Inhibitory Effects of Laminaria japonica Fucoidans Against Noroviruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Extraction of Fucoidans from Brown Algae
2.3. Cytotoxicity
2.4. Plaque Assay
2.5. Expression and Purification of HuNoV P Domains
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Carbohydrate Composition of the LJ Fucoidan
2.8. Sulfate Contents of the LJ Fucoidan
2.9. In Vivo Mouse Experiment
2.10. Statistical Analysis
3. Results
3.1. Preparation of the Fucoidans and Their Effects on Cell Viability
3.2. In Vitro MNoV and FCV Reduction by the Fucoidans
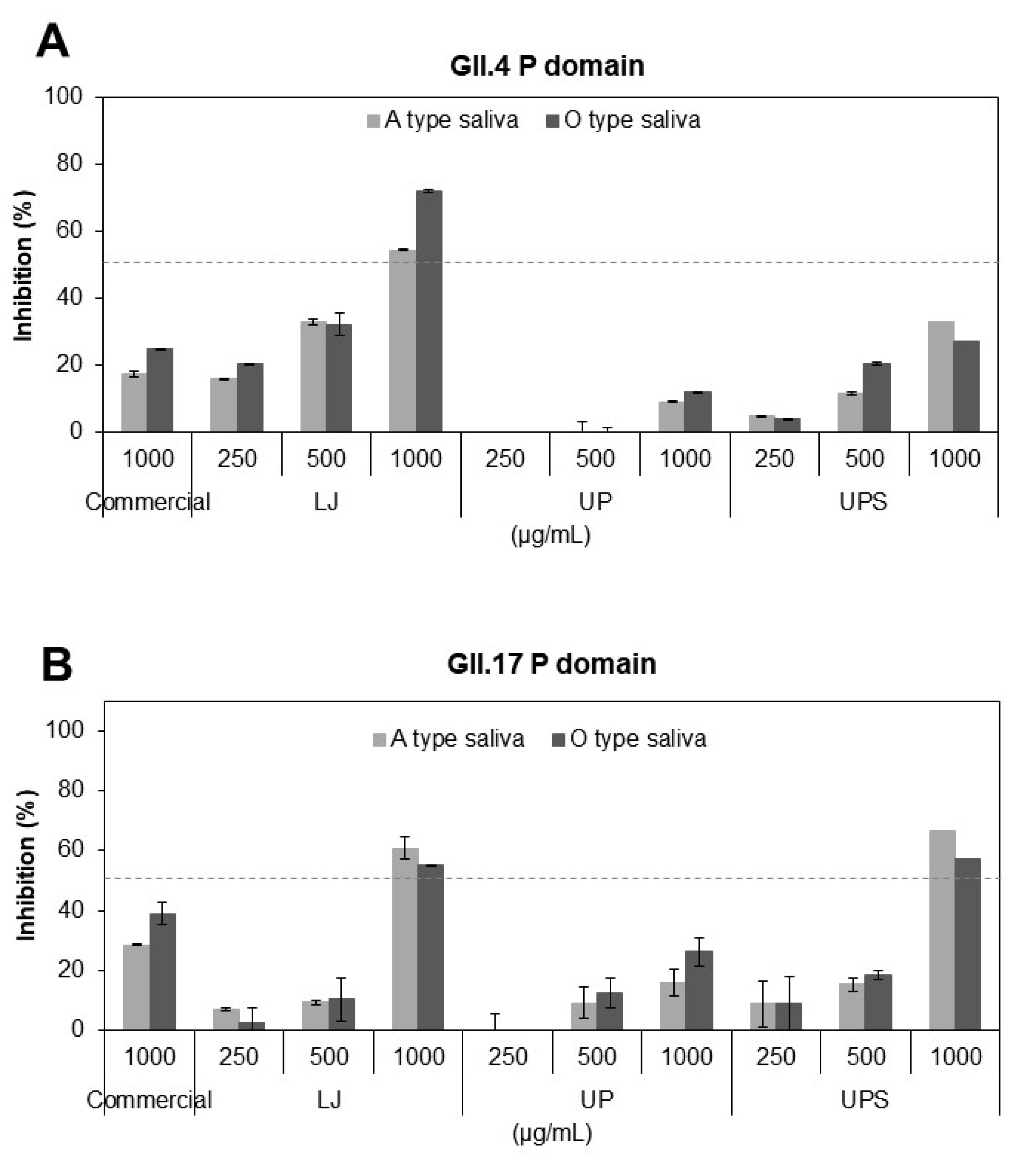
3.3. Inhibitory Effects of the Fucoidans on Binding of HuNoV P Domains to Receptors
3.4. The Chemical Composition of the LJ Fucoidan
3.5. Improvement of Survival Rates in Mice by the LJ Fucoidan
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef]
- Lindesmith, L.; Moe, C.; Marionneau, S.; Ruvoen, N.; Jiang, X.; Lindblad, L.; Stewart, P.; LePendu, J.; Baric, R. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 2003, 9, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Siebenga, J.J.; Beersma, M.F.; Vennema, H.; van Biezen, P.; Hartwig, N.J.; Koopmans, M. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: A model for in vivo molecular evolution. J. Infect. Dis. 2008, 198, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Teunis, P.F.; Moe, C.L.; Liu, P.; Miller, S.E.; Lindesmith, L.; Baric, R.S.; Le Pendu, J.; Calderon, R.L. Norwalk virus: How infectious is it? J. Med. Virol. 2008, 80, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef]
- de Graaf, M.; van Beek, J.; Vennema, H.; Podkolzin, A.T.; Hewitt, J.; Bucardo, F.; Templeton, K.; Mans, J.; Nordgren, J.; Reuter, G.; et al. Emergence of a novel GII.17 norovirus–end of the GII.4 era? Eurosurveillance 2015, 20, 21178. [Google Scholar] [CrossRef]
- Conley, M.J.; McElwee, M.; Azmi, L.; Gabrielsen, M.; Byron, O.; Goodfellow, I.G.; Bhella, D. Calicivirus VP2 forms a portal-like assembly following receptor engagement. Nature 2019, 565, 377–381. [Google Scholar] [CrossRef]
- Thorne, L.G.; Goodfellow, I.G. Norovirus gene expression and replication. J. Gen. Virol. 2014, 95, 278–291. [Google Scholar] [CrossRef]
- Koromyslova, A.; Tripathi, S.; Morozov, V.; Schroten, H.; Hansman, G.S. Human norovirus inhibition by a human milk oligosaccharide. Virology 2017, 508, 81–89. [Google Scholar] [CrossRef]
