Flavonoids as Antiviral Agents for Enterovirus A71 (EV-A71)
Abstract
1. Introduction
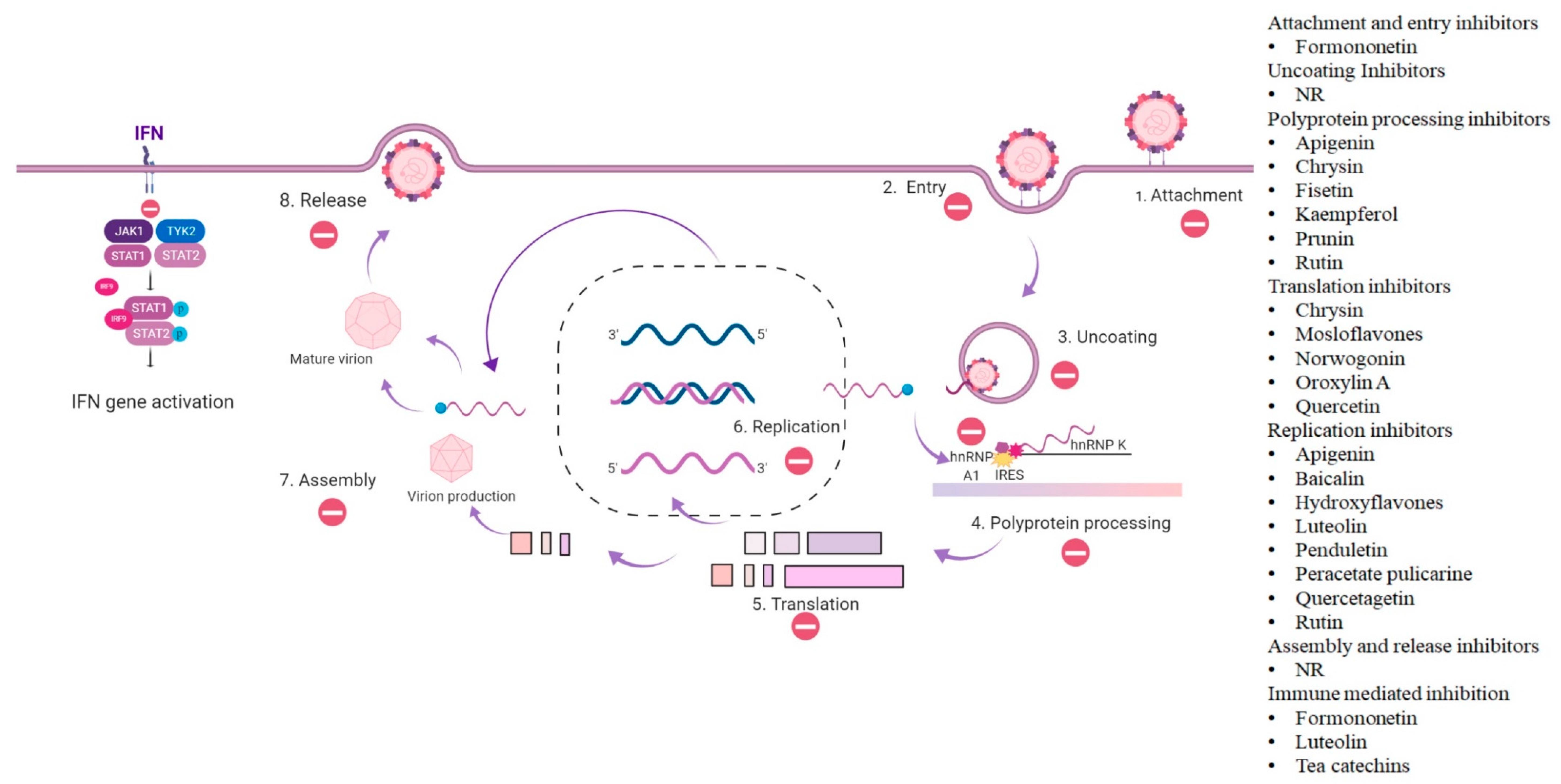
2. Flavonoids against Viruses
- Flavonoids that bind to specific extracellular regions of the virus such as viral proteins present on the capsids.
- Flavonoids that prevent attachment or entry of the virus into host cells. In some cases, flavonoids can bind to virions and modify the virus structure. Though the virus can still internalize, the process of viral uncoating is stalled.
- Early-stage replication inhibitors.
- Transcription and translation blockers.
- Inhibition of late stages of maturation such as inhibition of assembly/packaging and release.
- Flavonoids that can inhibit viral infections by interfering with host factors that are required for successful infection or modulating the immune system to reduce the viral load.
2.1. Flavonoids against Non-Picornaviruses
2.2. Flavonoids against Non-EV-A71 Picornaviruses
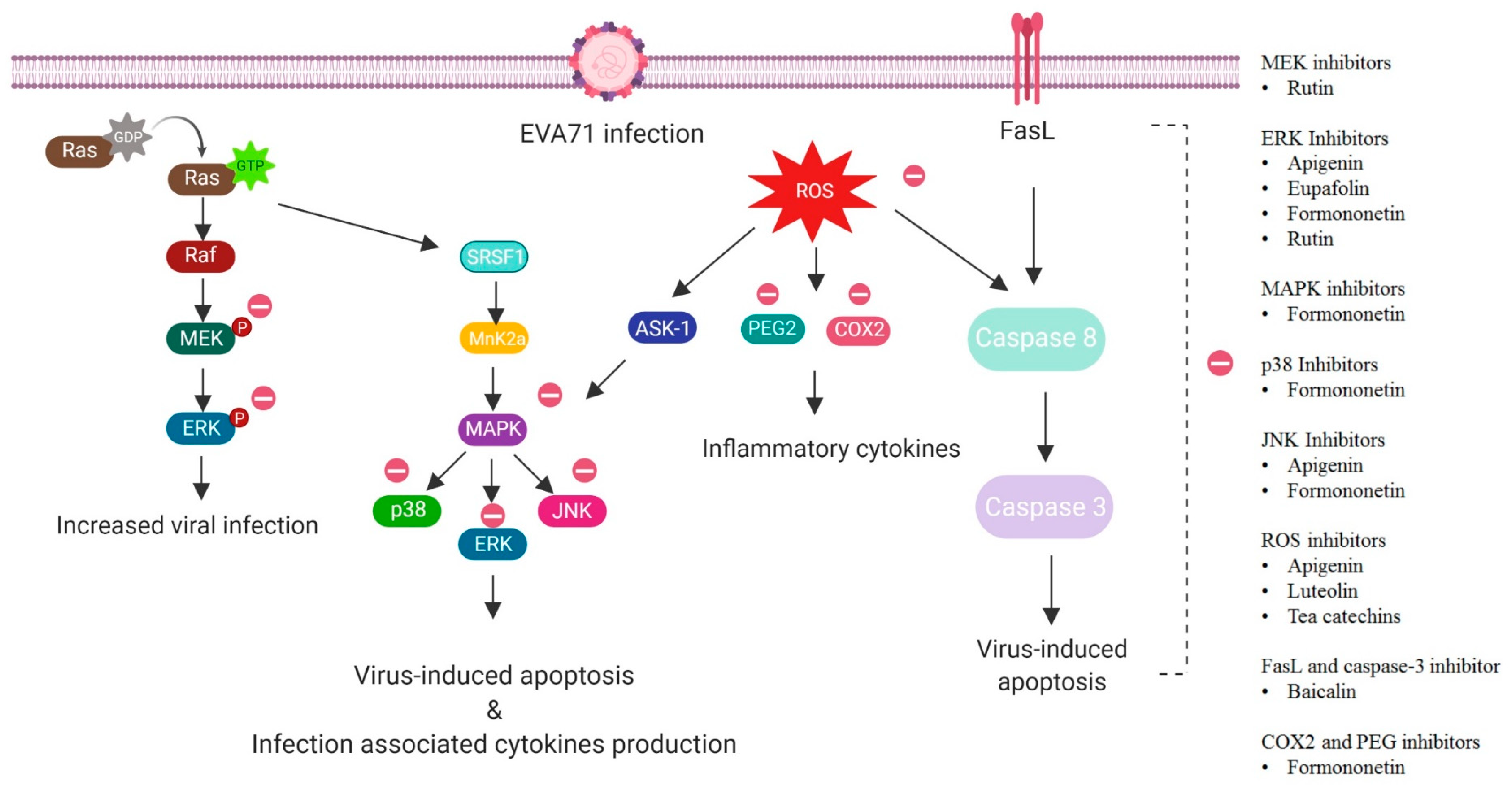
3. Flavonoids that Target EV-A71
3.1. Apigenin
3.2. Baicalin
3.3. Chrysin and Its Derivative
3.4. Fisetin
3.5. Formononetin
3.6. Hydroxyflavone and Its Derivatives
3.7. Kaempferol
3.8. Luteolin and Its Derivatives
3.9. Penduletin
3.10. Peracetate Pulicarine
3.11. Prunin
3.12. Quercetin and Its Derivatives
3.13. Flavonoids Isolated from Scutellaria Baicalensis Georgi
3.14. Flavonoids with Unknown Mechanisms of Action against EV-A71
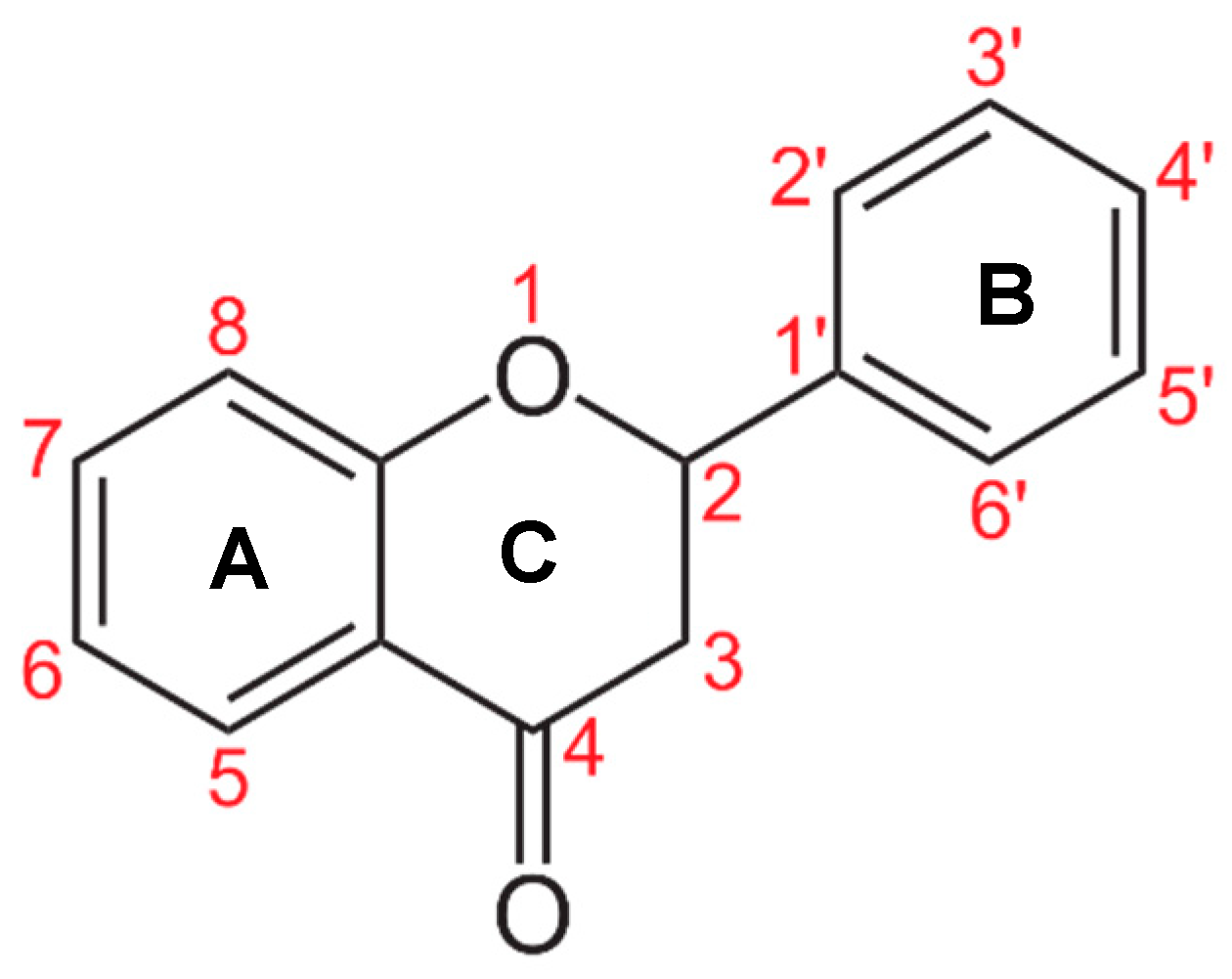
4. Structure–Activity Relationship among Potential Antiviral Flavonoids against EV-A71
5. Limitations of Flavonoids as Antivirals against EV-A71
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Int. J. Pharm. Sci. Technol. 2011, 100, 12–35. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Cutting, W.; Dreisbach, R.; Neff, B. Antiviral chemotherapy; flavones and related compounds. Stanf. Med. Bull. 1949, 7, 137. [Google Scholar]
- Veckenstedt, A.; Pusztai, R. Mechanism of antiviral action of quercetin against cardiovirus infection in mice. Antivir. Res. 1981, 1, 249–261. [Google Scholar] [CrossRef]
- Roschek, B., Jr.; Fink, R.C.; McMichael, M.D.; Li, D.; Alberte, R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry 2009, 70, 1255–1261. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses 2016, 8, 6. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, J.H.; Park, K.S.; Kwon, D.H. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur. J. Pharm. Sci. 2009, 37, 329–333. [Google Scholar] [CrossRef]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral. Hepat. 2012, 19, e81–e88. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Khachatoorian, R.; French, S.W. Quercetin: Bioflavonoids as part of interferon-free hepatitis C therapy? Expert Rev. Anti Infect. 2012, 10, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Kroeker, A.; He, S.; Kozak, R.; Audet, J.; Mbikay, M.; Chrétien, M. Prophylactic efficacy of quercetin 3-β-Od-glucoside against Ebola virus infection. Antimicrob. Agents Chemother. 2016, 60, 5182–5188. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Hu, Q.; Yang, J.; Li, R.; Li, X.; Lu, C.; Chen, C.; Wang, L.; Shattock, R.; Ben, K. In vitro anti-HIV and-HSV activity and safety of sodium rutin sulfate as a microbicide candidate. Antivir. Res. 2007, 75, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Shibata, C.; Ohno, M.; Otsuka, M.; Kishikawa, T.; Goto, K.; Muroyama, R.; Kato, N.; Yoshikawa, T.; Takata, A.; Koike, K. The flavonoid apigenin inhibits hepatitis C virus replication by decreasing mature microRNA122 levels. Virology 2014, 462, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Fu, T.; Dongyan, Y.; Mikovits, J.A.; Ruscetti, F.W.; Wang, J.M. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem. Biophys. Res. Commun. 2000, 276, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, E.; Teoh, B.T.; Sam, S.S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014, 4, 5452. [Google Scholar] [CrossRef]
- Nayak, M.K.; Agrawal, A.S.; Bose, S.; Naskar, S.; Bhowmick, R.; Chakrabarti, S.; Sarkar, S.; Chawla-Sarkar, M. Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of NS1-mediated cellular innate immune responses. J. Antimicrob. Chemother. 2014, 69, 1298–1310. [Google Scholar] [CrossRef]
- Chu, M.; Xu, L.; Zhang, M.-B.; Chu, Z.-Y.; Wang, Y.-D. Role of Baicalin in anti-influenza virus A as a potent inducer of IFN-gamma. Biomed. Res. Int. 2015, 2015, 11. [Google Scholar] [CrossRef]
