Effect of Postmortem Degradation on the Preservation of Viral Particles and Rabies Antigens in Mice Brains. Light and Electron Microscopic Study
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Animal Handling, Virus Inoculation, and Collection of the Infected Tissue
2.3. Histological and Immunohistochemical Processing
2.4. Electron Microscopy
2.5. Pre-Embedding Ultrastructural Immunocytochemistry
3. Results
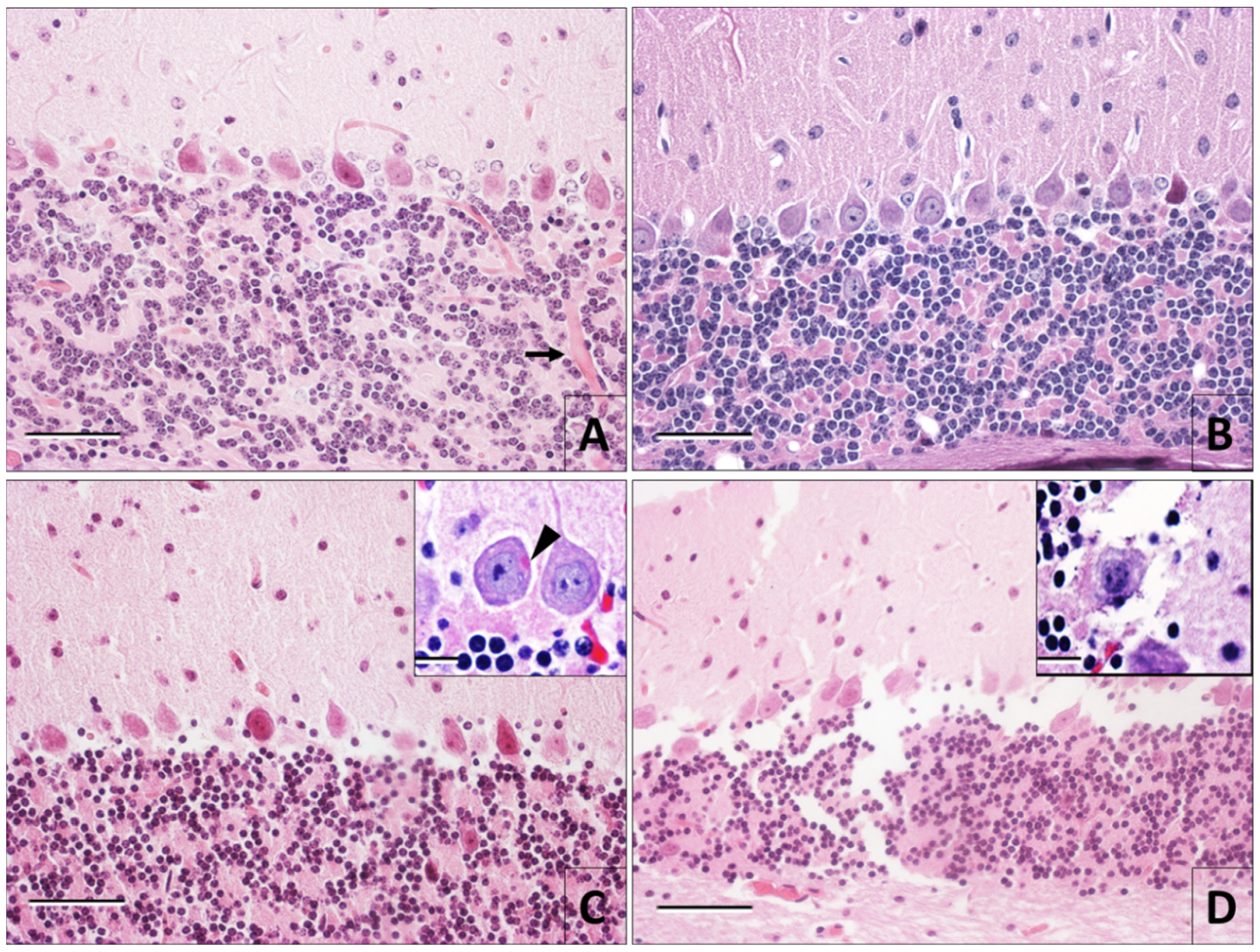
3.1. Hematoxylin and Eosin Staining
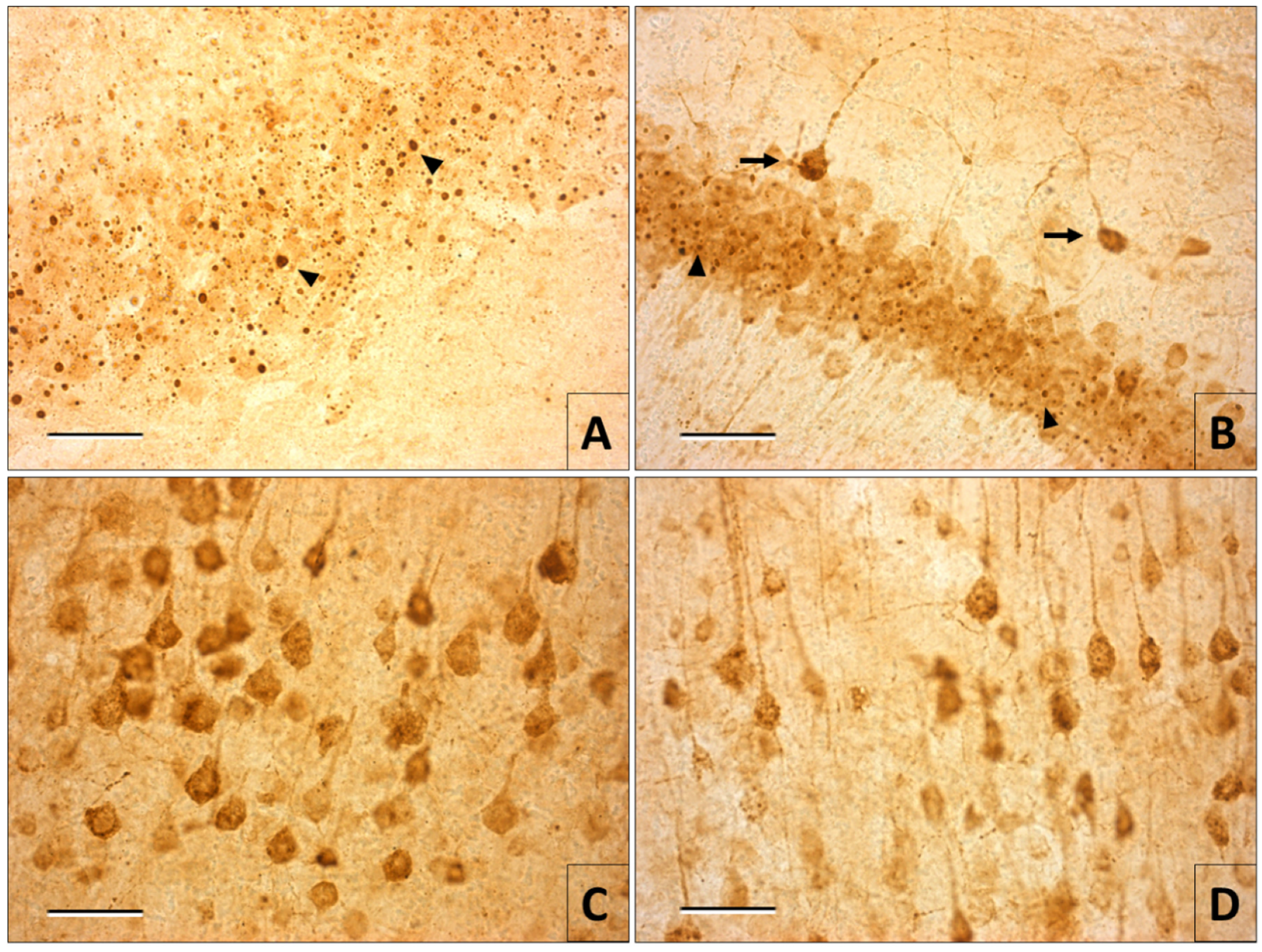
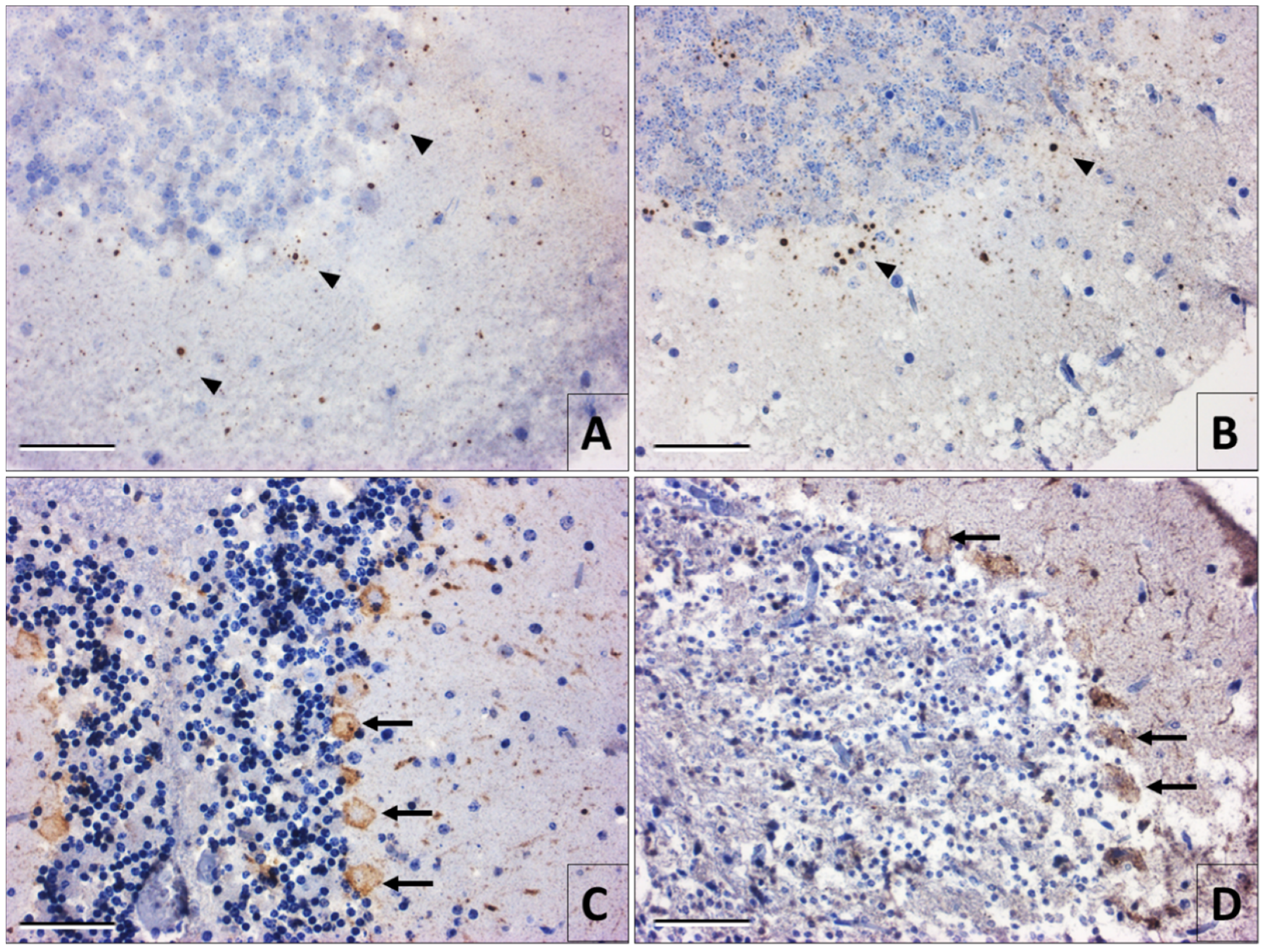
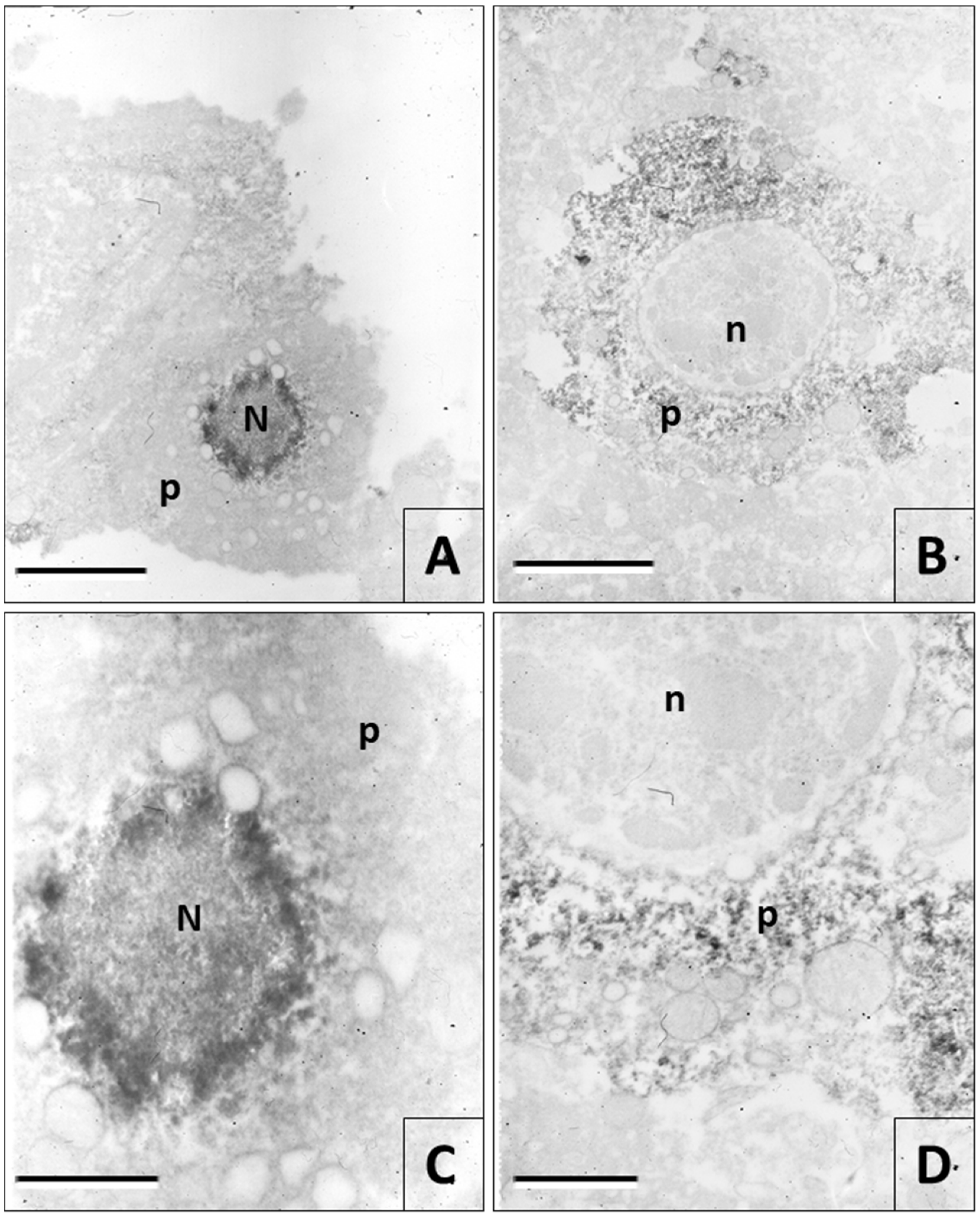
3.2. Immunohistochemistry of Rabies
