Moens, U.; Olsvik, Ø.; Traavik, T. Naturally occurring orthopoxviruses: Potential for recombination with vaccine vectors. J. Clin. Microbiol. 1998, 36, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Kik, M.J.L.; Liu, P.L.; Van Asten, J.A.M. Cowpoxvirus infection in the Patagonian cavy (Dolichotis patagonum) emerging disease in an educational animal park the first reported case. Vet. Q. 2006, 28, 42–44. [Google Scholar] [CrossRef]
- Tryland, M.; Sandvik, T.; Arnemo, J.M.; Stuve, G.; Olsvik, Ø.; Traavik, T. Antibodies against orthopoxviruses in wild carnivores from Fennoscandia. J. Wildl. Dis. 1998. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, D.; Brochier, B.; Crouch, A.; Bennett, M.; Gaskell, R.M.; Baxby, D.; Pastoret, P.P. Comparison of the susceptibility of the red fox (Vulpes vulpes) to a vaccinia-rabies recombinant virus and to cowpox virus. Vaccine 1995. [Google Scholar] [CrossRef]
- Schaller, V.K.; Pilaski, J. Pocken bei Breitmaulnashornern (Ceratotheriilm s. simum) im Zoologischen Garten Münster. Zool. Gatten N.F 1979, 49, 169–184. [Google Scholar]
- Kurth, A.; Straube, M.; Kucska, A.; Dunsche, A.J.; Meyer, H.; Nitsche, A. Cowpox virus outbreak in banded mongooses (mungos mungo) and jaguarundis (Herpailurus yagouaroundi) with a time-delayed infection to humans. PLoS ONE 2009, 4, e6883. [Google Scholar] [CrossRef]
- Zwart, P.; Gispen, R.; Peters, J.C. Cowpox in okapis Okapia johnstoni at Rotterdam zoo. Br. Vet. J. 1971. [Google Scholar] [CrossRef]
- Franke, A.; Kershaw, O.; Jenckel, M.; König, L.; Beer, M.; Hoffmann, B.; Hoffmann, D. Fatal cowpox virus infection in an aborted foal. Vector-Borne Zoonotic Dis. 2016, 16, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Wisser, J.; Pilaski, J.; Strauss, G.; Meyer, H.; Burck, G.; Truyen, U.; Rudolph, M.; Frölich, K. Cowpox virus infection causing stillbirth in an Asian elephant (Elphas maximus). Vet. Rec. 2001, 149, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Hentschke, J.; Meyer, H.; Wittstatt, U.; Ochs, A.; Burkhardt, S.; Aue, A. Kuhpocken bei kanadischen bibern (castor fiver canadensis) und katzenbaren (ailurus fulgens). Tierarztl. Umsch. 1999, 54. [Google Scholar]
- Cardeti, G.; Brozzi, A.; Eleni, C.; Polici, N.; D’Alterio, G.; Carletti, F.; Scicluna, M.T.; Castilletti, C.; Capobianchi, M.R.; di Caro, A.; et al. Cowpox virus in Llama, Italy. Emerg. Infect. Dis. 2011, 17, 1513–1515. [Google Scholar] [CrossRef]
- Schuppel, K.F.; Menger, S.; Eulenberger, K.; Bernhard, A.; Pilaski, J. Kuhpocken Infektion bei Alpakas (Lama glama pacos). Verh ber Erkrg Zootiere 1997, 38, 259–264. [Google Scholar]
- Essbauer, S.; Meyer, H. Genus Orthopoxvirus: Cowpox virus. In Poxviruses. Birkhäuser Advances in Infectious Diseases; Mercer, A.A., Schmidt, A., Weber, O., Eds.; Birkhäuser: Basel, Switzerland, 2007. [Google Scholar]
- Stemmler, M.; Neubauer, H.; Meyer, H. Comparison of closely related orthopoxvirus isolates by random amplified polymorphic DNA and restriction fragment length polymorphism analysis. J. Vet. Med. Ser. B 2001. [Google Scholar] [CrossRef]
- Baxby, D. Is cowpox misnamed? A review of 10 human cases. Br. Med. J. 1977, 1, 1379–1381. [Google Scholar] [CrossRef]