- Johari, J.; Kianmehr, A.; Mustafa, M.R.; Abubakar, S.; Zandi, K. Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef]
- Hassandarvish, P.; Rothan, H.A.; Rezaei, S.; Yusof, R.; Abubakar, S.; Zandi, K. In silico study on baicalein and baicalin as inhibitors of dengue virus replication. RSC Adv. 2016, 6, 31235–31247. [Google Scholar] [CrossRef]
- Sauter, D.; Schwarz, S.; Wang, K.; Zhang, R.; Sun, B.; Schwarz, W. Genistein as antiviral drug against HIV ion channel. Planta Med. 2014, 80, 682–687. [Google Scholar] [CrossRef]
- Miki, K.; Nagai, T.; Suzuki, K.; Tsujimura, R.; Koyama, K.; Kinoshita, K.; Furuhata, K.; Yamada, H.; Takahashi, K. Anti-influenza virus activity of biflavonoids. Bioorg. Med. Chem. Lett. 2007, 17, 772–775. [Google Scholar] [CrossRef]
- Lani, R.; Hassandarvish, P.; Shu, M.-H.; Phoon, W.H.; Chu, J.J.H.; Higgs, S.; Vanlandingham, D.; Bakar, S.A.; Zandi, K. Antiviral activity of selected flavonoids against Chikungunya virus. Antivir. Res. 2016, 133, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; AbuBakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Ryu, Y.B.; Park, S.-J.; Kim, J.H.; Kwon, H.-J.; Kim, J.H.; Park, K.H.; Rho, M.-C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg. Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Sauter, D.; Wang, K.; Zhang, R.; Sun, B.; Karioti, A.; Bilia, A.R.; Efferth, T.; Schwarz, W. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014, 80, 177–182. [Google Scholar] [CrossRef]
- Yi, L.; Li, Z.; Yuan, K.; Qu, X.; Chen, J.; Wang, G.; Zhang, H.; Luo, H.; Zhu, L.; Jiang, P. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004, 78, 11334–11339. [Google Scholar] [CrossRef]
- Yan, H.; Ma, L.; Wang, H.; Wu, S.; Huang, H.; Gu, Z.; Jiang, J.; Li, Y. Luteolin decreases the yield of influenza A virus in vitro by interfering with the coat protein I complex expression. J. Nat. Med. 2019, 73, 487–496. [Google Scholar] [CrossRef]
- Nagai, T.; Miyaichi, Y.; Tomimori, T.; Suzuki, Y.; Yamada, H. Inhibition of influenza virus sialidase and anti-influenza virus activity by plant flavonoids. Antivir. Res. 1990, 38, 1329–1332. [Google Scholar] [CrossRef]
- Nahmias, Y.; Goldwasser, J.; Casali, M.; van Poll, D.; Wakita, T.; Chung, R.T.; Yarmush, M.L. Apolipoprotein B–dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology 2008, 47, 1437–1445. [Google Scholar] [CrossRef]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A., Jr.; Dos Santos, C.N.D.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef]
- Keivan, Z.; Boon-Teong, T.; Sing-Sin, S.; Pooi-Fong, W.; Mustafa, M.R.; Sazaly, A. In vitro antiviral activity of fisetin, rutin and naringenin against dengue virus type-2. J. Med. Plants Res. 2011, 5, 5534–5539. [Google Scholar]
- Ferenci, P.; Scherzer, T.M.; Kerschner, H.; Rutter, K.; Beinhardt, S.; Hofer, H.; Schöniger–Hekele, M.; Holzmann, H.; Steindl–Munda, P. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology 2008, 135, 1561–1567. [Google Scholar] [CrossRef]
- Lin, A.-S.; Shibano, M.; Nakagawa-Goto, K.; Tokuda, H.; Itokawa, H.; Morris-Natschke, S.L.; Lee, K.-H. Cancer preventive agents. 7. Antitumor-promoting effects of seven active flavonolignans from milk thistle (Silybum marianum.) on epstein-barr virus activation. Pharm. Biol. 2007, 45, 735–738. [Google Scholar] [CrossRef][Green Version]
- Camini, F.C.; da Silva, T.F.; da Silva Caetano, C.C.; Almeida, L.T.; Ferraz, A.C.; Vitoreti, V.M.A.; de Mello Silva, B.; de Queiroz Silva, S.; de Magalhães, J.C.; de Brito Magalhães, C.L. Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress. Antivir. Res. 2018, 158, 8–12. [Google Scholar] [CrossRef]
- Wagoner, J.; Negash, A.; Kane, O.J.; Martinez, L.E.; Nahmias, Y.; Bourne, N.; Owen, D.M.; Grove, J.; Brimacombe, C.; McKeating, J.A. Multiple effects of silymarin on the hepatitis C virus lifecycle. J. Hepatol. 2010, 51, 1912–1921. [Google Scholar] [CrossRef]
- Lani, R.; Hassandarvish, P.; Chiam, C.W.; Moghaddam, E.; Chu, J.J.H.; Rausalu, K.; Merits, A.; Higgs, S.; Vanlandingham, D.; Bakar, S.A. Antiviral activity of silymarin against chikungunya virus. Sci. Rep. 2015, 5, 11421. [Google Scholar] [CrossRef]
- Qaddir, I.; Rasool, N.; Hussain, W.; Mahmood, S. Computer-aided analysis of phytochemicals as potential dengue virus inhibitors based on molecular docking, ADMET and DFT studies. J. Vector Borne Dis. 2017, 54, 255. [Google Scholar]