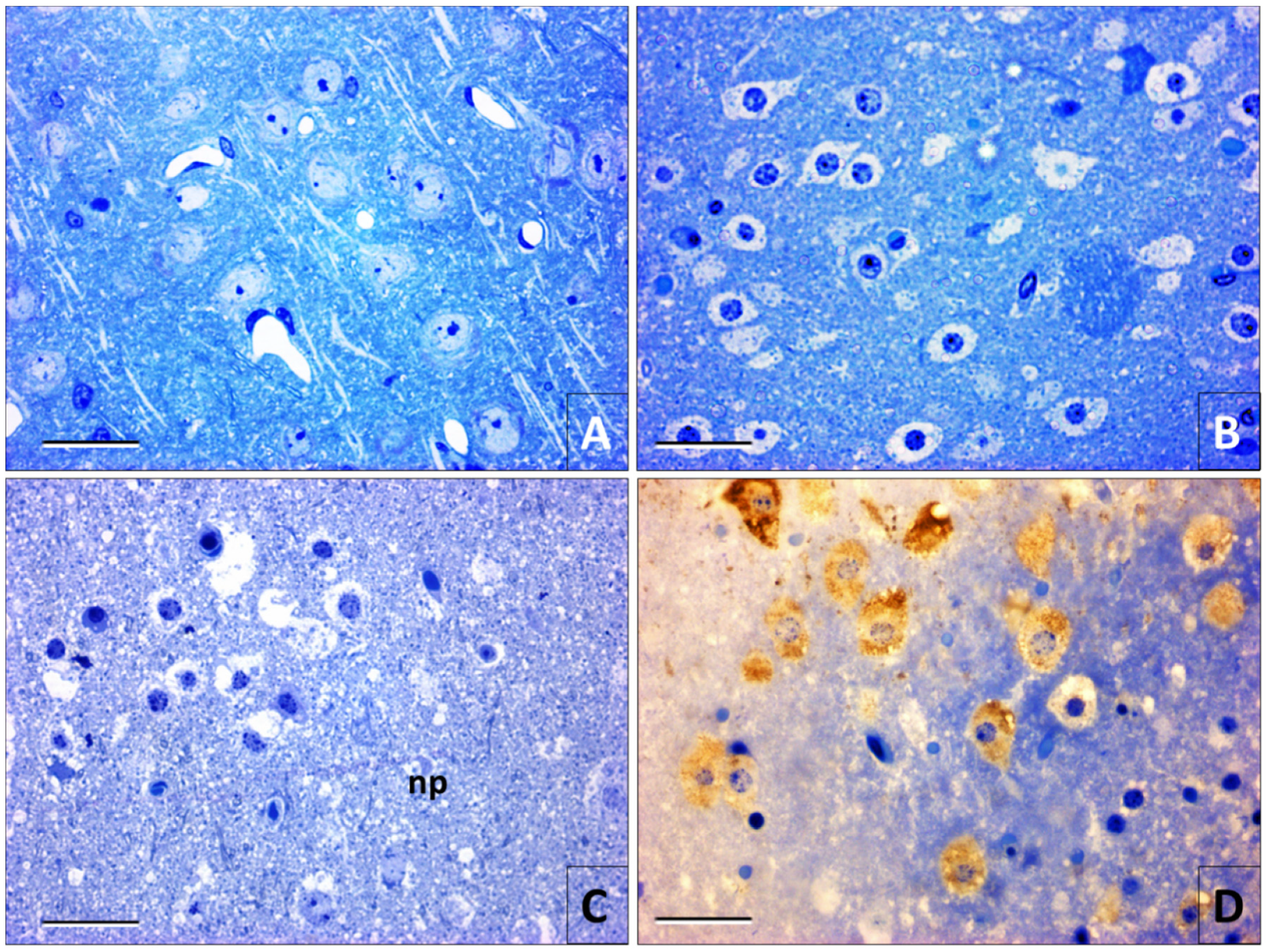
3.3. High-Resolution Optical Microscopy
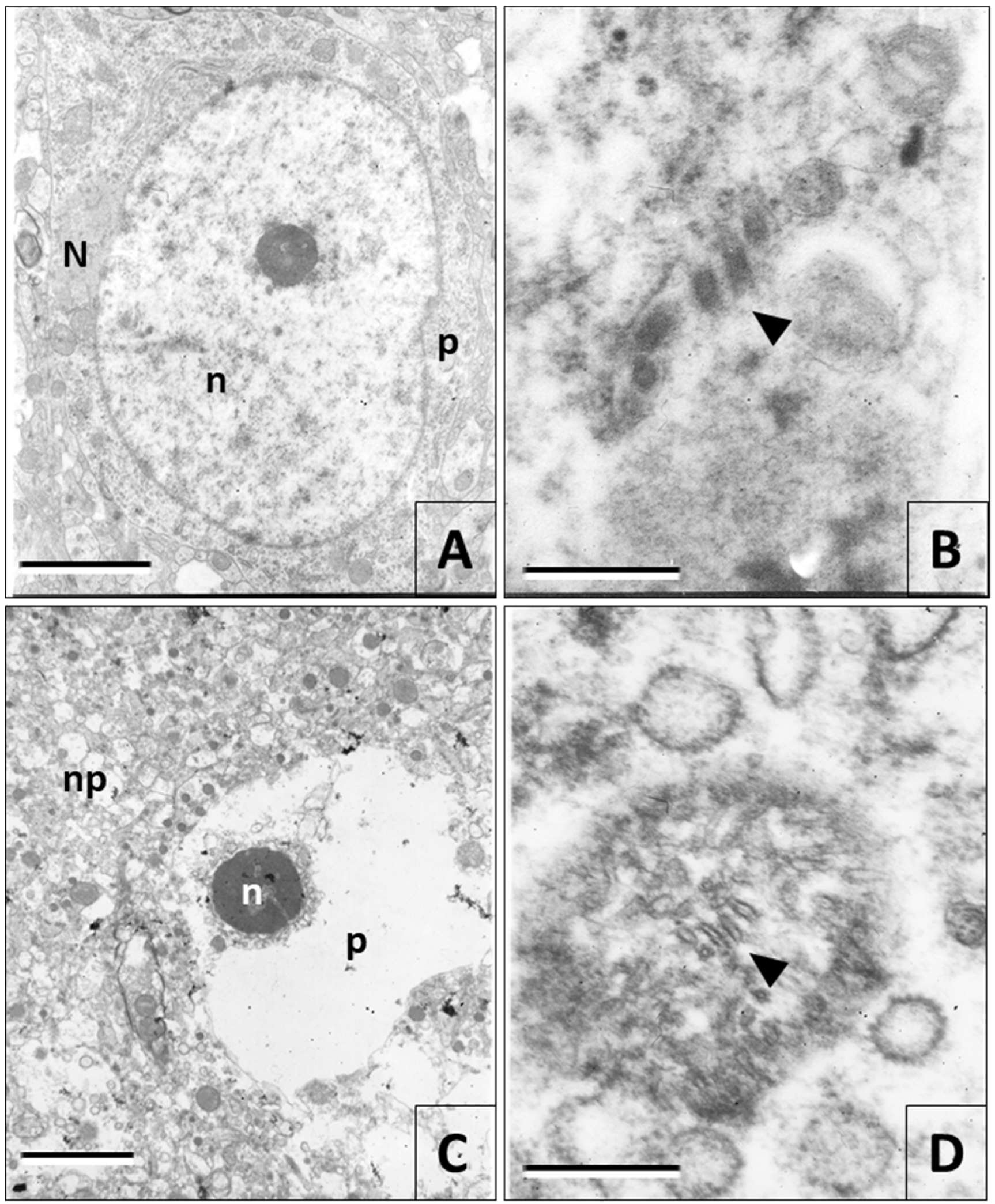
3.4. Electron Microscopy
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Expert Consultation on rabies. Technical Report Series No. 982; WHO Press: Geneva, Switzerland, 2013; pp. 1–139. [Google Scholar]
- Rodríguez, G.; Sarmiento, L. Rabia: El cuerpo de Negri. Biomédica 1999, 19, 196–197. [Google Scholar] [CrossRef]
- Trimarchi, C.; Smith, J. Diagnostic evaluation. In Rabies; Jackson, A.C., Wunner, W., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 307–349. [Google Scholar]
