Norovirus Seroprevalence among Adults in the United States: Analysis of NHANES Serum Specimens from 1999–2000 and 2003–2004
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Specimens
2.2. Norovirus VLP Panel
2.3. Anti-Norovirus IgG ELISA
2.4. Determination of OD Cut-Points
2.5. Statistical Analysis
2.6. Data Availability
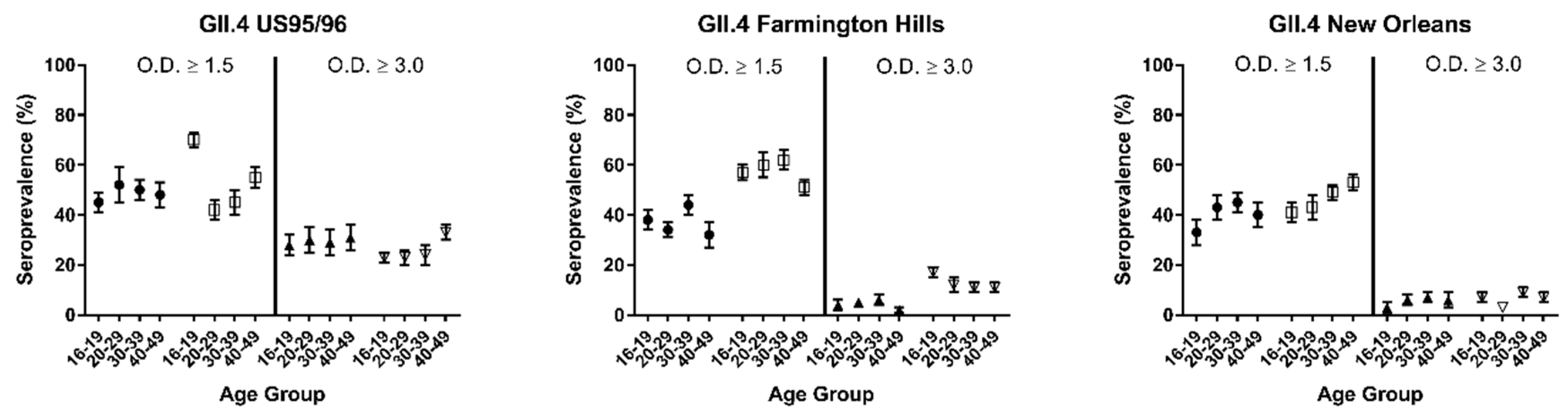
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hall, A.J.; Wikswo, M.E.; Manikonda, K.; Roberts, V.A.; Yoder, J.S.; Gould, L.H. Acute gastroenteritis surveillance through the National Outbreak Reporting System, United States. Emerg. Infect. Dis. 2013, 19, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States--major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.E.; Shi, J.; Montes, J.; Lichtenstein, M.; Moe, C.L. Disease course and viral shedding in experimental Norwalk virus and Snow Mountain virus infection. J. Med. Virol. 2014, 86, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Mattner, F.; Sohr, D.; Heim, A.; Gastmeier, P.; Vennema, H.; Koopmans, M. Risk groups for clinical complications of norovirus infections: An outbreak investigation. Clin. Microbio. Infect. 2006, 12, 69–74. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chiu, C.H. Worldwide molecular epidemiology of norovirus infection. Paediatr. Int. Child Health 2012, 32, 128–131. [Google Scholar] [CrossRef]
- Huang, P.; Farkas, T.; Zhong, W.; Tan, M.; Thornton, S.; Morrow, A.L.; Jiang, X. Norovirus and histo-blood group antigens: Demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol. 2005, 79, 6714–6722. [Google Scholar] [CrossRef]
- Lindesmith, L.; Moe, C.; Marionneau, S.; Ruvoen, N.; Jiang, X.; Lindblad, L.; Stewart, P.; LePendu, J.; Baric, R. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 2003, 9, 548–553. [Google Scholar] [CrossRef]
- Kindberg, E.; Akerlind, B.; Johnsen, C.; Knudsen, J.D.; Heltberg, O.; Larson, G.; Bottiger, B.; Svensson, L. Host genetic resistance to symptomatic norovirus (GGII.4) infections in Denmark. J. Clin. Microbiol. 2007, 45, 2720–2722. [Google Scholar] [CrossRef]
- Tan, M.; Jin, M.; Xie, H.; Duan, Z.; Jiang, X.; Fang, Z. Outbreak studies of a GII-3 and a GII-4 norovirus revealed an association between HBGA phenotypes and viral infection. J. Med. Virol. 2008, 80, 1296–1301. [Google Scholar] [CrossRef]
- Thorven, M.; Grahn, A.; Hedlund, K.O.; Johansson, H.; Wahlfrid, C.; Larson, G.; Svensson, L. A homozygous nonsense mutation (428G-->A) in the human secretor (fut2) gene provides resistance to symptomatic norovirus (GGII) infections. J. Virol. 2005, 79, 15351–15355. [Google Scholar] [CrossRef]
- Carlsson, B.; Kindberg, E.; Buesa, J.; Rydell, G.E.; Lidon, M.F.; Montava, R.; Abu Mallouh, R.; Grahn, A.; Rodriguez-Diaz, J.; Bellido, J.; et al. The g428a nonsense mutation in fut2 provides strong but not absolute protection against symptomatic GII.4 norovirus infection. PLoS ONE 2009, 4, e5593. [Google Scholar] [CrossRef] [PubMed]
