A Mouse Model of Oropharyngeal Papillomavirus-Induced Neoplasia Using Novel Tools for Infection and Nasal Anesthesia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. High-Titer Crude MmuPV1 Virus Preparation
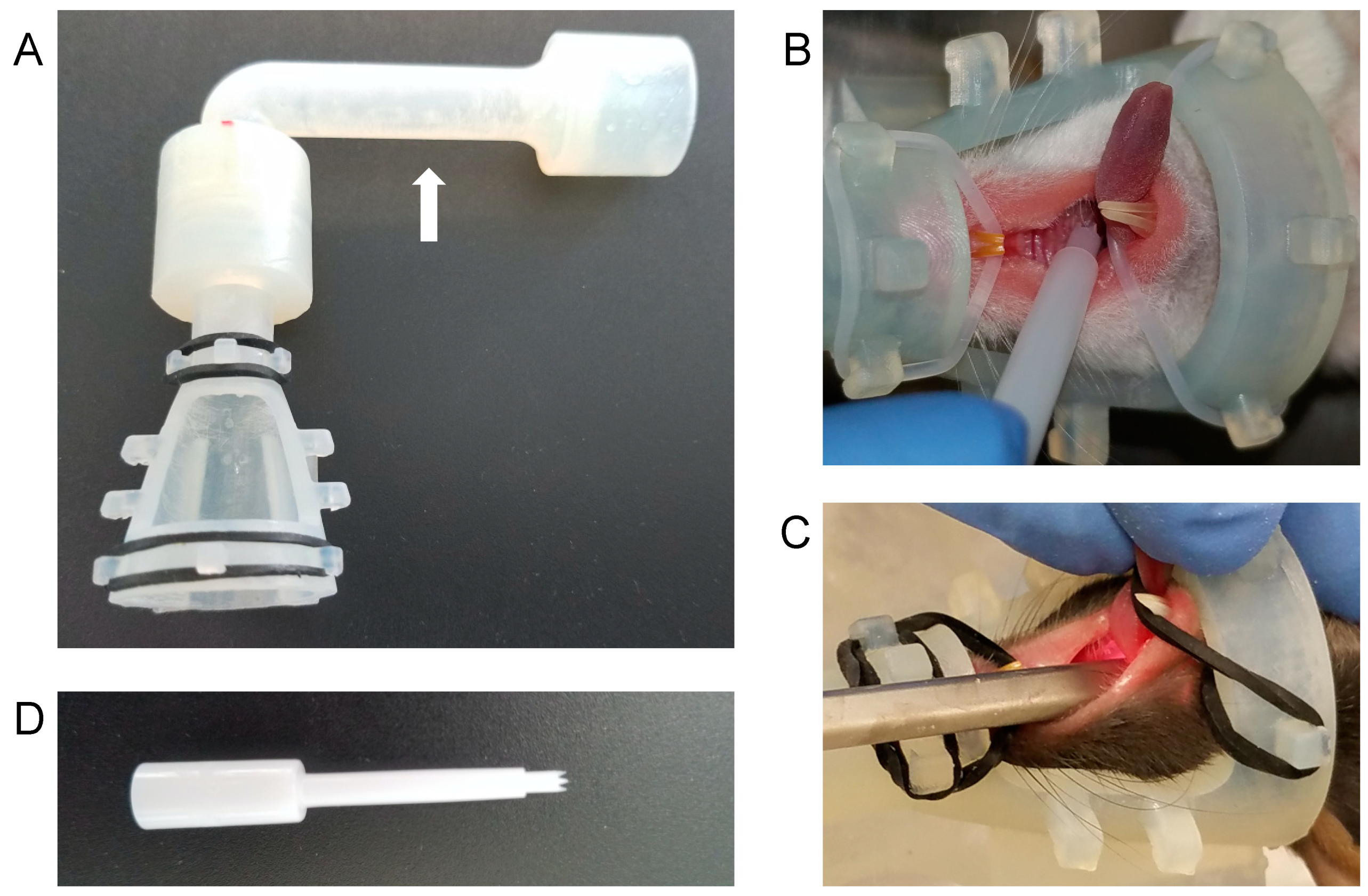
2.3. Nose Cone Design and Fabrication
2.4. Anesthesia and Infection
2.5. Oropharyngeal Endoscopy
2.6. Tissue Analysis
2.7. RNA/DNA in Situ Hybridization
3. Results
3.1. A Novel Isoflurane Cone Maintains