- Mani, R.S.; Madhusudana, S.N. Laboratory diagnosis of human rabies: Recent advances. Sci. World J. 2013, 2013, 569712. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, H.; Bidmon, H.; Oppermann, O.; Remmerbach, T. Influence of post-mortem delay and storage temperature on the immunohistochemical detection of antigens in the CNS of mice. Exp. Toxicol. Pathol. 2004, 56, 159–171. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, L.M.; Marston, D.A.; Brookes, S.M.; Fooks, A.R. Effects of carcase decomposition on rabies virus infectivity and detection. J. Virol. Methods 2014, 207, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Albas, A.; Ferrari, C.I.; da Silva, L.H.; Bernardi, F.; Ito, F.H. Influence of canine brain decomposition on laboratory diagnosis of rabies. Rev. Soc. Bras. Med. Trop. 1999, 32, 19–22. [Google Scholar] [CrossRef]
- David, D.; Yakobson, B.; Rotenberg, D.; Dveres, N.; Davidson, I.; Stram, Y. Rabies virus detection by RT-PCR in decomposed naturally infected brains. Vet. Microbiol. 2002, 20, 111–118. [Google Scholar] [CrossRef]
- Sarmiento, L.; Rodríguez, G.; De Serna, C.; Boshell, J.; Orozco, L. Detection of rabies virus antigens in tissue: Immunoenzymatic method. Patología 1999, 37, 7–10. [Google Scholar]
- Lamprea, N.; Ortega, L.; Santamaría, G.; Sarmiento, L.; Torres-Fernández, O. Elaboración y evaluación de un antisuero para la detección inmunohistoquímica