Extracellular Vesicles in HTLV-1 Communication: The Story of an Invisible Messenger
Abstract
:1. Introduction
1.1. Recent Trends in HTLV-1 Geographic Distribution
1.2. HTLV-1 Transmission
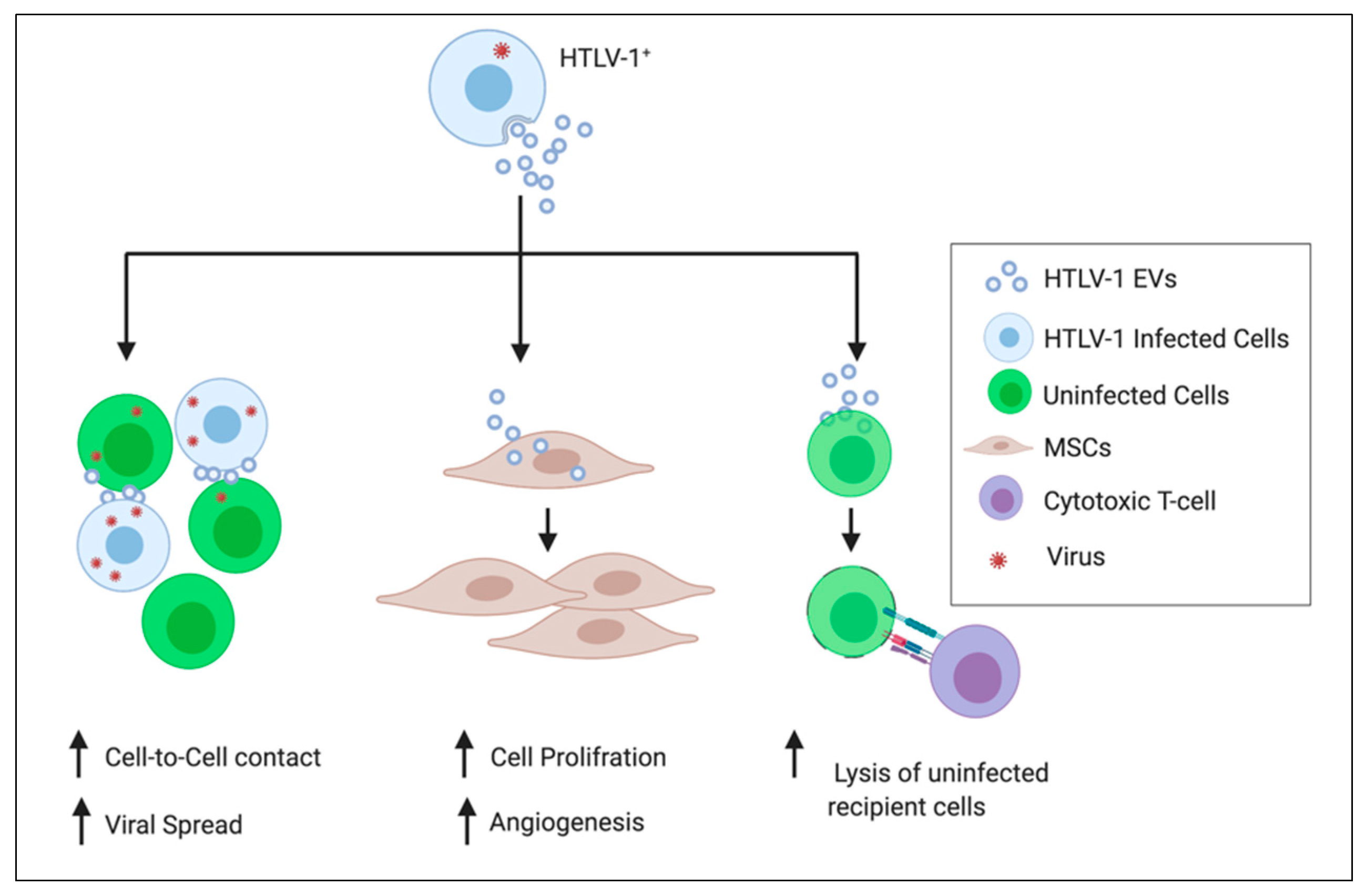
1.3. HTLV-1 Spread and Cell-Cell Contact
2. Extracellular Vesicles (EVs)
2.1. EV Biogenesis
2.2. EVs in Cell-Cell Communication
2.2.1. EV Cargo in Viral Infection
2.2.2. EV Cargo in Cancer
3. EVs in HTLV-1-Related Diseases
3.1. EVs in ATLL
3.2. EVs in HAM/TSP
3.3. EVs in Infective Dermatitis
3.4. EVs in Pulmonary Diseases
3.5. EVs in Uveitis
4. Treatment Options for HTLV-1-Associated Diseases
5. Treatments Used to modulate EV Biogenesis, Release, and Uptake
5.1. Inhibiting EV Trafficking
5.2. Inhibiting Lipid Metabolism
5.3. Inhibiting EV Uptake
5.4. Current Challenges to EV inhibition
6. Strategies for Preventing the Viral Transmission
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallo, R.C. Research and discovery of the first human cancer virus, HTLV-1. Best Pr. Res. Clin. Haematol. 2011, 24, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.C.; Willems, L.; Hasegawa, H. Global Virus Network’s Task Force on HTLV-1 Screening transplant donors for HTLV-1 and -2. Blood 2016, 128, 3029–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagaya, Y.; Gallo, R.C. The Exceptional Oncogenicity of HTLV-1. Front. Microbiol. 2017, 8, 1425. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Tagaya, Y.; Gallo, R. Time to eradicate HTLV-1: An open letter to WHO. Lancet 2018, 391, 1893–1894. [Google Scholar] [CrossRef]
- Barmpas, D.B.S.; Monteiro, D.L.M.; Taquette, S.R.; Rodrigues, N.C.P.; Trajano, A.J.B.; Cunha, J.D.C.; Nunes, C.L.; Villela, L.H.C.; Teixeira, S.A.M.; Sztajnbok, D.C.D.N.; et al. Pregnancy outcomes and mother-to-child transmission rate in HTLV-1/2 infected women attending two public hospitals in the metropolitan area of Rio de Janeiro. PLoS Neglected Trop. Dis. 2019, 13, e0007404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [Green Version]
- Gessain, A.; Cassar, O.; Domanovic, D. Europäisches Zentrum für die Prävention und die Kontrolle von Krankheiten. Geographical Distribution of Areas with a High Prevalence of HTLV-1 Infection; Technical Report; ECDC: Solna, Sweden, 2015; ISBN 978-92-9193-625-0. [Google Scholar]
- De Thé, G.; Kazanji, M. An HTLV-I/II vaccine: From animal models to clinical trials? J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996, 13 (Suppl. 1), S191–S198. [Google Scholar] [CrossRef]
- Gonçalves, D.U.; Proietti, F.A.; Ribas, J.G.R.; Araújo, M.G.; Pinheiro, S.R.; Guedes, A.C.; Carneiro-Proietti, A.B.F. Epidemiology, Treatment, and Prevention of Human T-Cell Leukemia Virus Type 1-Associated Diseases. Clin. Microbiol. Rev. 2010, 23, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Kondo, T.; Kono, H.; Miyamoto, N.; Yoshida, R.; Toki, H.; Matsumoto, I.; Hara, M.; Inoue, H.; Inatsuki, A.; Funatsu, T. Age- and sex-specific cumulative rate and risk of ATLL for HTLV-I carriers. Int. J. Cancer 1989, 43, 1061–1064. [Google Scholar] [CrossRef]