- Costantini, V.; Morantz, E.K.; Browne, H.; Ettayebi, K.; Zeng, X.L.; Atmar, R.L.; Estes, M.K.; Vinjé, J. Human norovirus in human intestinal enteroids as model to evaluate virus inactivation. Emerg. Infect. Dis. 2018, 24, 1453–1464. [Google Scholar] [CrossRef]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.L.; Qu, L.; et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Bhar, S.; Jones, M.K. In vitro replication of human norovirus. Viruses 2019, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J. Surrogate viruses for testing virucidal efficacy of chemical disinfectants. J. Hosp. Infect. 2004, 56, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wobus, C.E.; Thackray, L.B.; Virgin, H.W. Murine norovirus: A model system to study norovirus biology and pathogenesis. J. Virol. 2006, 80, 5104–5112. [Google Scholar] [CrossRef]
- Huang, P.; Farkas, T.; Marionneau, S.; Zhong, W.; Ruvoen-Clouet, N.; Morrow, A.L. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: Identification of 4 distinct strain-specific patterns. J. Infect. Dis. 2003, 188, 19–31. [Google Scholar] [CrossRef]
- Huang, P.; Farkas, T.; Zhong, W.; Tan, M.; Thornton, S.; Morrow, A.L.; Jiang, X. Norovirus and histo-blood group antigens: Demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol. 2005, 79, 6714–6722. [Google Scholar] [CrossRef]
- Kubota, T.; Kumagai, A.; Ito, H.; Furukawa, S.; Someya, Y.; Takeda, N.; Ishii, K.; Wakita, T.; Narimatsu, H.; Shirato, H. Structural basis for the recognition of Lewis antigens by genogroup I norovirus. J. Virol. 2012, 86, 11138–11150. [Google Scholar] [CrossRef]
- Shanker, S.; Choi, J.M.; Sankaran, B.; Atmar, R.L.; Estes, M.K.; Prasad, B.V. Structural Analysis of Histo-Blood Group Antigen Binding Specificity in a Norovirus GII.4 Epidemic Variant: Implications for Epochal Evolution. J. Virol. 2011, 85, 8635–8645. [Google Scholar] [CrossRef]
- Tan, M.; Jiang, X. Norovirus-host interaction: Multi-selections by human histo-blood group antigens. Trends Microbiol. 2011, 19, 382–388. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, P.; Zhong, W.; Tan, M.; Farkas, T.; Morrow, A.L.; Newburg, D.S.; Ruiz-Palacios, G.M.; Pickering, L.K. Human milk contains elements that block binding of noroviruses to human histo-blood group antigens in saliva. J. Infect. Dis. 2004, 190, 1850–1859. [Google Scholar] [CrossRef]
- Morozov, V.; Hansman, G.; Hanisch, F.G.; Schroten, H.; Kunz, C. Human milk oligosaccharides as promising antivirals. Mol. Nutr. Food Res. 2018, 62, e1700679. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Piskarev, V.E.; Xia, M.; Huang, P.; Jiang, X.; Likhosherstov, L.M.; Novikova, O.S.; Newburg, D.S.; Ratner, D.M. Identifying human milk glycans that inhibit norovirus binding using surface plasmon resonance. Glycobiology 2013, 23, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Weichert, S.; Koromyslova, A.; Singh, B.K.; Hansman, S.; Jennewein, S.; Schroten, H.; Hansman, G.S. Structural basis for norovirus inhibition by human milk oligosaccharides. J. Virol. 