- Mahnel, H.; Holejsovsky, J.; Bartak, P.; Czerny, C.P. Kongenitale “Ektromelie” bei Pelztieren durch Orthopoxvirus muris. Teirarztliche Prax. 1993, 21, 469–472. [Google Scholar]
- Vorou, R.M.; Papavassiliou, V.G.; Pierroutsakos, I.N. Cowpox virus infection: An emerging health threat. Curr. Opin. Infect. Dis. 2008, 21, 153–156. [Google Scholar] [CrossRef]
- Bennett, M.; Gaskell, C.J.; Baxbyt, D.; Gaskell, R.M.; Kelly, D.F.; Naidoot, J. Feline cowpox virus infection. J. Small Anim. Pract. 1990, 31, 167–173. [Google Scholar] [CrossRef]
- Thomsett, L.R.; Baxby, D.; Denham, E.M. Cowpox in the domestic cat. Vet. Rec. 1978, 103, 567. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, U.; Van De Poel, H.; Van Den Ingh, T.S.G.A.M. Necrotizing pneumonia in a cat caused by an orthopox virus. J. Comp. Pathol. 1999. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.; Pfleghaar, S.; von Bomhard, D.; Kaaden, O.R.; Meyer, H. Retrospective investigation of feline cowpox in Germany. Vet. Rec. 2002, 150, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L. Poxviruses, Birkhäuser Advances in Infectious Diseases; Mercer, A.A., Schmidt, A., Weber, O., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2007; ISBN 3-7643-7556-6. [Google Scholar]
- World Health Organization (WHO). The Global Eradication of Smallpox: Final Report of the Global Commission for the Certification of Smallpox Eradication, Geneva, December 1979. Available online: https://apps.who.int/iris/bitstream/handle/10665/39253/a41438.pdf?sequence=1&isAllowed=y (accessed on 30 September 2020).
- Henderson, D.A. Smallpox: The Death of a Disease: The Inside Story of Eradicating a Worldwide Killer; Prometheus Books: New York, NY, USA, 2009; ISBN 161592230X. [Google Scholar]
- Kroon, E.G.; Mota, B.E.F.; Abrahão, J.S.; da Fonseca, F.G.; de Souza Trindade, G. Zoonotic Brazilian Vaccinia virus: From field to therapy. Antiviral Res. 2011, 92, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Laboratory Acquired Vaccinia Exposures and Infections—United States, 2005–2007. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5715a3.htm (accessed on 30 September 2020).
- Drexler, I.; Staib, C.; Sutter, G. Modified vaccinia virus Ankara as antigen delivery system: How can we best use its potential? Curr. Opin. Biotechnol. 2004, 15, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Meseda, C.A.; Garcia, A.D.; Kumar, A.; Mayer, A.E.; Manischewitz, J.; King, L.R.; Golding, H.; Merchlinsky, M.; Weir, J.P. Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology 2005, 339, 164–175. [Google Scholar] [CrossRef]
- Trindade, G.S.; Emerson, G.L.; Carroll, D.S.; Kroon, E.G.; Damon, I.K. Brazilian vaccinia viruses and their origins. Emerg. Infect. Dis. 2007, 13, 965. [Google Scholar] [CrossRef]
- Miranda, J.B.; Borges, I.A.; Campos, S.P.S.; Vieira, F.N.; De Ázara, T.M.F.; Marques, F.A.; Costa, G.B.; Luis, A.P.M.F.; De Oliveira, J.S.; Ferreira, P.C.P.; et al. Serologic and molecular evidence of vaccinia virus circulation among small mammals from different biomes, Brazil. Emerg. Infect. Dis. 2017, 23, 931–938. [Google Scholar] [CrossRef]