- Coffin, J.M. The discovery of HTLV-1, the first pathogenic human retrovirus. Proc. Natl. Acad. Sci. USA 2015, 112, 15525–15529. [Google Scholar] [CrossRef] [Green Version]
- Hirai, H.; Fujisawa, J.; Suzuki, T.; Ueda, K.; Muramatsu, M.; Tsuboi, A.; Arai, N.; Yoshida, M. Transcriptional activator Tax of HTLV-1 binds to the NF-kappa B precursor p105. Oncogene 1992, 7, 1737–1742. [Google Scholar] [PubMed]
- Poiesz, B.J.; Ruscetti, F.W.; Reitz, M.S.; Kalyanaraman, V.S.; Gallo, R.C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature 1981, 294, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Hamada, C.; Ohno, S.; Miyakoshi, H.; Koide, H.; Robert-Guroff, M.; Ting, R.C.; Gallo, R.C. Location of human T-cell leukemia virus (HTLV) p19 antigen on virus-producing cells. Int. J. Cancer 1984, 33, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Bangham, C.R.M.; Araujo, A.; Yamano, Y.; Taylor, G.P. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat. Rev. Dis. Prim. 2015, 1, 15012. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.E.; Osame, M.; Kubota, H.; Igata, A.; Nishitani, H.; Maeda, Y.; Khabbaz, R.F.; Janssen, R.S. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J. Acquir. Immune Defic. Syndr. 1990, 3, 1096–1101. [Google Scholar]
- Naito, T.; Yasunaga, J.; Mitobe, Y.; Shirai, K.; Sejima, H.; Ushirogawa, H.; Tanaka, Y.; Nakamura, T.; Hanada, K.; Fujii, M.; et al. Distinct gene expression signatures induced by viral transactivators of different HTLV-1 subgroups that confer a different risk of HAM/TSP. Retrovirology 2018, 15, 72. [Google Scholar] [CrossRef]
- Gessain, A.; Barin, F.; Vernant, J.C.; Gout, O.; Maurs, L.; Calender, A.; de Thé, G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 1985, 2, 407–410. [Google Scholar] [CrossRef]
- Osame, M.; Usuku, K.; Izumo, S.; Ijichi, N.; Amitani, H.; Igata, A.; Matsumoto, M.; Tara, M. HTLV-I associated myelopathy, a new clinical entity. Lancet 1986, 1, 1031–1032. [Google Scholar] [CrossRef]
- LaGrenade, L.; Hanchard, B.; Fletcher, V.; Cranston, B.; Blattner, W. Infective dermatitis of Jamaican children: A marker for HTLV-I infection. Lancet 1990, 336, 1345–1347. [Google Scholar] [CrossRef]
- Mochizuki, M.; Watanabe, T.; Yamaguchi, K.; Takatsuki, K.; Yoshimura, K.; Shirao, M.; Nakashima, S.; Mori, S.; Araki, S.; Miyata, N. HTLV-I uveitis: A distinct clinical entity caused by HTLV-I. Jpn. J. Cancer Res. 1992, 83, 236–239. [Google Scholar] [CrossRef]
- Honarbakhsh, S.; Taylor, G.P. High prevalence of bronchiectasis is linked to HTLV-1-associated inflammatory disease. BMC Infect. Dis. 2015, 15, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, M.F.R.; Dos Santos, N.P.; Lírio, M.; Kritski, A.L.; Almeida, M.D.C.C.D.; Santana, L.P.; Lázaro, N.; Dias, J.; Netto, E.M.; Galvão-Castro, B. Tuberculosis incidence in a cohort of individuals infected with human T-lymphotropic virus type 1 (HTLV-1) in Salvador, Brazil. BMC Infect. Dis. 2016, 16, 491. [Google Scholar] [CrossRef] [Green Version]