Anesthesia during Manipulation of the Throat
3.2. The Greer “Pick” Efficiently and Effectively Delivers Virus to the Oropharynx
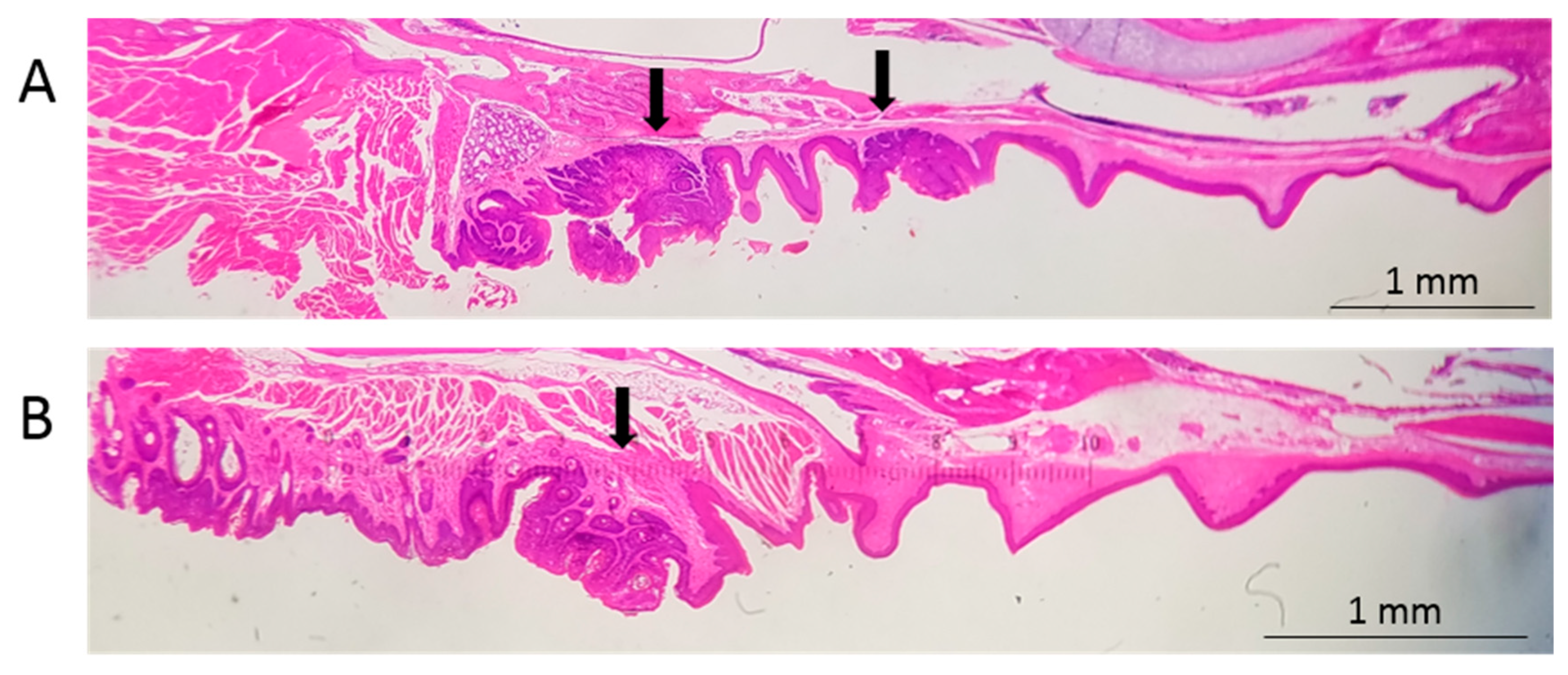
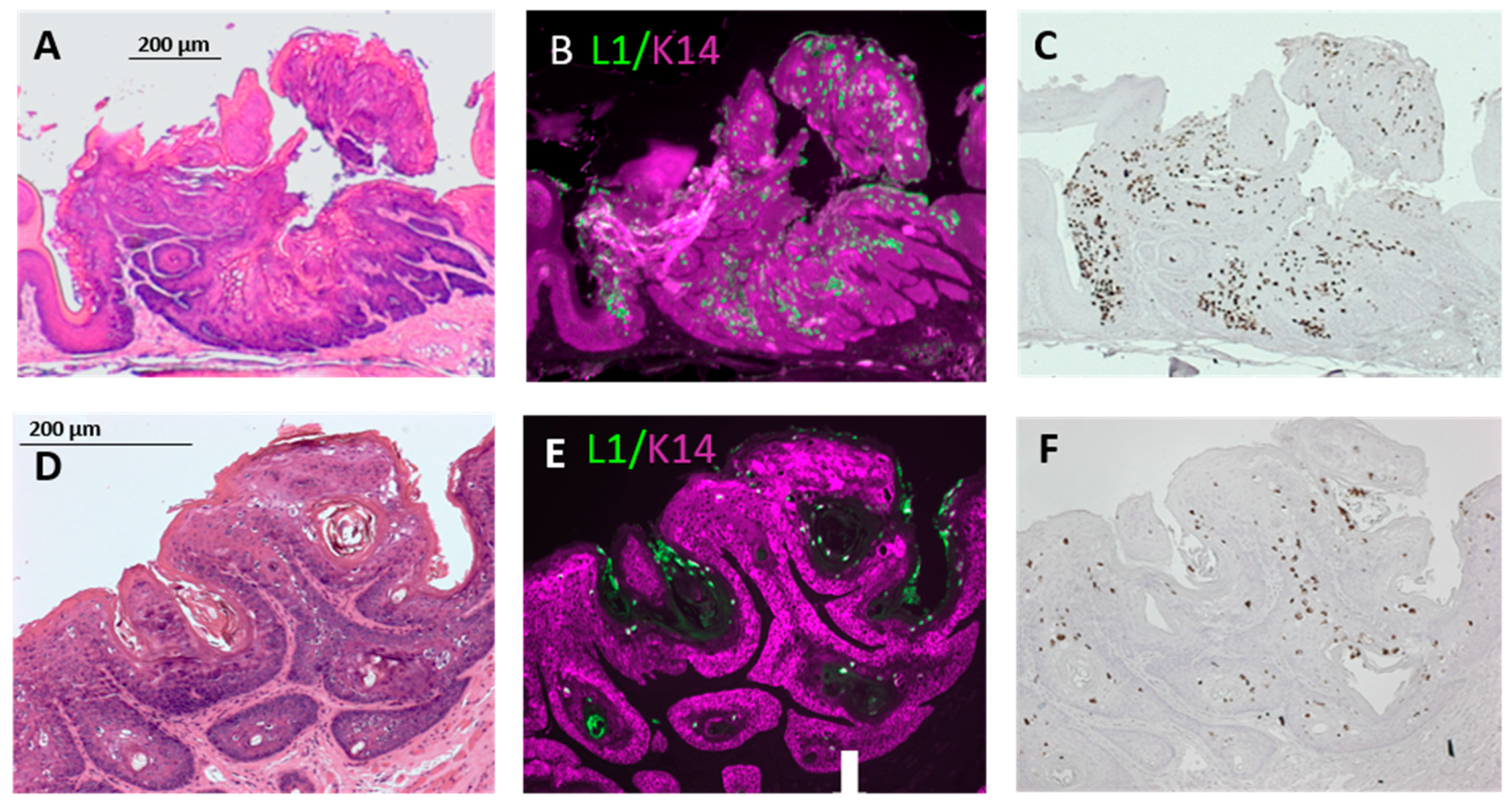