del virus de la rabia en tejido cerebral fijado en aldehídos. Biomédica 2010, 30, 146–151. [Google Scholar] [CrossRef][Green Version]
- Stein, L.T.; Rech, R.R.; Harrison, L.; Brown, C.C. Immunohistochemical study of rabies virus within the central nervous system of domestic and wildlife species. Vet. Pathol. 2010, 47, 630–636. [Google Scholar] [CrossRef]
- Lembo, T.; Niezgoda, M.; Velasco-Villa, A.; Cleaveland, S.; Ernest, E.; Rupprecht, C. Evaluation of a direct, rapid immunohistochemical test for rabies diagnosis. Emerg. Infect. Dis. 2006, 12, 310–313. [Google Scholar] [CrossRef]
- Dyer, J.L.; Niezgoda, M.; Orciari, L.A.; Yager, P.A.; Ellison, J.A.; Rupprecht, C.E. Evaluation of an indirect rapid immunohistochemistry test for the differentiation of rabies virus variants. J. Virol. Methods 2013, 190, 29–333. [Google Scholar] [CrossRef] [PubMed]
- Hummeler, K.; Atanasiu, P. Electron microscopy. In Laboratory Techniques in Rabies; Maslin, F.X., Kaplan, M.M., Koprowski, H., Eds.; World Health Organization: Geneva, Switzerland, 1996; pp. 209–217. [Google Scholar]
- Torres-Fernández, O.; Monroy-Gómez, J.; Sarmiento, L. Unusual Ultrastructural Findings in Dendrites of Pyramidal Neurons in the Cerebral Cortex of Rabies-Infected Mice. Available online: https://peerj.com/preprints/847v1/ (accessed on 23 April 2020).