- Nera, F.A.; Murphy, E.L.; Gam, A.; Hanchard, B.; Figueroa, J.P.; Blattner, W.A. Antibodies to Strongyloides stercoralis in healthy Jamaican carriers of HTLV-1. N. Engl. J. Med. 1989, 320, 252–253. [Google Scholar] [CrossRef]
- Brites, C.; Weyll, M.; Pedroso, C.; Badaró, R. Severe and Norwegian scabies are strongly associated with retroviral (HIV-1/HTLV-1) infection in Bahia, Brazil. AIDS 2002, 16, 1292–1293. [Google Scholar] [CrossRef] [PubMed]
- Einsiedel, L.J.; Pham, H.; Woodman, R.J.; Pepperill, C.; Taylor, K.A. The prevalence and clinical associations of HTLV-1 infection in a remote Indigenous community. Med. J. Aust. 2016, 205, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Einsiedel, L.; Pham, H.; Wilson, K.; Walley, R.; Turpin, J.; Bangham, C.; Gessain, A.; Woodman, R.J. Human T-Lymphotropic Virus type 1c subtype proviral loads, chronic lung disease and survival in a prospective cohort of Indigenous Australians. PLoS Negl. Trop. Dis. 2018, 12, e0006281. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C.; Chen, B.H. Seroindeterminate HTLV-1 prevalence and characteristics in blood donors in Taiwan. Int. J. Hematol. 2003, 77, 412–413. [Google Scholar] [CrossRef]
- Abbaszadegan, M.R.; Gholamin, M.; Tabatabaee, A.; Farid, R.; Houshmand, M.; Abbaszadegan, M. Prevalence of Human T-Lymphotropic Virus Type 1 among Blood Donors from Mashhad, Iran. J. Clin. Microbiol. 2003, 41, 2593–2595. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Ge, S.; Zhang, Y.; Lin, Y.; Ni, H.; Zhang, J.; Chen, C. The Prevalence of Human T-Lymphotropic Virus Infection among Blood Donors in Southeast China, 2004–2013. PLoS Negl. Trop. Dis. 2015, 9, e0003685. [Google Scholar] [CrossRef]
- Stienlauf, S.; Yahalom, V.; Schwartz, E.; Shinar, E.; Segal, G.; Sidi, Y. Epidemiology of Human T-cell Lymphotropic Virus Type 1 Infection in Blood Donors, Israel. Emerg Infect. Dis 2009, 15, 1116–1118. [Google Scholar] [CrossRef]
- Dumas, M.; Houinato, D.; Verdier, M.; Zohoun, T.; Josse, R.; Bonis, J.; Zohoun, I.; Massougbodji, A.; Denis, F. Seroepidemiology of human T-cell lymphotropic virus type I/II in Benin (West Africa). AIDS Res. Hum. Retrovir. 1991, 7, 447–451. [Google Scholar] [CrossRef]
- Anyanwu, N.C.J.; Ella, E.E.; Ohwofasa, A.; Aminu, M. Re-emergence of human T-lymphotropic viruses in West Africa. Braz. J. Infect. Dis. 2018, 22, 224–234. [Google Scholar] [CrossRef]
- Djuicy, D.D.; Mouinga-Ondémé, A.; Cassar, O.; Ramassamy, J.-L.; Idam Mamimandjiami, A.; Bikangui, R.; Fontanet, A.; Gessain, A. Risk factors for HTLV-1 infection in Central Africa: A rural population-based survey in Gabon. PLoS Negl. Trop. Dis. 2018, 12, e0006832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couroucé, A.M.; Pillonel, J.; Lemaire, J.M.; Saura, C. HTLV testing in blood transfusion. Vox Sang. 1998, 74 (Suppl. 2), 165–169. [Google Scholar] [CrossRef]