- Medaglia, M.L.G.; Moussatché, N.; Nitsche, A.; Dabrowski, P.W.; Li, Y.; Damon, I.K.; Lucas, C.G.O.; Arruda, L.B.; Damaso, C.R. Genomic Analysis, Phenotype, and Virulence of the Historical Brazilian Smallpox Vaccine Strain IOC: Implications for the Origins and Evolutionary Relationships of Vaccinia Virus. J. Virol. 2015, 89, 11909–11925. [Google Scholar] [CrossRef]
- Baxby, D.; Hill, B.J. Characteristics of a new poxvirus isolated from Indian buffaloes. Arch. Gesamte Virusforsch. 1971, 35, 70–79. [Google Scholar] [CrossRef]
- Bloch, B.; Lal, S.M. A study of the ultrastructure of the buffalo pox virus. Acta Pathol. Microbiol. Scand. Sect. B Microbiol. 1975, 83, 191–200. [Google Scholar] [CrossRef]
- Bera, B.C.; Shanmugasundaram, K.; Barua, S.; Anand, T.; Riyesh, T.; Vaid, R.K.; Virmani, N.; Bansal, M.; Shukla, B.N.; Malik, P. Sequence and phylogenetic analysis of host-range (E3L, K3L, and C7L) and structural protein (B5R) genes of buffalopox virus isolates from buffalo, cattle, and human in India. Virus Genes 2012, 45, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Eltom, K.H.; Samy, A.M.; Abd El Wahed, A.; Czerny, C.-P. Buffalopox Virus: An Emerging Virus in Livestock and Humans. Pathogens 2020, 9, 676. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.D.; Mauldin, M.R.; Nyayanit, D.A.; Albariño, C.G.; Sarkale, P.; Shete, A.; Guerrero, L.W.; Nakazawa, Y.; Nichol, S.T.; Mourya, D.T. Isolation and phylogenomic analysis of buffalopox virus from human and buffaloes in India. Virus Res. 2020, 277, 197836. [Google Scholar] [CrossRef]
- Goyal, T.; Varshney, A.; Bakshi, S.K.; Barua, S.; Bera, B.C.; Singh, R.K. Buffalo pox outbreak with atypical features: A word of caution and need for early intervention! Int. J. Dermatol. 2013, 52, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Bhanuprakash, V.; Venkatesan, G.; Balamurugan, V.; Hosamani, M.; Yogisharadhya, R.; Chauhan, R.S.; Pande, A.; Mondal, B.; Singh, R.K. Pox outbreaks in sheep and goats at Makhdoom (Uttar Pradesh), India: Evidence of sheeppox virus infection in goats. Transbound. Emerg. Dis. 2010, 57, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Venkatesan, G.; Singh, R.K. Buffalopox Virus. In Animal-Origin Viral Zoonoses—Livestock Diseases and Management; Malik, Y.S., Singh, R.K., Kuldeep, D., Eds.; Springer: Singapore, 2020; pp. 145–162. [Google Scholar]
- Zafar, A.; Swanepoel, R.; Hewson, R.; Nizam, M.; Ahmed, A.; Husain, A.; Grobbelaar, A.; Bewley, K.; Mioulet, V.; Dowsett, B. Nosocomial buffalopoxvirus infection, Karachi, Pakistan. Emerg. Infect. Dis. 2007, 13, 902. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bhat, P.P.; Mishra, B.P.; Singh, R.K. Biological transmissibility of buffalopox virus. J. Appl. Anim. Res. 1996, 9, 79–88. [Google Scholar] [CrossRef]
- Nalca, A.; Nichols, D.K. Rabbitpox: A model of airborne transmission of smallpox. J. Gen. Virol. 2011, 92, 31–35. [Google Scholar] [CrossRef]
- Christensen, L.R.; Bond, E.; Matanic, B. “Pock-less” rabbit pox. Lab. Anim. Care 1967, 17, 281. [Google Scholar]