- Song, J.; Choi, H. Silymarin efficacy against influenza A virus replication. Phytomedicine 2011, 18, 832–835. [Google Scholar] [CrossRef]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yamada, H.; Takuma, N.; Niino, H.; Sagesaka, Y.M. Effects of Green Tea Catechins and Theanine on Preventing Influenza Infection among Healthcare Workers: A Randomized Controlled Trial. BMC Complem. Altern. Med. 2011, 11, 15. [Google Scholar] [CrossRef]
- Imanishi, N.; Tuji, Y.; Katada, Y.; Maruhashi, M.; Konosu, S.; Mantani, N.; Terasawa, K.; Ochiai, H. Additional inhibitory effect of tea extract on the growth of influenza A and B viruses in MDCK cells. J. Microbiol. Immunol. 2002, 46, 491–494. [Google Scholar] [CrossRef]
- Kawai, K.; Tsuno, N.H.; Kitayama, J.; Okaji, Y.; Yazawa, K.; Asakage, M.; Hori, N.; Watanabe, T.; Takahashi, K.; Nagawa, H. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J. Allergy Clin. Immunol. 2003, 112, 951–957. [Google Scholar] [CrossRef]
- Isaacs, C.E.; Wen, G.Y.; Xu, W.; Jia, J.H.; Rohan, L.; Corbo, C.; Di Maggio, V.; Jenkins, E.C.; Hillier, S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 2008, 52, 962–970. [Google Scholar] [CrossRef]
- Weber, C.; Sliva, K.; von Rhein, C.; Kümmerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antivir. Res. 2015, 113, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-K.; Wei, T.-T.; Chiu, Y.-F.; Tung, C.-P.; Chuang, J.-Y.; Hung, S.-K.; Li, C.; Liu, S.-T. Inhibition of Epstein–Barr virus lytic cycle by (−)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 2003, 301, 1062–1068. [Google Scholar] [CrossRef]
- He, W.; Li, L.-X.; Liao, Q.-J.; Liu, C.-L.; Chen, X.-L. Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication-inducible cell line. World J. Gastroenterol. 2011, 17, 1507. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, J.; Shu, W.; Gao, B.; Xiong, S. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015, 6, e1770. [Google Scholar] [CrossRef]
- Nagai, T.; Moriguchi, R.; Suzuki, Y.; Tomimori, T.; Yamada, H. Mode of action of the anti-influenza virus activity of plant flavonoid, 5, 7, 4′-trihydroxy-8-methoxyflavone, from the roots of Scutellaria baicalensis. Antivir. Res. 1995, 26, 11–25. [Google Scholar] [CrossRef]
- Ahmadi, A.; Hassandarvish, P.; Lani, R.; Yadollahi, P.; Jokar, A.; Bakar, S.A.; Zandi, K. Inhibition of chikungunya virus replication by hesperetin and naringenin. RSC Adv. 2016, 6, 69421–69430. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef]
- Gonzalez, M.; Martínez-Abarca, F.; Carrasco, L. Flavonoids: Potent inhibitors of poliovirus RNA synthesis. Antivir. Chem. Chemother. 1990, 1, 203–209. [Google Scholar] [CrossRef]
- Vrijsen, R.; Everaert, L.; Van Hoof, L.; Vlietinck, A.; Berghe, D.V.; Boeye, A. The poliovirus-induced shut-off of cellular protein synthesis persists in the presence of 3-methylquercetin, a flavonoid which blocks viral protein and RNA synthesis. Antivir. Res. 1987, 7, 35–42. [Google Scholar] [CrossRef]
- Ishitsuka, H.; Ohsawa, C.; Ohiwa, T.; Umeda, I.; Suhara, Y. Antipicornavirus flavone Ro 09-0179. Antimicrob. Agents Chemother. 1982, 22, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Galochkina, A.V.; Anikin, V.B.; Babkin, V.A.; Ostrouhova, L.A.; Zarubaev, V.V. Virus-inhibiting activity of dihydroquercetin, a flavonoid from Larix sibirica, against coxsackievirus B4 in a model of viral pancreatitis. Arch. Virol. 2016, 161, 929–938. [Google Scholar] [CrossRef]
- Xu, L.; Su, W.; Jin, J.; Chen, J.; Li, X.; Zhang, X.; Sun, M.; Sun, S.; Fan, P.; An, D. Identification of luteolin as enterovirus 71 and coxsackievirus A16 inhibitors through reporter viruses and cell viability-based screening. Viruses 2014, 6, 2778–2795. [Google Scholar] [CrossRef]
- Vrijsen, R.; Everaert, L.; Boeyé, A. Antiviral activity of flavones and potentiation by ascorbate. J. Gen. Virol. 1988, 69, 1749–1751. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Huang, S.-C.; Lai, Z.-R.; Ho, Y.-L.; Jou, Y.-J.; Kung, S.-H.; Zhang, Y.; Chang, Y.-S.; Lin, C.-W. Eupafolin and ethyl acetate fraction of Kalanchoe gracilis stem extract show potent antiviral activities against enterovirus 71 and coxsackievirus A16. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Robin, V.; Irurzun, A.; Amoros, M.; Boustie, J.; Carrasco, L. Antipoliovirus flavonoids from Psiadia dentata. Antivir. Chem. Chemother. 2001, 12, 283–291. [Google Scholar] [CrossRef]