- Ireland, G.; Croxford, S.; Tosswill, J.; Raghu, R.; Davison, K.; Hewitt, P.; Simmons, R.; Taylor, G. Human T-lymphotropic viruses (HTLV) in England and Wales, 2004 to 2013: Testing and diagnoses. Eurosurveillance 2017, 22, 30539. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.L.; Figueroa, J.P.; Gibbs, W.N.; Holding-Cobham, M.; Cranston, B.; Malley, K.; Bodner, A.J.; Alexander, S.S.; Blattner, W.A. Human T-lymphotropic virus type I (HTLV-I) seroprevalence in Jamaica. I. Demographic determinants. Am. J. Epidemiol. 1991, 133, 1114–1124. [Google Scholar] [CrossRef]
- Vasquez, P.; Sanchez, G.; Volante, C.; Vera, L.; Ramirez, E.; Soto, G.; Lee, H. Human T-lymphotropic virus type I: New risk for Chilean population. Blood 1991, 78, 850–851. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.L. Infection with human T-lymphotropic virus types-1 and -2 (HTLV-1 and -2): Implications for blood transfusion safety. Transfus. Clin. Biol. 2016, 23, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Galvão-Castro, B.; Loures, L.; Rodriques, L.G.; Sereno, A.; Ferreira Júnior, O.C.; Franco, L.G.; Muller, M.; Sampaio, D.A.; Santana, A.; Passos, L.M.; et al. Distribution of human T-lymphotropic virus type I among blood donors: A nationwide Brazilian study. Transfusion 1997, 37, 242–243. [Google Scholar] [CrossRef]
- Einsiedel, L.; Fernandes, L.; Joseph, S.; Brown, A.; Woodman, R.J. Non-communicable diseases, infection and survival in a retrospective cohort of Indigenous and non-Indigenous adults in central Australia. BMJ Open 2013, 3, e003070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einsiedel, L.; Spelman, T.; Goeman, E.; Cassar, O.; Arundell, M.; Gessain, A. Clinical Associations of Human T-Lymphotropic Virus Type 1 Infection in an Indigenous Australian Population. PLoS Negl. Trop. Dis. 2014, 8, e2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, A.M.; Assone, T.; Haziot, M.E.J.; Smid, J.; Fonseca, L.A.M.; Luiz, O.D.C.; De Oliveira, A.C.P.; Casseb, J. Risk factors associated with HTLV-1 vertical transmission in Brazil: Longer breastfeeding, higher maternal proviral load and previous HTLV-1-infected offspring. Sci. Rep. 2018, 8, 7742. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.B.; Araújo, A.P.S.; Souza, A.P.C.; Gomes, C.M.; Silva-Oliveira, G.C.; Martins, L.C.; Fischer, B.; Machado, L.F.A.; Vallinoto, A.C.R.; Ishak, R.; et al. Human T-lymphotropic virus 1 and 2 among people who used illicit drugs in the state of Pará, northern Brazil. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Bittencourt, A.L.; Dourado, I.; Filho, P.B.; Santos, M.; Valadão, E.; Alcantara, L.C.; Galvão-Castro, B. Human T-cell lymphotropic virus type 1 infection among pregnant women in northeastern Brazil. J. Acquir. Immune Defic. Syndr. 