- Fenner, F. The biological characters of several strains of vaccinia, cowpox and rabbitpox viruses. Virology 1958, 5, 502–529. [Google Scholar] [CrossRef]
- Dumbell, K.; Richardson, M. Virological investigations of specimens from buffaloes affected by buffalopox in Maharashtra State, India between 1985 and 1987. Arch. Virol. 1993, 128, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.; Drumond, B.P.; Trindade, G.S.; Lobato, Z.I.P.; Da Fonseca, F.G.; Dos Santos, J.R.; Madureira, M.C.; Guedes, M.I.M.C.; Ferreira, J.M.S.; Bonjardim, C.A.; et al. Passatempo virus, a vaccinia virus strain, Brazil. Emerg. Infect. Dis. 2005, 11, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- Rivetti, A.V.; Guedes, M.I.M.C.; Rehfeld, I.S.; Oliveira, T.M.L.; Matos, A.C.D.; Abrahão, J.S.; Kroon, E.G.; Lobato, Z.I.P. Bovine vaccinia, a systemic infection: Evidence of fecal shedding, viremia and detection in lymphoid organs. Vet. Microbiol. 2013, 162, 103–111. [Google Scholar] [CrossRef]
- Matos, A.C.D.; Rehfeld, I.S.; Guedes, M.I.M.C.; Lobato, Z.I.P. Bovine vaccinia: Insights into the disease in cattle. Viruses 2018, 10, 120. [Google Scholar] [CrossRef]
- Damaso, C.R.A.; Esposito, J.J.; Condit, R.C.; Moussatché, N. An emergent poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virology 2000, 277, 439–449. [Google Scholar] [CrossRef]
- De Souza Trindade, G.; Da Fonseca, F.G.; Marques, J.T.; Nogueira, M.L.; Mendes, L.C.N.; Borges, A.S.; Peiró, J.R.; Pituco, E.M.; Bonjardim, C.A.; Ferreira, P.C.P.; et al. Araçatuba virus: A vaccinia-like virus associated with infection in humans and cattle. Emerg. Infect. Dis. 2003, 9, 155–160. [Google Scholar] [CrossRef]
- Campos, R.K.; Brum, M.C.S.; Nogueira, C.E.W.; Drumond, B.P.; Alves, P.A.; Siqueira-Lima, L.; Assis, F.L.; Trindade, G.S.; Bonjardim, C.A.; Ferreira, P.C.; et al. Assessing the variability of Brazilian Vaccinia virus isolates from a horse exanthematic lesion: Coinfection with distinct viruses. Arch. Virol. 2011, 156, 275–283. [Google Scholar] [CrossRef]
- Lima, M.T.; Oliveira, G.P.; Assis, F.L.; de Oliveira, D.B.; Vaz, S.M.; de Souza Trindade, G.; Abrahão, J.S.; Kroon, E.G. Ocular vaccinia infection in dairy worker, Brazil. Emerg. Infect. Dis. 2018, 24, 161. [Google Scholar] [CrossRef]
- Trindade, G.d.S.; Emerson, G.L.; Sammons, S.; Frace, M.; Govil, D.; Mota, B.E.F.; Abrahão, J.S.; de Assis, F.L.; Olsen-Rasmussen, M.; Goldsmith, C.S.; et al. Serro 2 virus highlights the fundamental genomic and biological features of a natural vaccinia virus infecting humans. Viruses 2016, 8, 328. [Google Scholar] [CrossRef]
- Drumond, B.P.; Leite, J.A.; da Fonseca, F.G.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Brazilian Vaccinia virus strains are genetically divergent and differ from the Lister vaccine strain. Microbes Infect. 2008, 10, 185–197. [Google Scholar] [CrossRef] [PubMed]