- Lindesmith, L.; Moe, C.; Lependu, J.; Frelinger, J.A.; Treanor, J.; Baric, R.S. Cellular and humoral immunity following Snow Mountain virus challenge. J. Virol. 2005, 79, 2900–2909. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, J.; Kindberg, E.; Lindgren, P.E.; Matussek, A.; Svensson, L. Norovirus gastroenteritis outbreak with a secretor-independent susceptibility pattern, sweden. Emerg. Infect. Dis. 2010, 16, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Rockx, B.H.; Vennema, H.; Hoebe, C.J.; Duizer, E.; Koopmans, M.P. Association of histo-blood group antigens and susceptibility to norovirus infections. J. Infect. Dis. 2005, 191, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Gaensslen, R.E.; Bell, S.C.; Lee, H.C. Distributions of genetic markers in United States populations: I. Blood group and secretor systems. J. Forensic Sci. 1987, 32, 1016–1058. [Google Scholar] [CrossRef]
- Currier, R.L.; Payne, D.C.; Staat, M.A.; Selvarangan, R.; Shirley, S.H.; Halasa, N.; Boom, J.A.; Englund, J.A.; Szilagyi, P.G.; Harrison, C.J.; et al. Innate susceptibility to norovirus infections influenced by fut2 genotype in a United States pediatric population. Clin. Infect. Dis. 2015, 60, 1631–1638. [Google Scholar] [CrossRef]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef]
- Matthews, J.E.; Dickey, B.W.; Miller, R.D.; Felzer, J.R.; Dawson, B.P.; Lee, A.S.; Rocks, J.J.; Kiel, J.; Montes, J.S.; Moe, C.L.; et al. The epidemiology of published norovirus outbreaks: A review of risk factors associated with attack rate and genogroup. Epidemiol. Infect. 2012, 140, 1161–1172. [Google Scholar] [CrossRef]
- Cannon, J.L.; Barclay, L.; Collins, N.R.; Wikswo, M.E.; Castro, C.J.; Magana, L.C.; Gregoricus, N.; Marine, R.L.; Chhabra, P.; Vinje, J. Genetic and epidemiologic trends of norovirus outbreaks in the United States from 2013 to 2016 demonstrated emergence of novel GII.4 recombinant viruses. J. Clin. Microbiol. 2017, 55, 2208–2221. [Google Scholar] [CrossRef]
- Shanker, S.; Choi, J.M.; Sankaran, B.; Atmar, R.L.; Estes, M.K.; Prasad, B.V. Structural analysis of histo-blood group antigen binding specificity in a norovirus GII.4 epidemic variant: Implications for epochal evolution. J. Virol. 2011, 85, 8635–8645. [Google Scholar] [CrossRef]
- Siebenga, J.J.; Vennema, H.; Renckens, B.; de Bruin, E.; van der Veer, B.; Siezen, R.J.; Koopmans, M. Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J. Virol. 2007, 81, 9932–9941. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Pourshaban, M.; Iaconelli, M.; Muscillo, M. Detection of genogroup IV noroviruses in environmental and clinical samples and partial sequencing through rapid amplification of cDNA ends. Arch. Virol. 2008, 153, 2077–2083. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, J.M.; Fumian, T.M.; Rocha, M.S.; Dos Santos, I.A.L.; Carvalho-Costa, F.A.; de Assis, M.R.; Rodrigues, J.S.; Leite, J.P.G.; Miagostovich, M.P. Surveillance of noroviruses in Rio de Janeiro, Brazil: Occurrence of new GIV genotype in clinical and wastewater samples. Food Environ. Virol. 2018, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Parnaudeau, S.; Grodzki, M.; Okabe, S.; Atmar, R.L.; Le Guyader, F.S. Environmental detection of genogroup I, II, and IV noroviruses by using a generic real-time reverse transcription-PCR assay. Appl. Environ. Microbiol. 2013, 79, 6585–6592. [Google Scholar] [CrossRef]
- La Rosa, G.; Iaconelli, M.; Pourshaban, M.; Fratini, M.; Muscillo, M. Molecular detection and genetic diversity of norovirus genogroup IV: A yearlong monitoring of sewage throughout Italy. Arch. Virol. 2010, 155, 589–593. [Google Scholar] [CrossRef]
- Yen, C.; Hall, A.J. Editorial commentary: Challenges to estimating norovirus disease burden. J. Pediatr. Infect. Dis. Soc. 2013, 2, 61–62. [Google Scholar] [CrossRef]
- Zheng, D.P.; Ando, T.; Fankhauser, R.L.; Beard, R.S.; Glass, R.I.; Monroe, S.S. Norovirus classification and proposed strain nomenclature. Virology 2006, 346, 312–323. [Google Scholar] [CrossRef]
- Bull, R.A.; Eden, J.S.; Rawlinson, W.D.; White, P.A. Rapid evolution of pandemic noroviruses of the GII.4 lineage. PLoS Pathog. 2010, 6, e1000831. [Google Scholar] [CrossRef]
- Bull, R.A.; White, P.A. Mechanisms of GII.4 norovirus evolution. Trends Microbiol. 2011, 19, 233–240. [Google Scholar] [CrossRef]
- Debbink, K.; Lindesmith, L.C.; Donaldson, E.F.; Baric, R.S. Norovirus immunity and the great escape. PLoS Pathog. 2012, 8, e1002921. [Google Scholar] [CrossRef]
- Simmons, K.; Gambhir, M.; Leon, J.; Lopman, B. Duration of immunity to norovirus gastroenteritis. Emerg. Inf. Dis. 2013, 19, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Opekun, A.R.; Gilger, M.A.; Estes, M.K.; Crawford, S.E.; Neill, F.H.; Graham, D.Y. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 2008, 14, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Gastanaduy, P.A.; Hall, A.J.; Curns, A.T.; Parashar, U.D.; Lopman, B.A. Burden of norovirus gastroenteritis in the ambulatory setting-United States, 2001–2009. J. Infect. Dis. 2013, 207, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Curns, A.T.; McDonald, L.C.; Parashar, U.D.; Lopman, B.A. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin. Infect. Dis. 2012, 55, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Rosenthal, M.; Gregoricus, N.; Greene, S.A.; Ferguson, J.; Henao, O.L.; Vinje, J.; Lopman, B.A.; Parashar, U.D.; Widdowson, M.A. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004–2005. Emerg. Infect. Dis. 2011, 17, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Cubitt, W.D.; Green, K.Y.; Payment, P. Prevalence of antibodies to the Hawaii strain of human calicivirus as measured by a recombinant protein based immunoassay. J. Med. Virol. 1998, 54, 135–139. [Google Scholar] [CrossRef]