- Kim, N.; Park, S.; Nhiem, N.X.; Song, J.-H.; Ko, H.-J.; Kim, S.H. Cycloartane-type triterpenoid derivatives and a flavonoid glycoside from the burs of Castanea crenata. Phytochemistry 2019, 158, 135–141. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Q.; Yang, W.; Wu, K.; Wu, J. Virucidal Capacity of Novel ProtecTeaV Sanitizer Formulations Containing Lipophilic Epigallocatechin-3-Gallate (EGCG). Antivir. Antiretrovir. Res. 2016, 1, 1–5. [Google Scholar]
- Kant, K.; Lal, U.R.; Ghosh, M. In-silico discovery of natural lead hits from the genus of arisaema against Human Rhino Virus. In Proceedings of the 20th International Electronic Conference on Synthetic Organic Chemistry, 1–31 November 2016. [Google Scholar]
- Choi, H.-J. In Vitro Antiviral Activity of Sakuranetin against Human Rhinovirus 3. Osong Public Health Res. Perspect. 2017, 8, 415. [Google Scholar] [CrossRef] [PubMed]
- Desideri, N.; Conti, C.; Sestili, I.; Tomao, P.; Stein, M.L.; Orsi, N. In vitro Evaluation of the Anti-Picornavirus Activities of New Synthetic Flavonoids. Antivir. Chem. Chemother. 1995, 6, 298–306. [Google Scholar] [CrossRef]
- Li, Y.; Leung, K.-T.; Yao, F.; Ooi, L.S.; Ooi, V.E. Antiviral Flavans from the Leaves of Pithecellobium C lypearia. J. Nat. Prod. 2006, 69, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.; Nobbs, S.; Pyke, S.; Reynolds, G.; Flower, R. Antiviral flavonoid from Pterocaulon sphacelatum, an Australian Aboriginal medicine. J. Ethnopharmacol. 1999, 68, 283–288. [Google Scholar] [CrossRef]
- Van, N.T.H.; Vien, T.A.; Nhiem, N.X.; Van Kiem, P.; Van Minh, C.; Long, P.Q.; Anh, L.T.; Cuong, N.M.; Song, J.H.; Ko, H.J. Chemical components of Ardisia splendens leaves and their activity against Coxsackie A16 viruses. Nat. Prod. Commun. 2014, 9. [Google Scholar] [CrossRef]
- Ortega, J.T.; Serrano, M.L.; Suárez, A.I.; Baptista, J.; Pujol, F.H.; Cavallaro, L.V.; Campos, H.R.; Rangel, H.R. Antiviral activity of flavonoids present in aerial parts of Marcetia taxifolia against Hepatitis B virus, Poliovirus, and Herpes Simplex Virus in vitro. Excli J. 2019, 18, 1037. [Google Scholar]
- Gunaseelan, S.; Wong, K.Z.; Min, N.; Sun, J.; Ismail, N.K.B.M.; Tan, Y.J.; Lee, R.C.H.; Chu, J.J.H. Prunin suppresses viral IRES activity and is a potential candidate for treating enterovirus A71 infection. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, H.; Lv, Y.; Wang, J.; Chen, X.; Hou, Y.; Tan, R.; Li, E. Apigenin inhibits enterovirus-71 infection by disrupting viral RNA association with trans-acting factors. PLoS ONE 2014, 9, e110429. [Google Scholar] [CrossRef]
- Lv, X.; Qiu, M.; Chen, D.; Zheng, N.; Jin, Y.; Wu, Z. Apigenin inhibits enterovirus 71 replication through suppressing viral IRES activity and modulating cellular JNK pathway. Antivir. Res. 2014, 109, 30–41. [Google Scholar] [CrossRef]
- Dai, W.; Bi, J.; Li, F.; Wang, S.; Huang, X.; Meng, X.; Sun, B.; Wang, D.; Kong, W.; Jiang, C. Antiviral Efficacy of Flavonoids against Enterovirus 71 Infection in Vitro and in Newborn Mice. Viruses 2019, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Wu, T.; Jin, Y.; Cheng, J.; Wan, C.; Qian, W.; Xing, F.; Shi, W. The antiviral effect of baicalin on enterovirus 71 in vitro. Viruses 2015, 7, 4756–4771. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, T.; Du, J.; Cui, S.; Yang, F.; Jin, Q. Anti-enterovirus 71 effects of chrysin and its phosphate ester. PLoS ONE 2014, 9, e89668. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chang, Y.-C.; Hsiao, N.-W.; Hsieh, J.-L.; Wang, C.-Y.; Kung, S.-H.; Tsai, F.-J.; Lan, Y.-C.; Lin, C.-W. Fisetin and rutin as 3C protease inhibitors of enterovirus A71. J. Virol. Methods 2012, 182, 93–98. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Ge, M.; Li, Z.; Jiang, J.; Li, Y. Formononetin inhibits enterovirus 71 replication by regulating COX-2/PGE 2 expression. Virol. J. 2015, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gao, Q.; Yuan, S.; Wang, L.; Altmeyer, R.; Lan, K.; Yin, F.; Zou, G. Characterization of three small molecule inhibitors of enterovirus 71 identified from screening of a library of natural products. Antivir. Res. 2017, 143, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, H.; Zhang, T.; Du, J.; Cui, S.; Yang, F.; Jin, Q. Inhibition of Enterovirus 71 replication by 7-hydroxyflavone and diisopropyl-flavon7-yl Phosphate. PLoS ONE 2014, 9, e92565. [Google Scholar] [CrossRef]
- Tsai, F.-J.; Lin, C.-W.; Lai, C.-C.; Lan, Y.-C.; Lai, C.-H.; Hung, C.-H.; Hsueh, K.-C.; Lin, T.-H.; Chang, H.C.; Wan, L. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011, 128, 312–322. [Google Scholar] [CrossRef]