- de Souza Trindade, G.; Li, Y.; Olson, V.A.; Emerson, G.; Regnery, R.L.; da Fonseca, F.G.; Damon, I. Real-time PCR assay to identify variants of Vaccinia virus: Implications for the diagnosis of bovine vaccinia in Brazil. J. Virol. Methods 2008, 152, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.B.; Miranda, J.B.; Almeida, G.G.; Silva de Oliveira, J.; Pinheiro, M.S.; Gonçalves, S.A.; Pimenta Dos Reis, J.K.; Gonçalves, R.; Ferreira, P.C.P.; Bonjardim, C.A.; et al. Detection of Vaccinia Virus in Urban Domestic Cats, Brazil. Emerg. Infect. Dis. 2017, 23, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Dutra, L.A.L.; de Freitas Almeida, G.M.; Oliveira, G.P.; Abrahão, J.S.; Kroon, E.G.; Trindade, G.d.S. Molecular evidence of Orthopoxvirus DNA in capybara (Hydrochoerus hydrochaeris) stool samples. Arch. Virol. 2017, 162, 439–448. [Google Scholar] [CrossRef]
- Brum, M.C.S.; dos Anjos, B.L.; Nogueira, C.E.W.; Amaral, L.A.; Weiblen, R.; Flores, E.F. An outbreak of orthopoxvirus-associated disease in horses in southern Brazil. J. Vet. Diagn. Investig. 2010, 22, 143–147. [Google Scholar] [CrossRef]
- Abrahão, J.S.; de Souza Trindade, G.; Pereira-Oliveira, G.; de Oliveira Figueiredo, P.; Costa, G.; Moreira Franco-Luiz, A.P.; Lopes Assis, F.; Bretas de Oliveira, D.; Mattos Paim, L.R.; de Araújo Oliveira, C.E.; et al. Detection of Vaccinia virus during an outbreak of exanthemous oral lesions in Brazilian equids. Equine Vet. J. 2017. [Google Scholar] [CrossRef]
- Lima, M.T.; Oliveira, G.P.; Afonso, J.A.B.; Souto, R.J.C.; De Mendonça, C.L.; Dantas, A.F.M.; Abrahao, J.S.; Kroon, E.G. An update on the known host range of the brazilian vaccinia virus: An outbreak in Buffalo Calves. Front. Microbiol. 2019, 9, 3327. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Guedes, M.I.M.; Trindade, G.S.; Fonseca, F.G.; Campos, R.K.; Mota, B.F.; Lobato, Z.I.P.; Silva-Fernandes, A.T.; Rodrigues, G.O.L.; Lima, L.S.; et al. One more piece in the VACV ecological puzzle: Could peridomestic rodents be the link between wildlife and bovine vaccinia outbreaks in Brazil? PLoS ONE 2009, 4, e7428. [Google Scholar] [CrossRef]
- Martins da Costa, P.S.P.; Oliveira, J.S.; Domingos, I.J.d.S.; e Silva, P.H.B.; Dutra, A.G.S.; Amaral, C.D.; Abrahão, J.S.; Richini Pereira, V.B.; Kroon, E.G.; Barbosa Costa, G.; et al. Circulation of vaccinia virus in southern and south-eastern wildlife, Brazil. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef]
- Costa, G.B.; de Almeida, L.R.; Cerqueira, A.G.R.; Mesquita, W.U.; de Oliveira, J.S.; Miranda, J.B.; Saraiva-Silva, A.T.; Abrahão, J.S.; Drumond, B.P.; Kroon, E.G.; et al. Vaccinia virus among domestic dogs and wild coatis, Brazil, 2013–2015. Emerg. Infect. Dis. 2018, 24, 2338–2342. [Google Scholar] [CrossRef]
- Assis, F.L.; Borges, I.A.; Peregrino Ferreira, P.C.; Bonjardim, C.A.; de Souza Trindade, G.; Portela Lobato, Z.I.; Maldonado Guedes, M.I.; Mesquita, V.; Kroon, E.G.; Abrahão, J.S. Group 2 vaccinia virus, Brazil. Emerg. Infect. Dis. 2012. [Google Scholar] [CrossRef]