- Dimitrov, D.H.; Dashti, S.A.; Ball, J.M.; Bishbishi, E.; Alsaeid, K.; Jiang, X.; Estes, M.K. Prevalence of antibodies to human caliciviruses (huCVs) in Kuwait established by ELISA using baculovirus-expressed capsid antigens representing two genogroups of huCVs. J. Med. Virol. 1997, 51, 115–118. [Google Scholar] [CrossRef]
- Gabbay, Y.B.; Glass, R.I.; Monroe, S.S.; Carcamo, C.; Estes, M.K.; Mascarenhas, J.D.; Linhares, A.C. Prevalence of antibodies to Norwalk virus among Amerindians in isolated Amazonian communities. Am. J. Epidemiol. 1994, 139, 728–733. [Google Scholar] [CrossRef]
- Gray, J.J.; Jiang, X.; Morgan-Capner, P.; Desselberger, U.; Estes, M.K. Prevalence of antibodies to Norwalk virus in England: Detection by enzyme-linked immunosorbent assay using baculovirus-expressed Norwalk virus capsid antigen. J. Clin. Microbiol. 1993, 31, 1022–1025. [Google Scholar] [CrossRef]
- Jing, Y.; Qian, Y.; Huo, Y.; Wang, L.P.; Jiang, X. Seroprevalence against Norwalk-like human caliciviruses in Beijing, China. J. Med. Virol. 2000, 60, 97–101. [Google Scholar] [CrossRef]
- Lew, J.F.; Valdesuso, J.; Vesikari, T.; Kapikian, A.Z.; Jiang, X.; Estes, M.K.; Green, K.Y. Detection of Norwalk virus or Norwalk-like virus infections in Finnish infants and young children. J. Infect. Dis. 1994, 169, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Honma, S.; Numata, K.; Kogawa, K.; Ukae, S.; Adachi, N.; Jiang, X.; Estes, M.K.; Gatheru, Z.; Tukei, P.M.; et al. Prevalence of human calicivirus infections in Kenya as determined by enzyme immunoassays for three genogroups of the virus. J. Clin. Microbiol. 1998, 36, 3160–3163. [Google Scholar] [CrossRef] [PubMed]
- O’Ryan, M.L.; Vial, P.A.; Mamani, N.; Jiang, X.; Estes, M.K.; Ferrecio, C.; Lakkis, H.; Matson, D.O. Seroprevalence of Norwalk virus and Mexico virus in Chilean individuals: Assessment of independent risk factors for antibody acquisition. Clin. Infect. Dis. 1998, 27, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.P.; Cubitt, W.D.; Jiang, X. Enzyme immunoassay using baculovirus-expressed human calicivirus (Mexico) for the measurement of IgG responses and determining its seroprevalence in London, UK. J. Med. Virol. 1995, 46, 194–200. [Google Scholar] [CrossRef]
- Peasey, A.E.; Ruiz-Palacios, G.M.; Quigley, M.; Newsholme, W.; Martinez, J.; Rosales, G.; Jiang, X.; Blumenthal, U.J. Seroepidemiology and risk factors for sporadic norovirus/Mexico strain. J. Infect. Dis. 2004, 189, 2027–2036. [Google Scholar] [CrossRef]
- Smit, T.K.; Steele, A.D.; Peenze, I.; Jiang, X.; Estes, M.K. Study of Norwalk virus and Mexico virus infections at Ga-Rankuwa Hospital, Ga-Rankuwa, South Africa. J. Clin. Microbiol. 1997, 35, 2381–2385. [Google Scholar] [CrossRef]
- CDC. National Health and Nutrition Examination Survey Biospecimen Program. Available online: https://www.cdc.gov/nchs/nhanes/biospecimens/biospecimens.htm (accessed on 13 June 2018).