- Torres-Fernández, O.; Monroy-Gómez, J.; Sarmiento, L. Ultraestructura dendrítica en neuronas piramidales de ratones inoculados con virus de la rabia. Biosalud 2016, 15, 9–16. [Google Scholar] [CrossRef]
- Monroy-Gómez, J.; Torres-Fernández, O. Distribución de calbindina y parvoalbúmina y efecto del virus de la rabia sobre su expresión en la médula espinal de ratones. Biomédica 2013, 33, 564–573. [Google Scholar] [CrossRef]
- Monroy-Gómez, J.; Santamaría, S.; Torres-Fernández, O. Overexpression of MAP2 and NF-H associated with dendritic pathology in the spinal cord of mice infected with rabies virus. Viruses 2018, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Da-Silva, A.; Priestleym, J.; Cuello, C. Pre-embedding ultrastructural immunocytochemistry. In Immunohistochemistry II; Cuello, C., Ed.; John Wiley & Sons Inc.: Chichester, UK, 1993; pp. 181–227. [Google Scholar]
- Santos, B.; Del-Bel, E.; Homem, J.; Tumas, V. Influence of external factors on the preservation of human nervous tissue for histological studies: Review article. J. Bras. Patol. Med. Lab. 2014, 50, 438–444. [Google Scholar] [CrossRef]
- Lavenex, P.; Lavenex, P.B.; Bennett, J.L.; Amaral, D.G. Post mortem changes in the neuroanatomical characteristics of the primate brain: Hippocampal formation. J. Comp. Neurol. 2009, 512, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Tagawa, K.; Ito, H.; Okazawa, H. Dynamic changes of the phosphoproteome in post mortem mouse brains. PLoS ONE 2011, 6, e21405. [Google Scholar] [CrossRef]
- Yin, J.F.; Ding, Y.L.; Huang, Y.; Tao, X.Y.; Li, H.; Yu, P.C.; Shen, X.X.; Jiao, W.T.; Liang, G.D.; Tang, Q.; et al. Comparative analysis of the pathogenic mechanisms of street rabies virus strains with different virulence levels. Biomed. Environ. Sci. 2014, 27, 749–762. [Google Scholar] [CrossRef]
- Torres-Fernández, O.; Santamaría, G.; Rengifo, A.; Monroy-Gómez, J.; Hurtado, A.; Rivera, J.; Sarmiento, L. Patología dendrítica en rabia: Estudio neurohistológico, inmunohistoquímico y ultraestructural en ratones. Rev. Asoc. Colomb. Cienc. Biol. 2014, 26, 96–107. [Google Scholar]
- Rossiter, J.; Jackson, A.C. Pathology. In Rabies; Jackson, A.C., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 351–386. [Google Scholar]
- Jackson, A.C.; Randle, E.; Lawrance, G.; Rossiter, J. Neuronal apoptosis does not play an important role in human rabies encephalitis. J. Neurovirol. 2008, 14, 368–375. [Google Scholar] [CrossRef]
- Suja, M.; Mahadevan, A.; Madhusudana, S.; Shankar, S. Role of apoptosis in rabies viral encephalitis: A comparative study in mice, canine, and human brain with a review of literature. Pathol. Res. Int. 2011, 2011, 374286. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kondo, M.; Inoue, S.; Noguchi, A.; Oyamada, T.; Yoshikawa, H.; Yamada, A. The histopathogenesis of paralytic rabies in six-week-old C57BL/6J mice following inoculation of the CVS-11 strain into the right triceps surae muscle. J. Vet. Med. Sci. 2006, 68, 589–595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kojima, D.; Park, C.H.; Tsujikawa, S.; Kohara, K.; Hatai, H.; Oyamada, T.; Noguchi, A.; Inoue, S. Lesions of the central nervous system induced by intracerebral inoculation of BALB/c mice with rabies virus (CVS-11). J. Vet. Med. Sci. 2010, 72, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- King, A.A.; Fooks, A.R.; Aubert, M.; Wandeler, A.I. Historical Perspectives of Rabies in Europe and the Mediterranean Basin Paris; World Organization for Animal Health (OIE): Paris, France, 2004; pp. 1–383. [Google Scholar]