2001, 26, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Hino, S. Establishment of the milk-borne transmission as a key factor for the peculiar endemicity of human T-lymphotropic virus type 1 (HTLV-1): The ATL Prevention Program Nagasaki. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Biggar, R.J.; Ng, J.; Kim, N.; Hisada, M.; Li, H.-C.; Cranston, B.; Hanchard, B.; Maloney, E.M. Human leukocyte antigen concordance and the transmission risk via breast-feeding of human T cell lymphotropic virus type I. J. Infect. Dis. 2006, 193, 277–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujino, T.; Nagata, Y. HTLV-I transmission from mother to child. J. Reprod. Immunol. 2000, 47, 197–206. [Google Scholar] [CrossRef]
- Sagara, Y.; Iwanaga, M.; Morita, M.; Sagara, Y.; Nakamura, H.; Hirayama, H.; Irita, K. Fine-scale geographic clustering pattern of human T-cell leukemia virus type 1 infection among blood donors in Kyushu-Okinawa, Japan. J. Med. Virol. 2018, 90, 1658–1665. [Google Scholar] [CrossRef] [Green Version]
- Nunes, D.; Boa-Sorte, N.; Grassi, M.F.R.; Taylor, G.P.; Teixeira, M.G.; Barreto, M.L.; Dourado, I.; Galvão-Castro, B. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLoS ONE 2017, 12, e0171303. [Google Scholar] [CrossRef]
- Dourado, I.; Andrade, T.; Carpenter, C.L.; Galvão-Castro, B. Risk Factors for Human T Cell Lymphotropic Virus Type I among Injecting Drug Users in Northeast Brazil: Possibly Greater Efficiency of Male to Female Transmission. Memórias do Instituto Oswaldo Cruz 1999, 94. [Google Scholar] [CrossRef] [Green Version]
- Osame, M.; Janssen, R.; Kubota, H.; Nishitani, H.; Igata, A.; Nagataki, S.; Mori, M.; Goto, I.; Shimabukuro, H.; Khabbaz, R. Nationwide survey of HTLV-I-associated myelopathy in Japan: Association with blood transfusion. Ann. Neurol. 1990, 28, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Jeang, K.-T. Human T cell leukemia virus type 1 (HTLV-1) and leukemic transformation: Viral infectivity, Tax, HBZ, and therapy. Oncogene 2011, 30, 1379–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, C.; Thoma-Kress, A.K. Molecular Mechanisms of HTLV-1 Cell-to-Cell Transmission. Viruses 2016, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Maali, Y.; Journo, C.; Mahieux, R.; Dutartre, H. Microbial Biofilms: Human T-cell Leukemia Virus Type 1 First in Line for Viral Biofilm but Far Behind Bacterial Biofilms. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Omsland, M.; Pise-Masison, C.; Fujikawa, D.; Galli, V.; Fenizia, C.; Parks, R.W.; Gjertsen, B.T.; Franchini, G.; Andresen, V. Inhibition of Tunneling Nanotube (TNT) Formation and Human T-cell Leukemia Virus Type 1 (HTLV-1) Transmission by Cytarabine. Sci. Rep. 2018, 8, 11118. [Google Scholar] [CrossRef]
- Pinto, D.O.; DeMarino, C.; Pleet, M.L.; Cowen, M.; Branscome, H.; Al Sharif, S.; Jones, J.; Dutartre, H.; Lepene, B.; Liotta, L.A.; et al. HTLV-1 Extracellular Vesicles Promote Cell-to-Cell Contact. Front. Microbiol 2019, 10. [Google Scholar] [CrossRef]