- Green, K.Y.; Lew, J.F.; Jiang, X.; Kapikian, A.Z.; Estes, M.K. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J. Clin. Microbiol. 1993, 31, 2185–2191. [Google Scholar] [CrossRef]
- Widdowson, M.A.; Cramer, E.H.; Hadley, L.; Bresee, J.S.; Beard, R.S.; Bulens, S.N.; Charles, M.; Chege, W.; Isakbaeva, E.; Wright, J.G.; et al. Outbreaks of acute gastroenteritis on cruise ships and on land: Identification of a predominant circulating strain of norovirus--United States, 2002. J. Infect. Dis. 2004, 190, 27–36. [Google Scholar] [CrossRef]
- Mossong, J.; Hens, N.; Jit, M.; Beutels, P.; Auranen, K.; Mikolajczyk, R.; Massari, M.; Salmaso, S.; Tomba, G.S.; Wallinga, J.; et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008, 5, e74. [Google Scholar] [CrossRef]
- Vinje, J.; Altena, S.A.; Koopmans, M.P. The incidence and genetic variability of small round-structured viruses in outbreaks of gastroenteritis in the Netherlands. J. Infect. Dis. 1997, 176, 1374–1378. [Google Scholar] [CrossRef]
- Noel, J.S.; Fankhauser, R.L.; Ando, T.; Monroe, S.S.; Glass, R.I. Identification of a distinct common strain of “Norwalk-like viruses” having a global distribution. J. Infect. Dis. 1999, 179, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Vega, E.; Barclay, L.; Gregoricus, N.; Williams, K.; Lee, D.; Vinje, J. Novel surveillance network for norovirus gastroenteritis outbreaks, United States. Emerg. Infect. Dis. 2011, 17, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.S.; Kingsley, D.H.; Montes, J.S.; Richards, G.P.; Lyon, G.M.; Abdulhafid, G.M.; Seitz, S.R.; Fernandez, M.L.; Teunis, P.F.; Flick, G.J.; et al. Randomized, double-blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl. Environ. Microbiol. 2011, 77, 5476–5482. [Google Scholar] [CrossRef] [PubMed]
- Lopman, B.; Vennema, H.; Kohli, E.; Pothier, P.; Sanchez, A.; Negredo, A.; Buesa, J.; Schreier, E.; Reacher, M.; Brown, D.; et al. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet 2004, 363, 682–688. [Google Scholar] [CrossRef]
- Le Pendu, J.; Ruvoen-Clouet, N.; Kindberg, E.; Svensson, L. Mendelian resistance to human norovirus infections. Semin. Immunol. 2006, 18, 375–386. [Google Scholar] [CrossRef]
- Eftim, S.E.; Hong, T.; Soller, J.; Boehm, A.; Warren, I.; Ichida, A.; Nappier, S.P. Occurrence of norovirus in raw sewage—A systematic literature review and meta-analysis. Water Res. 2017, 111, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Kim, S.C.; Kim, S.T.; Chung, C.H.; Chung, J.Y. Detection of norovirus genogroup IV, klassevirus, and pepper mild mottle virus in sewage samples in South Korea. Arch. Virol. 2014, 159, 457–463. [Google Scholar] [CrossRef]
- Kitajima, M.; Rachmadi, A.T.; Iker, B.C.; Haramoto, E.; Gerba, C.P. Genetically distinct genogroup IV norovirus strains identified in wastewater. Arch. Virol. 2016, 161, 3521–3525. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Profio, F.; Ceci, C.; Di Felice, E.; Green, K.Y.; Bok, K.; De Grazia, S.; Giammanco, G.M.; Massirio, I.; Lorusso, E.; et al. Seroprevalence of norovirus genogroup IV antibodies among humans, Italy, 2010–2011. Emerg. Infect. Dis. 2014, 20, 1828–1832. [Google Scholar] [CrossRef]