2016, 90, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Yang, H.; Zeng, M.; Dong, S.; Liu, Z.; Li, R. Anti-proliferative activity of phlorotannin extracts from brown algae Laminaria japonica Aresch. Chin. J. Oceanol. Limnol. 2010, 28, 122–130. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Cao, M.J.; Liu, G.M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Q.; Wang, J.; Zhang, W. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef]
- Dinesh, S.; Menon, T.; Hanna, L.E.; Suresh, V.; Sathuvan, M.; Manikannan, M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016, 82, 83–88. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Zhang, X.; Hao, C.; Zhao, X.; Jiao, G. Inhibition of influenza A virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci. Rep. 2017, 7, 40760. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Sun, H.; Wang, Z.; Ermakova, S.P.; Xiao, J.; Lu, T.; Xue, P.; Zvyagintseva, T.N.; Xiong, H.; Shao, C.; et al. PDZ-binding kinase/T-LAK cell-originated protein kinase is a target of the fucoidan from brown alga Fucus evanescens in the prevention of EGF-induced neoplastic cell transformation and colon cancer growth. Oncotarget 2016, 7, 18763–18773. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.; Kim, J.; Kim, H.; Seok, J.H.; Lee, D.B.; Kim, K.H.; Chung, M.S. Inactivation of norovirus surrogates by kimchi fermentation in the presence of black raspberry. Food Control 2018, 91, 390–396. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.J.; Park, Y.B.; Woo, H.C.; Chun, B.S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Kilic, T.; Koromyslova, A.; Malak, V.; Hansman, G.S. Atomic structure of the murine norovirus protruding domain and soluble CD300lf receptor complex. J. Virol. 2018, 92, e00413-18. [Google Scholar] [CrossRef]
- Manns, D.; Deutschle, A.L.; Saake, B.; Meyer, A.S. Methodology for quantitative determination of the carbohydrate composition of brown seaweeds (Laminariaceae). RSC Adv. 2014, 4, 25736–25746. [Google Scholar] [CrossRef]
- Tabatabai, M.A. A rapid method for determination of sulfate in water samples. Environ. Lett. 1974, 7, 237–243. [Google Scholar] [CrossRef]
- Bartsch, S.M.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B.Y. Global economic burden of norovirus gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef]