- Zhu, Q.-C.; Wang, Y.; Liu, Y.-P.; Zhang, R.-Q.; Li, X.; Su, W.-H.; Long, F.; Luo, X.-D.; Peng, T. Inhibition of enterovirus 71 replication by chrysosplenetin and penduletin. Eur. J. Pharm. Sci. 2011, 44, 392–398. [Google Scholar] [CrossRef]
- Min, N.; Leong, P.T.; Lee, R.C.H.; Khuan, J.S.E.; Chu, J.J.H. A flavonoid compound library screen revealed potent antiviral activity of plant-derived flavonoids on human enterovirus A71 replication. Antivir. Res. 2018, 150, 60–68. [Google Scholar] [CrossRef]
- Yao, C.; Xi, C.; Hu, K.; Gao, W.; Cai, X.; Qin, J.; Lv, S.; Du, C.; Wei, Y. Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virol. J. 2018, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, P.; Chen, X.; Wang, W.; Jin, Y. Saururus chinensis (Lour.) Baill blocks enterovirus 71 infection by hijacking MEK1–ERK signaling pathway. Antivir. Res. 2015, 119, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Song, H.-H.; Lee, J.-S.; Ko, H.-J.; Song, J.-H. Inhibitory effects of norwogonin, oroxylin A, and mosloflavone on enterovirus 71. Biomol. Ther. 2016, 24, 552. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xu, Y.; Ou, Z.; Yang, X.; Liu, H. An antiviral drug screening system for enterovirus 71 based on an improved plaque assay: A potential high-throughput method. J. Med. Virol. 2019, 91, 1440–1447. [Google Scholar] [CrossRef]
- Zhang, D.; Ji, X.; Gao, R.; Wang, H.; Meng, S.; Zhong, Z.; Li, Y.; Jiang, J.; Li, Z. Synthesis and antiviral activities of a novel class of thioflavone and flavonoid analogues. Acta Pharm. Sin. B 2012, 2, 575–580. [Google Scholar] [CrossRef]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef]
- Sarwar, M.W.; Riaz, A.; Dilshad, S.M.R.; Al-Qahtani, A.; Nawaz-Ul-Rehman, M.S.; Mubin, M. Structure activity relationship (SAR) and quantitative structure activity relationship (QSAR) studies showed plant flavonoids as potential inhibitors of dengue NS2B-NS3 protease. BMC Struct. Biol. 2018, 18, 6. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Saraf, S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar]
| Flavonoid | Virus | Virus Family | Model | Stage of Virus Inhibition | Suggested Mechanism | Reference |
|---|---|---|---|---|---|---|
| Apigenin | Hepatitis C virus (HCV) | Flaviviridae | In vitro | Host factor modulation | Reduction in mature miRNA122 | [12] |
| Baicalin | Dengue virus-2 (DENV-2) | Flaviviridae | In vitro | Attachment | Blockade of attachment of the virus to Vero cells | [14] |
| Human immunodeficiency virus-1 (HIV-1) | Retroviridae | In vitro | Fusion | Inhibition of the fusion of virus envelope protein with T cells and monocytes expressing CD4/CXCR4 or CD4/CCR5 | [13] | |
| Influenza A virus | Orthomyxoviridae | In vitro | Indirect: Immune-mediated infection control | Directly binds to NS1–p85β (RNA binding domain) to down-regulate IFN-ɣ and activates the JAK/STAT1 pathway that reduced the viral load | [15,16] | |
| Baicalein | Chikungunya virus (CHIKV) | Togaviridae | In vitro | Prophylaxis | Inhibition of attachment by inhibiting extracellular particles such as nsP1, nsP3, and E2 proteins | [21] |
| DENV-2 | Flaviviridae | In silico | Replication | Binds to the NS3/NS2B and NS5 proteins | [18] | |
| Japanese encephalitis virus (JEV) | Flaviviridae | In vitro | Entry | Unknown Postulated to be an accumulation of the compound in cells to prevent entry or interaction with structural and/or non-structural protein(s) | [17] | |
| Epigallocatechin (ECG) | Influenza A and B viruses | Orthomyxoviridae | In vitro | Replication | Acidification of the lysosomal and endosomal environment through clathrin-mediated endocytosis | [40] |
| Epigallocatechin gallate (EGGC) | CHIKV | Togaviridae | In vitro | Entry | Competitor for cellular co-receptors of target cells such as heparan sulfate or sialic acid | [43] |
| Epstein–Barr virus (EBV) | Herpesviridae | Replication | Inhibition of Zta, Rta and EA-D genes by interrupting the MEK/ERK1/2 and PI3-K/Akt signaling pathway of the lytic cycle of a virus | [44] | ||
| Hepatitis B virus (HBV) | Hepadnaviridae | In vitro | Replication | Impair the production of pre-core mRNA and replicative intermediates of DNA | [45] | |
| Acidification in lysosomes to make an unfavorable environment for virus replication | [47] | |||||
| Herpes simplex virus (HSV) | Herpesviridae | In vitro | Entry | Binds to glycoprotein B and D of virus | [42] | |
| HIV-1 | Retroviridae | In vitro | Entry | Directly binds to CD4+ T-cells and blocks binding of envelope protein gp120 to cells | [41] | |