- Madhusudana, S.; Paul, J.; Abhilash, V.; Suja, M. Rapid diagnosis of rabies in humans and animals by a dot blot enzyme immunoassay. Int. J. Infect. Dis. 2004, 8, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Panning, M.; Baumgarte, S.; Pfefferl, S.; Maier, T.; Martens, A.; Drosten, C. Comparative analysis of rabies virus reverse transcription-PCR and virus isolation using samples from a patient infected with rabies virus. J. Clin. Microbiol. 2010, 48, 2960–2962. [Google Scholar] [CrossRef]
- Monroy-Gómez, J.; Torres-Fernández, O. Efecto de la degradación post mortem sobre la detección inmunohistoquímica de antígenos en el cerebro de ratón. Rev. Investig. Salud Univ. Boyacá 2014, 1, 45–62. [Google Scholar] [CrossRef]
- Gigante, C.M.; Dettinger, L.; Powell, J.W.; Seiders, M.; Condori, R.E.C.; Griesser, R.; Okogi, K.; Carlos, M.; Pesko, K. Multi-site evaluation of the LN34 pan-lyssavirus real-time RT-PCR assay for post-mortem rabies diagnostics. PLoS ONE 2018, 13, e0197074. [Google Scholar] [CrossRef]
- Araújo, D.; Langoni, H.; Almeida, M.F.; Megid, J. Heminested reverse-transcriptase polymerase chain reaction (hnRT-PCR) as a tool for rabies virus detection in stored and decomposed samples. BMC Res. Notes 2008, 1, 17. [Google Scholar] [CrossRef]
- Lopes, M.C.; Venditti, L.L.; Queiroz, L.H. Comparison between RT-PCR and the mouse inoculation test for detection of rabies virus in samples kept for long periods under different conditions. J. Virol. Methods 2010, 164, 19–23. [Google Scholar] [CrossRef]
- Beltran, F.J.; Dohmen, F.G.; Del Pietro, H.; Cisterna, D.M. Diagnosis and molecular typing of rabies virus in samples stored in inadequate conditions. J. Infect. Dev. Ctries. 2014, 8, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Baskin, D. Fixation and tissue processing in immunohistochemistry. In A Dynamic Encyclopedia of Disease Mechanisms Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: Amsterdam, The Netherlands, 2014; pp. 3797–3806. [Google Scholar]
- Jurado, G.; Montoya-Flórez, L.; Betancur, C.; Pedraza-Ordoñez, F. Uso de la inmunohistoquímica como herramienta epidemiológica para el diagnóstico de rabia bovina a partir de casos no conclusivos. Rev. Mvz. Córdoba 2012, 17, 3065–3070. [Google Scholar] [CrossRef][Green Version]
- Dürr, S.; Naïssengar, S.; Mindekem, R.; Diguimbye, C.; Niezgoda, M.; Kuzmin, I.; Rupprecht, C.; Zinsstag, J. Rabies diagnosis for developing countries. PLoS Negl. Trop. Dis. 2008, 2, e206. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroy-Gómez, J.; Santamaría, G.; Sarmiento, L.; Torres-Fernández, O. Effect of Postmortem Degradation on the Preservation of Viral Particles and Rabies Antigens in Mice Brains. Light and Electron Microscopic Study. Viruses 2020, 12, 938. https://doi.org/10.3390/v12090938
Monroy-Gómez J, Santamaría G, Sarmiento L, Torres-Fernández O. Effect of Postmortem Degradation on the Preservation of Viral Particles and Rabies Antigens in Mice Brains. Light and Electron Microscopic Study. Viruses. 2020; 12(9):938. https://doi.org/10.3390/v12090938
Chicago/Turabian StyleMonroy-Gómez, Jeison, Gerardo Santamaría, Ladys Sarmiento, and Orlando Torres-Fernández. 2020. "Effect of Postmortem Degradation on the Preservation of Viral Particles and Rabies Antigens in Mice Brains. Light and Electron Microscopic Study" Viruses 12, no. 9: 938. https://doi.org/10.3390/v12090938
APA StyleMonroy-Gómez, J., Santamaría, G., Sarmiento, L., & Torres-Fernández, O. (2020). Effect of Postmortem Degradation on the Preservation of Viral Particles and Rabies Antigens in Mice Brains. Light and Electron Microscopic Study. Viruses, 12(9), 938. https://doi.org/10.3390/v12090938