- Joshi, S.S.; Howell, A.B.; D’Souza, D.H. Reduction of enteric viruses by blueberry juice and blueberry proanthocyanidins. Food Environ. Virol. 2016, 8, 235–243. [Google Scholar] [CrossRef]
- Falcó, I.; Randazzo, W.; Gómez-Mascaraque, L.G.; Aznar, R.; López-Rubio, A.; Sánchez, G. Fostering the antiviral activity of green tea extract for sanitizing purposes through controlled storage conditions. Food Control 2018, 84, 485–492. [Google Scholar] [CrossRef]
- Li, D.; Baert, L.; Zhang, D.; Xia, M.; Zhong, W.; Van Coillie, E.; Jiang, X.; Uyttendaele, M. Effect of grape seed extract on human norovirus GII.4 and murine norovirus 1 in viral suspensions, on stainless steel discs, and in lettuce wash water. Appl. Environ. Microbiol. 2012, 78, 7572–7578. [Google Scholar] [CrossRef]
- Yang, M.; Lee, G.; Si, J.; Lee, S.J.; You, H.J.; Ko, G. Curcumin shows antiviral properties against norovirus. Molecules 2016, 21, 1401–1442. [Google Scholar] [CrossRef]
- Oh, M.; Lee, J.H.; Bae, S.Y.; Seok, J.H.; Kim, S.; Chung, Y.B.; Han, K.R.; Kim, K.H.; Chung, M.S. Protective effects of red wine and resveratrol for foodborne virus surrogates. Food Control 2015, 47, 502–509. [Google Scholar] [CrossRef]
- Hanisch, F.G.; Hansman, G.S.; Morozov, V.; Kunz, C.; Schroten, H. Avidity of α-fucose on human milk oligosaccharides and blood group-unrelated oligo/polyfucoses is essential for potent norovirus-binding targets. J. Biol. Chem. 2018, 293, 11955–11965. [Google Scholar] [CrossRef]
- Koromyslova, A.D.; White, P.A.; Hansman, G.S. Treatment of norovirus particles with citrate. Virology 2015, 485, 199–204. [Google Scholar] [CrossRef]
- Karst, S.M.; Wobus, C.E.; Lay, M.; Davidson, J.; Virgin, H.W. STAT1-dependent innate immunity to a Norwalk-like virus. Science 2003, 299, 1575–1578. [Google Scholar] [CrossRef]
- Mumphrey, S.M.; Changotra, H.; Moore, T.N.; Heimann-Nichols, E.R.; Wobus, C.E.; Reilly, M.J.; Moghadamfalahi, M.; Shukla, D.; Karst, S.M. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J. Virol. 2007, 81, 3251–3263. [Google Scholar] [CrossRef]
- Rocha-Pereira, J.; Kolawole, A.O.; Verbeken, E.; Wobus, C.E.; Neyts, J. Post-exposure antiviral treatment of norovirus infections effectively protects against diarrhea and reduces virus shedding in the stool in a mortality mouse model. Antivir. Res. 2016, 132, 76–84. [Google Scholar] [CrossRef]
- FAO. The global status of seaweed production, trade and utilization. Globefish Res. Programme 2018, 124, 120. [Google Scholar]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef]