| Zika virus | Flaviviridae | In vitro | Entry | Interaction with the lipid envelope of virus | [46] | |
| Fisetin | CHIKV | Togaviridae | In vitro | Replication | Inhibition of NS protein 1 and 3 and downregulation of E2 protein and its precursor pE2 | [21] |
| DENV-2 | Flaviviridae | In vitro | Replication | Directly binds to the viral RNA to impede polymerases activity | [22] | |
| Genistein | HIV | Retroviridae | In vitro | Assembly and release | Inhibition of Vpu protein involved in the formation of ion channels in infected cells | [19] |
| Ginkgetin | Influenza A virus | Orthomyxoviridae | In vitro | Assembly and release | Inhibition of sialidase | [20] |
| Kaempferol | Coronavirus | Coronaviridae | In vitro | Assembly and release | Inhibition of the release of progeny virus by blocking 3a channels of virus | [24] |
| Influenza A virus | Orthomyxoviridae | In silico | Entry | Inhibition of neuraminidase enzyme | [23] | |
| Luteolin | Influenza A virus | Orthomyxoviridae | In vitro | Entry | Interaction with hemagglutinins of virus | [25] |
| Severe acute respiratory syndrome coronavirus (SARS-CoV) | Coronaviridae | In vitro | Entry | Binds to the S2 protein of virus | [25] | |
| Methoxyflavone, isoscutellarein, and 8-methoxy-isoscutellarein | Influenza A virus | Orthomyxoviridae | In vitro | Early replication | Reduction in sialidase activity, lysosomal fusion and RNA polymerase activity | [27,48] |
| Naringenin | CHIKV | Togaviridae | In vitro | Replication | Reduction in RNA and proteins | [49] |
| DENV-2 and 4 | Flaviviridae | In vitro | Replication | Reduction in RNA levels | [29,30] | |
| HCV | Flaviviridae | In vitro | Replication | Inhibition of RNA and core protein | [28] | |
| Quercetin | HCV | Flaviviridae | In vitro | Transcription | Inactivation of the NS3 helicase and NS5 protease | [8,9] |
| HSV-1, HSV-2, drug-resistant HSV-1 | Herpesviridae | In vitro | Binding and entry | N/R | [6] | |
| Influenza A virus | Orthomyxoviridae | In vitro | Binding and entry | Inhibition of neuraminidase activity by interaction with the viral subunit 2 of the hemagglutinin | [6] | |
| Rabies virus | Rhabdoviridae | In vivo | Cell protection | N/R | [3] | |
| Vesicular stomatitis virus (VSV) | Rhabdoviridae | In vivo | Indirect: Immune-mediated infection control | Activation of macrophages | [4] | |
| Quercetin 3-β-O-d-glucoside | Ebola virus | Filoviridae | In vivo | Prophylaxis | Unknown | [10] |
| Quercitrin | Influenza A virus | Orthomyxoviridae | In vitro | Early replication | Reduction in mRNA synthesis | [7] |
| Rabies virus | Rhabdoviridae | In vivo | Prophylaxis | Unknown | [3] | |
| Rutin (sulfated) | HIV-1 | Retroviridae | In vitro | Fusion | Inhibition of glycoprotein-mediated cell-cell fusion | [11] |
| HSV | Herpesviridae | In vitro | Adsorption | Unknown | [11] | |
| Silibinin | HCV | Flaviviridae | Phase II Clinical trial | Replication or immune-mediated infection control | Unknown. Postulated to be IFN-JAK/STAT independent immune-mediated antiviral mechanisms such as Regulation by interferon regulatory factor 3, Toll-like receptor 7 and p38 protein kinase pathways | [31] |
| Silymarin | CHIKV | Togaviridae | In vitro | Replication | Inhibition of viral proteins | [35] |
| DENV-2 | Flaviviridae | In silico | Replication | Inhibition of NS4B protein | [36] | |
| EBV | Herpesviridae | In vitro | Early antigen inactivation | N/R | [32] | |
| HCV | Flaviviridae | In vitro | Entry and fusion | Inhibition of viral pseudoparticles (pp) fusion with liposomes | [34] | |
| Influenza A virus | Orthomyxoviridae | In vitro | Late replication | Inhibition of viral mRNA synthesis | [37] | |
| Mayaro virus | Togaviridae | In vitro | Replication | Inhibition of reactive oxygen species (ROS) and reduction in levels of malondialdehyde (MDA) | [33] | |
| Tea catechins | Influenza A virus | Orthomyxoviridae | In vitro | Adsorption and entry | Interaction with hemagglutinins of virus | [38] |
| Tetra-O-methyl quercetin | Influenza A virus | Orthomyxoviridae | In vitro | Entry | Interaction with mannose-rich hemagglutinin domains of virus | [5] |
| Flavonoid | Picornavirus | Model | Stage of Virus Inhibition | Suggested Mechanism | Reference |
|---|---|---|---|---|---|
| 3-Methylquercetin | Poliovirus | In vitro | Late replication | Blocked of genomic RNA synthesis | [51] |
| Reduction in viral protein and RNA synthesis | [52] | ||||