- Peres, M.G.; Barros, C.B.; Appolinário, C.M.; Antunes, J.M.A.P.; Mioni, M.S.R.; Bacchiega, T.S.; Allendorf, S.D.; Vicente, A.F.; Fonseca, C.R.; Megid, J. Dogs and opossums positive for vaccinia virus during outbreak affecting cattle and humans, São Paulo State, Brazil. Emerg. Infect. Dis. 2016, 22, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.M.S.; Drumond, B.P.; Guedes, M.I.M.C.; Pascoal-Xavier, M.A.; Almeida-Leite, C.M.; Arantes, R.M.E.; Mota, B.E.F.; Abrahão, J.S.; Alves, P.A.; Oliveira, F.M.; et al. Virulence in murine model shows the existence of two distinct populations of Brazilian Vaccinia virus strains. PLoS ONE 2008, 3, e3043. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Assis, F.; Almeida, G.; Albarnaz, J.; Lima, M.; Andrade, A.C.; Calixto, R.; Oliveira, C.; Neto, J.D.; Trindade, G.; et al. From lesions to viral clones: Biological and molecular diversity amongst autochthonous Brazilian Vaccinia virus. Viruses 2015, 7, 1218–1237. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Oliveira, T.M.L.; Campos, R.K.; Madureira, M.C.; Kroon, E.G.; Lobato, Z.I.P. Bovine vaccinia outbreaks: Detection and isolation of vaccinia virus in milk samples. Foodborne Pathog. Dis. 2009, 6, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, I.S.; Matos, A.C.D.; Guedes, M.I.M.C.; Costa, A.G.; Fraiha, A.L.S.; Lobato, Z.I.P. Subclinical bovine vaccinia: An important risk factor in the epidemiology of this zoonosis in cattle. Res. Vet. Sci. 2017, 114, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Silva-Fernandes, A.T.; Lima, L.S.; Campos, R.K.; Guedes, M.I.M.C.; Cota, M.M.G.; Assis, F.L.; Borges, I.A.; Souza-Júnior, M.F.; Lobato, Z.I.P.; et al. Vaccinia virus infection in monkeys, Brazilian Amazon. Emerg. Infect. Dis. 2010, 16, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.G.; Bacchiega, T.S.; Appolinário, C.M.; Vicente, A.F.; De Souza Ribeiro Mioni, M.; Ribeiro, B.L.D.; Fonseca, C.R.S.; Pelícia, V.C.; Ferreira, F.; Oliveira, G.P.; et al. Vaccinia virus in blood samples of humans, domestic and wild mammals in Brazil. Viruses 2018, 10, 42. [Google Scholar] [CrossRef]
- Peres, M.G.; Bacchiega, T.S.; Appolinário, C.M.; Vicente, A.F.; Allendorf, S.D.; Antunes, J.M.A.P.; Moreira, S.A.; Legatti, E.; Fonseca, C.R.; Pituco, E.M.; et al. Serological study of vaccinia virus reservoirs in areas with and without official reports of outbreaks in cattle and humans in São Paulo, Brazil. Arch. Virol. 2013, 158, 2433–2441. [Google Scholar] [CrossRef]
- Barbosa, A.V.; Medaglia, M.L.G.; Soares, H.S.; Quixabeira-Santos, J.C.; Gennari, S.M.; Damaso, C.R. Presence of neutralizing antibodies to orthopoxvirus in capybaras (hydrochoerus hydrochaeris) in Brazil. J. Infect. Dev. Ctries. 2014, 8, 1646–1649. [Google Scholar] [CrossRef]
- de Assis, F.L.; Pereira, G.; Oliveira, C.; Rodrigues, G.O.L.; Cotta, M.M.G.; Silva-Fernandes, A.T.; Ferreira, P.C.P.; Bonjardim, C.A.; de Souza Trindade, G.; Kroon, E.G.; et al. Serologic evidence of orthopoxvirus infection in buffaloes, Brazil. Emerg. Infect. Dis. 2012, 18, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Franco-Luiz, A.P.M.; Fagundes Pereira, A.; de Oliveira, C.H.S.; Barbosa, J.D.; Oliveira, D.B.; Bonjardim, C.A.; Ferreira, P.C.P.; de Souza Trindade, G.; Abrahão, J.S.; Kroon, E.G. The detection of Vaccinia virus confirms the high circulation of Orthopoxvirus in buffaloes living in geographical isolation, Marajó Island, Brazilian Amazon. Comp. Immunol. Microbiol. Infect. Dis. 2016. [Google Scholar] [CrossRef]