- Lu, J.; Shi, K.K.; Chen, S.; Wang, J.; Hassouna, A.; White, L.N.; Merien, F.; Xie, M.; Kong, Q.; Li, J.; et al. Fucoidan extracted from the New Zealand Undaria pinnatifida—physicochemical comparison against five other fucoidans: Unique low molecular weight fraction bioactivity in breast cancer cell lines. Mar. Drugs 2018, 16, 461. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, J.; Ren, S.; Song, N.; Zhang, Q. Structural analysis of a heteropolysaccharide from Saccharina japonica by electrospray mass spectrometry in tandem with collision-induced dissociation tandem mass spectrometry (ESI-CID-MS/MS). Mar. Drugs 2012, 10, 2138–2152. [Google Scholar] [CrossRef] [PubMed]

| Sample | Conc. (µg/mL) | MNoV | FCV | ||
|---|---|---|---|---|---|
| Titer (log PFU/mL) | Log Reduction | Titer (log PFU/mL) | Log Reduction | ||
| PBS | 6.18 ± 0.03 a | - | 6.66 ± 0.03 a | - | |
| Commercial | 10 | 6.10 ± 0.04 a | 0.08 | 6.48 ± 0.09 b | 0.18 |
| 100 | 5.79 ± 0.09 b | 0.39 | 6.27 ± 0.02 c | 0.39 | |
| 1000 | 5.11 ± 0.08 c | 1.07 | 5.72 ± 0.03 d | 0.94 | |
| LJ | 10 | 6.07 ± 0.03 a | 0.11 | 6.46 ± 0.02 b | 0.20 |
| 100 | 5.86 ± 0.05 b | 0.32 | 6.14 ± 0.06 c | 0.52 | |
| 1000 | 4.80 ± 0.10 c | 1.38 | 5.48 ± 0.07 d | 1.18 | |
| UP | 10 | 6.01 ± 0.10 a | 0.17 | 6.54 ± 0.02 ab | 0.12 |
| 100 | 5.95 ± 0.07 b | 0.23 | 6.45 ± 0.06 b | 0.21 | |
| 1000 | 5.50 ± 0.04 c | 0.66 | 5.90 ± 0.03 c | 0.76 | |
| UPS | 10 | 5.89 ± 0.12 bc | 0.29 | 6.38 ± 0.08 bc | 0.28 |
| 100 | 5.83 ± 0.04 c | 0.35 | 6.27 ± 0.01 c | 0.39 | |
| 1000 | 5.08 ± 0.05 d | 1.10 | 5.32 ± 0.10 d | 1.34 | |
| Sulfate (%) | Monosaccharides (%) | ||||||
|---|---|---|---|---|---|---|---|
| Fucose | Galactose | Glucose | Mannose | Rhamnose | Xylose | ||
| Laminaria japonica | 25.8 | 23.6 | 2.8 | 0.3 | 1.5 | 0 | 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Lim, C.Y.; Lee, D.B.; Seok, J.H.; Kim, K.H.; Chung, M.S. Inhibitory Effects of Laminaria japonica Fucoidans Against Noroviruses. Viruses 2020, 12, 997. https://doi.org/10.3390/v12090997
Kim H, Lim CY, Lee DB, Seok JH, Kim KH, Chung MS. Inhibitory Effects of Laminaria japonica Fucoidans Against Noroviruses. Viruses. 2020; 12(9):997. https://doi.org/10.3390/v12090997
Chicago/Turabian StyleKim, Hyojin, Chae Yeon Lim, Dan Bi Lee, Jong Hyeon Seok, Kyung Hyun Kim, and Mi Sook Chung. 2020. "Inhibitory Effects of Laminaria japonica Fucoidans Against Noroviruses" Viruses 12, no. 9: 997. https://doi.org/10.3390/v12090997
APA StyleKim, H., Lim, C. Y., Lee, D. B., Seok, J. H., Kim, K. H., & Chung, M. S. (2020). Inhibitory Effects of Laminaria japonica Fucoidans Against Noroviruses. Viruses, 12(9), 997. https://doi.org/10.3390/v12090997