| 3-Methylkaempferol | Poliovirus-1 | In vitro | Replication | Inhibition of positive-strand of viral RNA | [58] |
| 5,3′-Dihydroxy-3,6,7,8,4′-pentamethoxyflavone and 5-hydroxy-3,6,7,3′,4′-pentamethoxyflavone | Poliovirus-1 | In vitro | Replication | Postulated to be inhibition of cellular processes (apoptosis and downstream signaling pathways) | [67] |
| 5,7,4′-Trihydroxy-3′-methoxyflavone | Rhinovirus (HRV) | In silico | Entry | Inhibition by binding to human rhinovirus protein grid | [61] |
| 6-Chloro-4′-oxazolinylflavanone | Poliovirus-2 | In vitro | Replication | N/R | [63] |
| HRV-1B | In vitro | Replication | N/R | [63] | |
| 7-O-galloyltricetifavan and 7,4′-di-Ogalloyltricetifavan | Coxsackievirus B3 (CV-B3) | In vitro | N/R | N/R | [64] |
| Chrysosplenol C | Poliovirus | In vitro | Replication | N/R | [65] |
| Desmanthin-1 | Coxsackieviruses A16 (CV-A16) | In vitro | Replication | N/R | [66] |
| Dihydroquercetin | Coxsackievirus B4 (CV-B4) | In vivo | Indirect: Immune-mediated infection control | Reduction in viral immune mediators (ROS-mediated signaling and oxidative stress | [54] |
| Epigallocatechin-3-Gallate | Poliovirus-1 | In vitro | Virucidal effect (irreversible) | N/R | [60] |
| Eupafolin | CV-A16 | In vitro | Attachment | Reduction in IL-6 and RANTES and inactivation of downstream signaling pathways (ERK1/2, c-Jun, and STAT3) | [57] |
| Kaempferol-3-O-[2″,6″-di-O-Z-p-coumaroyl]-β-d-glucopyranoside and derivatives | CV-B3 | In vitro | Replication | N/R | [59] |
| HRV-1B | In vitro | Replication | N/R | [59] | |
| Luteolin | CV-A16 | In vitro | Replication | Inhibition of viral RNA synthesis | [55] |
| Poliovirus | In vitro | Replication | N/R | [56] | |
| Myricitrin | CV-A16 | In vitro | Replication | N/R | [66] |
| Pachypodol (RO 09-0179) | CV | In vitro | Early replication | Interference with viral replications between the uncoating and RNA synthesis stage | [53] |
| Poliovirus | In vitro | Late replication | Blocked the synthesis of positive-strand RNA | [51] | |
| HRV | In vitro | Early replication | Interference with viral replications between the uncoating and RNA synthesis stage | [53] | |
| Prunin | Enteroviruses A and B | In vitro and in vivo | Translation and replication | Inhibition of IRES activity and protein synthesis | [68] |
| Quercetin | Encephalomyocarditis virus (EMCV) | In vivo | Indirect: Immune-mediated infection control | Activation of macrophages | [4] |
| Mengo virus | In vivo | Indirect: Immune-mediated infection control | Activation of macrophages | [4] | |
| HRV | In vitro | Transcription and translation | Reduction in endocytosis of virus and phosphorylation of Akt (effector of phosphoinositol 3-kinase). Repression of interferon and interleukin-8 response resulted in lower viral RNA and capsid protein production. | [50] | |
| HRV | In vivo | Indirect: Immune mediated infection control | Suppression of viral immune mediators | [50] | |
| RO 09-0298 | CV-B1 | In vivo | N/R | N/R | [53] |
| Sakuranetin | HRV-3 | In vitro | Replication | Antioxidant activity through inhibition of viral adsorption | [62] |
| Flavonoid | In Vitro EC50 (µM) | Lethal Dose of Challenge Virus | In Vivo Dose of Flavonoid | Survival Rate | Duration of Treatment | Reference |
|---|---|---|---|---|---|---|
| Apigenin | 24.74 | 600,000 TCID50 | 50 mg/Kg | 88.89% | Once a day for 7 days, starting from 2 h post-infection | [72] |
| Chrysosplenetin | 0.68 | 600,000 TCID50 | 5 and 1 mg/Kg | 30% | Once a day for 7 days, starting from 2 h post-infection | [72] |
| Formononetin | 12.5 | 600,000 TCID50 | 10 mg/Kg | 75% | Once a day for 7 days, starting from 2 h post-infection | [72] |
| Isorhamnetin | 60.7 | 600,000 TCID50 | 10 mg/Kg | 100% | Once a day for 7 days, starting from 2 h post-infection | [72] |
| Kaempferol | 52.75 | 600,000 TCID50 | 50 mg/Kg | 88.89% | Once a day for 7 days, starting from 2 h post-infection | [72] |
| Luteolin | 13.5 | 600,000 TCID50 | 10 mg/Kg | 91.67% | Once a day for 7 days, starting from 2 h post-infection | [72] |
| Penduletin | 0.63 | 600,000 TCID50 | 5 mg/Kg | 66.67% | Once a day for 7 days, starting from 2 h post-infection | [72] |
| Prunin | 0.115 | 2 × 107 PFU | 3 and 10 mg/Kg | 100% | Once a day for 7 days, starting from 1 or 6 h post infection | [68] |
| Quercetin | 1.2 | 600,000 TCID50 | 10 mg/Kg | 50% | Once a day for 7 days, starting from 2 h post infection | [72] |
| Flavonoid | Structure | EV-A71 Strain * (Genotype/Subgenotype) | Antiviral Activity/IC50 | Cytoxicity/CC50 | Reference |