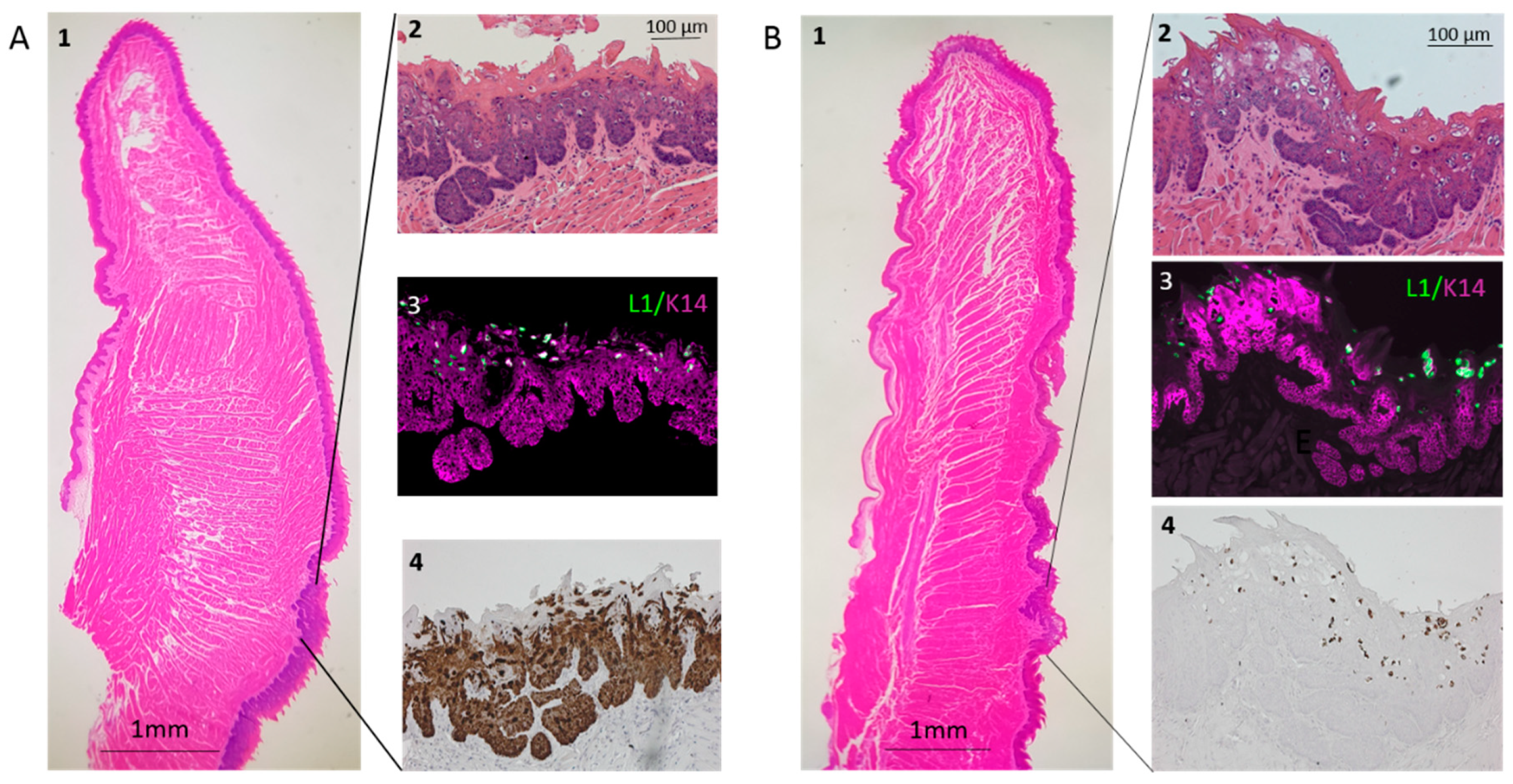
3.3. Infection Yields Dysplastic Lesions, Including Exophytic Tumors, in the Soft Palate and Base of the Tongue
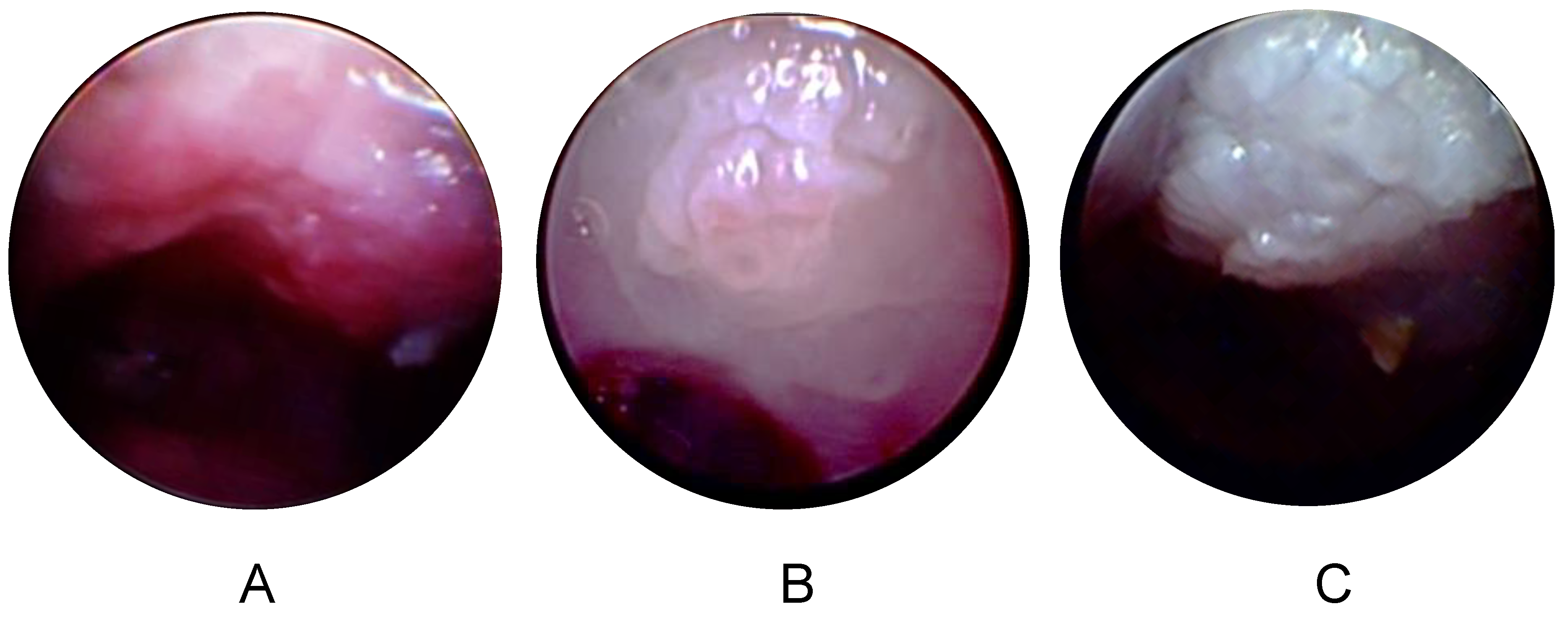
3.4. Longitudinal Endoscopy Reveals Morphological Changes during Tumor Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- How Many Cancers Are Linked with HPV Each Year? CDC. Available online: https://www.cdc.gov/cancer/hpv/statistics/cases.htm (accessed on 20 December 2019).