- Siqueira Ferreira, J.M.; Abrahão, J.S.; Drumond, B.P.; Oliveira, F.M.; Alves, P.A.; Pascoal-Xavier, M.A.; Lobato, Z.I.P.; Bonjardim, C.A.; Peregrino Ferreira, P.C.; Kroon, E.G. Vaccinia virus: Shedding and horizontal transmission in a murine model. J. Gen. Virol. 2008, 89, 2986–2991. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.B.; Lavergne, A.; Darcissac, E.; Lacoste, V.; Drumond, B.P.; Abrahão, J.S.; Kroon, E.G.; de Thoisy, B.; de Souza Trindade, G. Absence of vaccinia virus detection in a remote region of the Northern Amazon forests, 2005–2015. Arch. Virol. 2017, 162, 2369–2373. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; de Souza Trindade, G.; Ferreira, J.M.S.; Campos, R.K.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Long-lasting stability of Vaccinia virus strains in murine feces: Implications for virus circulation and environmental maintenance. Arch. Virol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, P.; Zange, S.; Ibrahim, S.; Zoeller, G.; Herbstreit, F.; Meyer, H. Generalized cowpox virus infection in a patient with HIV, Germany, 2012. Emerg. Infect. Dis. 2016, 22, 553. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.S.; Costa, G.B.; Franco Luiz, A.P.M.; Leite, J.A.; Bonjardim, C.A.; Abrahão, J.S.; Drumond, B.P.; Kroon, E.G.; Trindade, G.d.S. Cross-sectional study involving healthcare professionals in a Vaccinia virus endemic area. Vaccine 2017, 35, 3281–3285. [Google Scholar] [CrossRef] [PubMed]
- Lobato, Z.I.P.; Trindade, G.S.; Frois, M.C.M.; Ribeiro, E.B.T.; Dias, G.R.C.; Teixeira, B.M.; Lima, F.A.; Almeida, G.M.F.; Kroon, E.G. Outbreak of exantemal disease caused by Vaccinia virus in human and cattle in Zona da Mata region, Minas Gerais. Arq. Bras. Med. Vet. e Zootec. 2005. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Doty, J.B.; McCollum, A.M.; Olson, V.A.; Nakazawa, Y. Monkeypox re-emergence in Africa: A call to expand the concept and practice of One Health. Expert Rev. Anti. Infect. Ther. 2019, 17, 129–139. [Google Scholar] [CrossRef]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and Disease Emergence: Dynamics at the Wildlife–Livestock–Human Interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef]
- Oliveira, G.P.; Fernandes, A.T.S.; De Assis, F.L.; Alves, P.A.; Luiz, A.P.M.F.; Figueiredo, L.B.; De Almeida, C.M.C.; Travassos, C.E.P.F.; De Souza Trindade, G.; Abrahão, J.S.; et al. Short report: Intrafamilial transmission of Vaccinia virus during a bovine vaccinia outbreak in Brazil: A new insight in viral transmission chain. Am. J. Trop. Med. Hyg. 2014. [Google Scholar] [CrossRef]
- Laiton-Donato, K.; Ávila-Robayo, P.; Páez-Martinez, A.; Benjumea-Nieto, P.; Usme-Ciro, J.A.; Pinzón-Nariño, N.; Giraldo, I.; Torres-Castellanos, D.; Nakazawa, Y.; Patel, N.; et al. Progressive vaccinia acquired through zoonotic transmission in a patient with HIV/AIDS, Colombia. Emerg. Infect. Dis. 2020, 26, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Abubakar, I.; Ihekweazu, C.; Heymann, D.; Ntoumi, F.; Blumberg, L.; Asogun, D.; Mukonka, V.; Lule, S.A.; Bates, M.; et al. Monkeypox—Enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int. J. Infect. Dis. 2019, 78, 78–84. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).