- Reeck, A.; Kavanagh, O.; Estes, M.K.; Opekun, A.R.; Gilger, M.A.; Graham, D.Y.; Atmar, R.L. Serological correlate of protection against norovirus-induced gastroenteritis. J. Infect. Dis. 2010, 202, 1212–1218. [Google Scholar] [CrossRef]

| 1999–2000 | 2003–2004 | ||||
|---|---|---|---|---|---|
| Seroprevalence (%) | 95% Confidence Limits | Seroprevalence (%) | 95% Confidence Limits | p-Value a | |
| Seropositive for at least one antigen | |||||
| OD ≥ 1.5 | 90.0 | 87.5, 92.5 | 95.9 | 94.1, 97.6 | 0.007 |
| OD ≥ 3.0 | 51.8 | 48.4, 55.3 | 59.1 | 55.4, 62.9 | 0.003 |
| Seropositive for all 7 antigens | |||||
| OD ≥ 1.5 | 1.0 | 4.0, 2.5 | 3.0 | 1.5, 3.7 | 0.120 |
| OD ≥ 3.0 | 0.0 | 0.0, 0.0 | 0.0 | 0.0, 0.0 | - |
| Seronegative for all 7 antigens | |||||
| OD < 1.5 | 10.0 | 7.5, 12.5 | 4.1 | 2.4, 5.9 | < 0.001 |
| Antigen | 1999–2000 Study Cycle | 2003–2004 Study Cycle | p-Value a | ||
|---|---|---|---|---|---|
| Seroprevalence (%) | 95% Confidence Limits | Seroprevalence (%) | 95% Confidence Limits | ||
| Cut-point OD ≥ 1.5 | |||||
| GI.1 Norwalk | 59.7 | 53.9, 65.5 | 56.4 | 51.4, 61.4 | 0.360 |
| GI.4 | 27.6 | 24.4, 30.9 | 27.0 | 22.7, 31.3 | 0.812 |
| GII.3 | 53.7 | 49.1, 58.4 | 50.0 | 44.1, 55.9 | 0.302 |
| GII.4 US 95/96 | 49.6 | 45.6, 53.5 | 49.9 | 44.1, 55.8 | 0.927 |
| GII.4 Farmington Hills | 36.9 | 33.6, 40.3 | 57.5 | 53.5, 61.5 | <0.001 |
| GII.4 New Orleans | 41.6 | 37.0, 46.1 | 48.1 | 44.3, 51.9 | 0.019 |
| GIV.1 | 19.1 | 14.9, 23.4 | 25.9 | 21.9, 29.9 | 0.014 |
| Cut-point OD ≥ 3.0 | |||||
| GI.1 Norwalk | 15.8 | 12.3, 19.2 | 19.5 | 15.8, 23.3 | 0.126 |
| GI.4 | 3.4 | 1.2, 5.6 | 6.0 | 3.7, 8.3 | 0.081 |
| GII.3 | 26.1 | 22.9, 29.2 | 28.1 | 22.4, 33.8 | 0.523 |
| GII.4 US 95/96 | 29.9 | 25.4, 34.4 | 26.8 | 23.2, 30.3 | 0.249 |
| GII.4 Farmington Hills | 4.5 | 2.4, 6.6 | 11.7 | 9.5, 13.9 | <0.001 |
| GII.4 New Orleans | 6.1 | 3.2, 9.0 | 6.4 | 4.1, 8.7 | 0.866 |
| GIV.1 | 0.9 | 0.2, 1.5 | 2.3 | 1.0, 3.7 | 0.040 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirby, A.E.; Kienast, Y.; Zhu, W.; Barton, J.; Anderson, E.; Sizemore, M.; Vinje, J.; Moe, C.L. Norovirus Seroprevalence among Adults in the United States: Analysis of NHANES Serum Specimens from 1999–2000 and 2003–2004. Viruses 2020, 12, 179. https://doi.org/10.3390/v12020179
Kirby AE, Kienast Y, Zhu W, Barton J, Anderson E, Sizemore M, Vinje J, Moe CL. Norovirus Seroprevalence among Adults in the United States: Analysis of NHANES Serum Specimens from 1999–2000 and 2003–2004. Viruses. 2020; 12(2):179. https://doi.org/10.3390/v12020179
Chicago/Turabian StyleKirby, Amy E., Yvonne Kienast, Wanzhe Zhu, Jerusha Barton, Emeli Anderson, Melissa Sizemore, Jan Vinje, and Christine L. Moe. 2020. "Norovirus Seroprevalence among Adults in the United States: Analysis of NHANES Serum Specimens from 1999–2000 and 2003–2004" Viruses 12, no. 2: 179. https://doi.org/10.3390/v12020179
APA StyleKirby, A. E., Kienast, Y., Zhu, W., Barton, J., Anderson, E., Sizemore, M., Vinje, J., & Moe, C. L. (2020). Norovirus Seroprevalence among Adults in the United States: Analysis of NHANES Serum Specimens from 1999–2000 and 2003–2004. Viruses, 12(2), 179. https://doi.org/10.3390/v12020179