- Doorbar, J. Model systems of human papillomavirus-associated disease. J. Pathol. 2016, 238, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.F. Transgenic Mouse Models of Tumor Virus Action. Annu. Rev. Virol. 2016, 3, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Nyman, P.E.; Buehler, D.; Lambert, P.F. Loss of Function of Canonical Notch Signaling Drives Head and Neck Carcinogenesis. Clin. Cancer Res. 2018, 24, 6308–6318. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.; Ghim, S.; Joh, J.; Chepkoech, I.; Bennett Jenson, A.; Sundberg, J.P. Novel laboratory mouse papillomavirus (MusPV) infection. Vet. Pathol. 2011, 48, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Cooper, T.K.; Hu, J.; Christensen, N.D. A Novel Pre-Clinical Murine Model to Study the Life Cycle and Progression of Cervical and Anal Papillomavirus Infections. PLoS ONE 2015, 10, e0120128. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, A.; Yoshida, S.; Frazer, I.H.; Pitot, H.C.; Lambert, P.F. Role of Ultraviolet Radiation in Papillomavirus-Induced Disease. PLoS Pathog. 2016, 12, e1005664. [Google Scholar] [CrossRef]
- Sundberg, J.P.; Stearns, T.M.; Joh, J.; Proctor, M.; Ingle, A.; Silva, K.A.; Dadras, S.S.; Jenson, A.B.; Ghim, S. Immune status, strain background, and anatomic site of inoculation affect mouse papillomavirus (MmuPV1) induction of exophytic papillomas or endophytic trichoblastomas. PLoS ONE 2014, 9, e113582. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; Uberoi, A.; McGregor, S.M.; Wei, T.; Ward-Shaw, E.; Lambert, P.F. A Novel In Vivo Infection Model To Study Papillomavirus-Mediated Disease of the Female Reproductive Tract. mBio 2019, 10, 00180-19. [Google Scholar] [CrossRef]
- Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Cooper, T.K.; Hu, J.; Christensen, N.D. Mouse papillomavirus MmuPV1 infects oral mucosa and preferentially targets the base of the tongue. Virology 2016, 488, 73–80. [Google Scholar] [CrossRef]
- Haeggblom, L.; Ramqvist, T.; Tommasino, M.; Dalianis, T.; Näsman, A. Time to change perspectives on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Res. Amst. Neth. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Gargiulo, S.; Greco, A.; Gramanzini, M.; Esposito, S.; Affuso, A.; Brunetti, A.; Vesce, G. Mice anesthesia, analgesia, and care, Part I: Anesthetic considerations in preclinical research. ILAR J. 2012, 53, E55–E69. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.N.; Buck, C.B.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, D.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007, 13, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, A. MmuPV1 L1- cytokeratin dual immunofluorescence using home-based tyramide signal amplification. Protocols.io 2017. [Google Scholar] [CrossRef]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.-C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.-T.; Ma, X.-J.; Luo, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef]
- Carr, W.W.; Martin, B.; Howard, R.S.; Cox, L.; Borish, L. Immunotherapy Committee of the American Academy of Allergy, Asthma and Immunology Comparison of test devices for skin prick testing. J. Allergy Clin. Immunol. 2005, 116, 341–346. [Google Scholar] [CrossRef]
- Stubenrauch, F.; Laimins, L.A. Human papillomavirus life cycle: Active and latent phases. Semin. Cancer Biol. 1999, 9, 379–386. [Google Scholar] [CrossRef]
- Gono, K. Narrow Band Imaging: Technology Basis and Research and Development History. Clin. Endosc. 2015, 48, 476–480. [Google Scholar] [CrossRef]