|---|---|---|---|---|---|
| Apigenin | 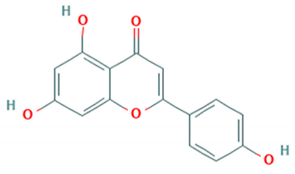 | Fuyang 0805 (C4a) | 10.3 μM | 79.0 μM (RD cells) | [70] |
| Fuyang0805 (C4a) BrCr (A) | Not reported | >200 μM (RD and Vero cells) | [71] | ||
| Baicalin | 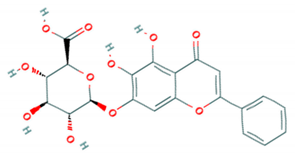 | BrCr (A) | 4.96 μg/mL | 823.53 µg/mL (RD cells) | [73] |
| Chrysin Ester of chrysin (CR) | 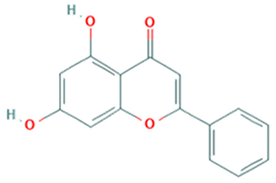 | SHZH-98 (C4) | C = 13.86 μM | >200 μM (RD cells) | [74] |
| CR = 24.12 μM | >200 μM (RD cells) | ||||
| Chrysosplenetin | 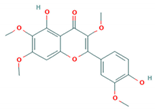 | GZ-08-02 (Accession # FJ360545) | 0.17 μM (Vero cells) | 18.27 μM (Vero cells) | [80] |
| 0.20 μM (RD cells) | 13.90 μM (RD cells) | ||||
| Eupafolin | 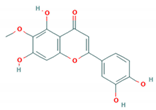 | Not reported | 0.44 µg/mL (RD cells) | 355.87 µg/mL (RD cells) | [57] |
| Fisetin | 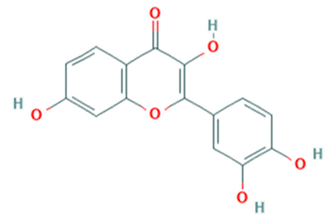 | CMUH01 (B5) | 85 μM | >1000 μM (RD cells) | [75] |
| Formononetin | 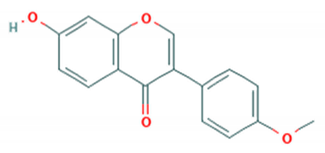 | SHZH-98 (C4) JS-52 (C4) H BrCr (A) |
3.45–3.95 μM 17.87 ± 8.51 μM 11.11 ± 9.23 μM 6.47 ± 4.40 μM | 149.38 μM (Vero cells) 198.80 μM (SK-N-SH cells) | [76] |
| Galangin | 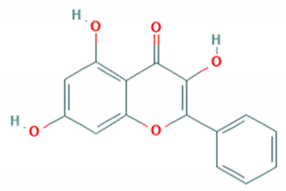 | C4b | Not reported | Not reported | [55] |
| Hesperetin | 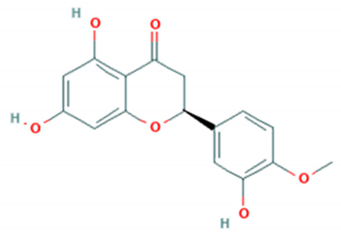 | Cmuh-050530-5 (Accession # HM807310) | Not reported | >50 μM (RD cells) | [79] |
| Hesperidin | 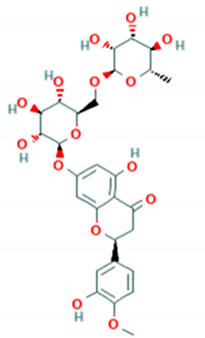 | Cmuh-050530-5 (Accession # HM807310) | Not reported | >50 μM (RD cells) | [79] |
| Hydroxyflavone (HF) and its phosphate ester | 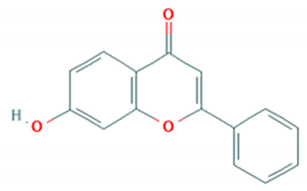 | SHZH-98 (C4) | 23.45 μM | >200 μM (RD cells) | [78] |
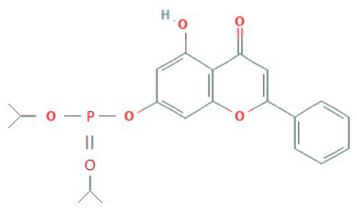 | SHZH-98 (C4) | 13.63 μM | >200 μM (RD cells) | [78] | |
| Kaempferol | 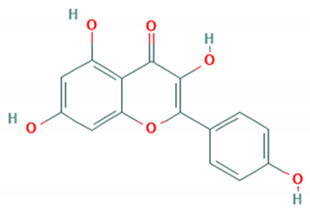 | Cmuh-050530-5 (Accession # HM807310) | Not reported 6 log reduction at 24 hrs | >50 μM (RD cells) | [79] |
| Luteolin | 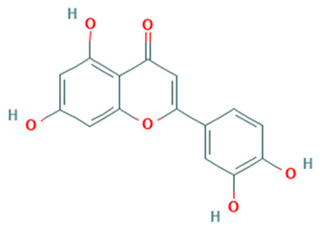 | C4b | 10 μM | 148.02 μM (RDS cells) | [55] |
| 292.00 μM (RD cells) | |||||
| Fuyang0805 (C4a) BrCr (A) | Not reported | 178.65 μM (Vero cells) | [71] | ||
| 157 μM (Vero cells) | |||||
| 200 μM (RD cells) | |||||
| Penduletin | 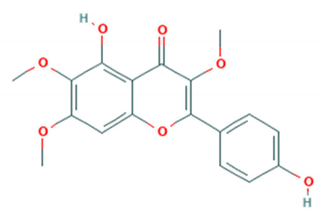 | GZ-08-02 (Accession # FJ360545) | 0.17 µM (Vero cells) | 111.46 µM (Vero cells) | [80] |
| 0.37 µM (RD cells) | 74.18 µM (RD cells) | ||||
| Peracetate pulicarine | 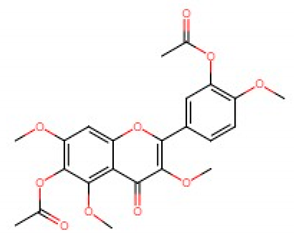 | 5865/sin/000009 (B4) | Not reported. | >20 µg/mL (RD cells) | [81] |
| 5511-SIN-00 (B5) | 2.5 Log reduction | ||||
| Prunin | 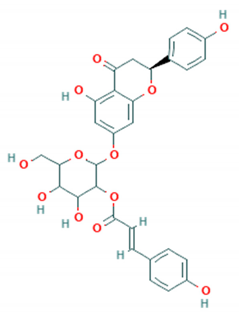 | EV-A71 clinical isolates, EV-A71 strains H, B5 and C4 genotypes | 115.3 nM | 2715 nM (RD cells) | [68] |
| Quercetagetin | 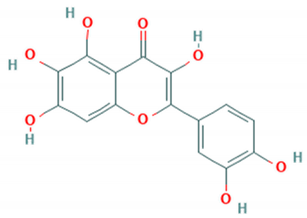 | 5865/sin/000009 (B4) | Not reported. | >20 µg/mL (RD cells) | [81] |
| 5511-SIN-00 (B5) | 3.5 Log reduction | ||||
| Quercetin | 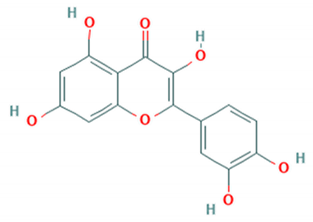 | SK-EV006/Malaysia/97 (Accession # AB469182) | 12.1 μM (RD cells) | >200 μM (RD and Vero cells) | [82] |
| 8.8 μM (Vero cells) | |||||
| Rutin | 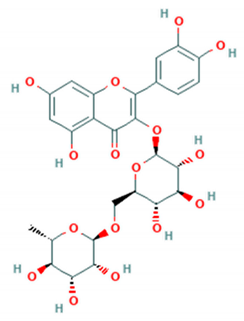 | CMUH01 (B5) | 110 μM | >1000 μM (RD cells) | [75] |
| Thio flavones (Multiple) 4b 7d 7i 8b 9b | 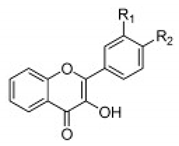 4b = R1 = OCH3, R2 = H 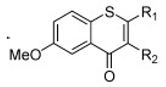 7d = R1 = 4-methoxyphenyl, R2 = H 7i = R1 = CH3, R2 = Cl 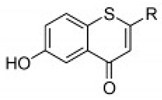 8b = R = n-propyl 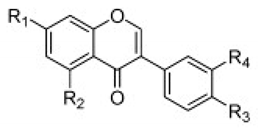 9b = R1 = OH, R2 = H, R3 = OH, R4 = OH | SHZH-98 (C4) | 4b = 16.9 μM | 4d = 29.23 μM | [86] |
| 7d = 8.27 μM | 7d = 107.34 μM | ||||
| 7i = 39.63 μM | 7i = 133.15 μM | ||||
| 8b = 100.86 μM | 8b = 174.41 μM | ||||
| 9b = 5.48 μM | 9b = 23.75 μM (Vero cells) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalani, S.; Poh, C.L. Flavonoids as Antiviral Agents for Enterovirus A71 (EV-A71). Viruses 2020, 12, 184. https://doi.org/10.3390/v12020184
Lalani S, Poh CL. Flavonoids as Antiviral Agents for Enterovirus A71 (EV-A71). Viruses. 2020; 12(2):184. https://doi.org/10.3390/v12020184
Chicago/Turabian StyleLalani, Salima, and Chit Laa Poh. 2020. "Flavonoids as Antiviral Agents for Enterovirus A71 (EV-A71)" Viruses 12, no. 2: 184. https://doi.org/10.3390/v12020184
APA StyleLalani, S., & Poh, C. L. (2020). Flavonoids as Antiviral Agents for Enterovirus A71 (EV-A71). Viruses, 12(2), 184. https://doi.org/10.3390/v12020184