- Muto, M.; Nakane, M.; Katada, C.; Sano, Y.; Ohtsu, A.; Esumi, H.; Ebihara, S.; Yoshida, S. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer 2004, 101, 1375–1381. [Google Scholar] [CrossRef]
- Cosway, B.; Drinnan, M.; Paleri, V. Narrow band imaging for the diagnosis of head and neck squamous cell carcinoma: A systematic review. Head Neck 2016, 38 (Suppl. S1), E2358–E2367. [Google Scholar] [CrossRef]
- Pan, C.; Issaeva, N.; Yarbrough, W.G. HPV-driven oropharyngeal cancer: Current knowledge of molecular biology and mechanisms of carcinogenesis. Cancers Head Neck 2018, 3, 12. [Google Scholar] [CrossRef]
- Mestre, V.F.; Medeiros-Fonseca, B.; Estêvão, D.; Casaca, F.; Silva, S.; Félix, A.; Silva, F.; Colaço, B.; Seixas, F.; Bastos, M.M.; et al. HPV16 is sufficient to induce squamous cell carcinoma specifically in the tongue base in transgenic mice. J. Pathol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Griffin, H. Refining our understanding of cervical neoplasia and its cellular origins. Papillomavirus Res. Amst. Neth. 2019, 7, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Spurgeon, M.E.; Lambert, P.F. Sexual transmission of murine papillomavirus (MmuPV1) in Mus musculus. eLife 2019, 8, e50056. [Google Scholar] [CrossRef] [PubMed]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Tomita, H.; Nakashima, T.; Hirata, A.; Tanaka, T.; Shibata, T.; Hara, A. Current mouse models of oral squamous cell carcinoma: Genetic and chemically induced models. Oral Oncol. 2017, 73, 16–20. [Google Scholar] [CrossRef]
| Degree of Dysplasia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incidence | Tongue | Soft Palate | Genome Equivalents | Duration (Weeks) | ||||||
| Strain | Tongue | Palate | Mild | Moderate | Severe | Mild | Moderate | Severe | ||
| Nude | NA | 1/1 | NA | 0 | 0 | 1 | 108 | 14 | ||
| Nude | 6/6 | 6/6 | 0 | 3 | 3 | 2 | 2 | 2 | 3 × 109 | 21 |
| NSG | 3/3 | 3/3 | 2 | 1 | 0 | 2 | 1 | 0 | 8 × 109 | 21 |
| NSG | 3/3 | NA | 3 | 0 | 0 | NA | 3 × 109 | 10 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilger, A.; King, R.E.; Schroeder, J.P.; Piette, J.T.; Hinshaw, L.A.; Kurth, A.D.; AlRamahi, R.W.; Barthel, M.V.; Ward-Shaw, E.T.; Buehler, D.; et al. A Mouse Model of Oropharyngeal Papillomavirus-Induced Neoplasia Using Novel Tools for Infection and Nasal Anesthesia. Viruses 2020, 12, 450. https://doi.org/10.3390/v12040450
Bilger A, King RE, Schroeder JP, Piette JT, Hinshaw LA, Kurth AD, AlRamahi RW, Barthel MV, Ward-Shaw ET, Buehler D, et al. A Mouse Model of Oropharyngeal Papillomavirus-Induced Neoplasia Using Novel Tools for Infection and Nasal Anesthesia. Viruses. 2020; 12(4):450. https://doi.org/10.3390/v12040450
Chicago/Turabian StyleBilger, Andrea, Renee E. King, Josh P. Schroeder, Jared T. Piette, Louis A. Hinshaw, Andrew D. Kurth, Ronnie W. AlRamahi, Matthew V. Barthel, Ella T. Ward-Shaw, Darya Buehler, and et al. 2020. "A Mouse Model of Oropharyngeal Papillomavirus-Induced Neoplasia Using Novel Tools for Infection and Nasal Anesthesia" Viruses 12, no. 4: 450. https://doi.org/10.3390/v12040450
APA StyleBilger, A., King, R. E., Schroeder, J. P., Piette, J. T., Hinshaw, L. A., Kurth, A. D., AlRamahi, R. W., Barthel, M. V., Ward-Shaw, E. T., Buehler, D., Masters, K. S., Thibeault, S. L., & Lambert, P. F. (2020). A Mouse Model of Oropharyngeal Papillomavirus-Induced Neoplasia Using Novel Tools for Infection and Nasal Anesthesia. Viruses, 12(4), 450. https://doi.org/10.3390/v12040450