- Pinto, D.O.; AlSharif, S.; Mensah, G.; Cowen, M.; Khatka, P.; Erickson, J.; Branscome, H.; Latanze, T.; DeMarino, C.; Magni, R.; et al. Extracellular Vesicles From HTLV-1 Infected Cells Modulate Target Cells and Viral Spread. Retrovirology (under review) 2020. [Google Scholar]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17. [Google Scholar] [CrossRef]
- Tkach, M.; Kowal, J.; Théry, C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [Green Version]
- Fleming, A.; Sampey, G.; Chung, M.-C.; Bailey, C.; van Hoek, M.L.; Kashanchi, F.; Hakami, R.M. The carrying pigeons of the cell: Exosomes and their role in infectious diseases caused by human pathogens. Pathog. Dis. 2014, 71, 109–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastelowitz, N.; Yin, H. Exosomes and Microvesicles: Identification and Targeting By Particle Size and Lipid Chemical Probes. ChemBioChem 2014, 15, 923–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, S.; Sanderson, M.P.; Stoeck, A.; Altevogt, P. Exosomes: From biogenesis and secretion to biological function. Immunol. Lett. 2006, 107, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Sampey, G.C.; Meyering, S.S.; Zadeh, M.A.; Saifuddin, M.; Hakami, R.M.; Kashanchi, F. Exosomes and their role in CNS viral infections. J. Neurovirol. 2014, 20, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Vader, P.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Emerging targets for cancer therapy. Trends Mol. Med. 2014, 20, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Van Dongen, H.M.; Masoumi, N.; Witwer, K.W.; Pegtel, D.M. Extracellular Vesicles Exploit Viral Entry Routes for Cargo Delivery. Microbiol. Mol. Biol. Rev. 2016, 80, 369–386. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; Andaloussi, S.E.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juan, T.; Fürthauer, M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin. Cell Dev. Biol. 2018, 74, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Ridinger, J.; Rupp, A.-K.; Janssen, J.W.G.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poliakov, A.; Spilman, M.; Dokland, T.; Amling, C.L.; Mobley, J.A. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate 2009, 69, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.L.; Kaddour, H.; Winchester, L.; Fletcher, C.V.; Stapleton, J.T.; Okeoma, C.M. Semen Extracellular Vesicles From HIV-1-Infected Individuals Inhibit HIV-1 Replication In Vitro, and Extracellular Vesicles Carry Antiretroviral Drugs In Vivo. J. Acquir. Immune Defic. Syndr. 2020, 83, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Shen, R.-F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [Green Version]
- Lässer, C.; O’Neil, S.E.; Ekerljung, L.; Ekström, K.; Sjöstrand, M.; Lötvall, J. RNA-containing exosomes in human nasal secretions. Am. J. Rhinol. Allergy 2011, 25, 89–93. [Google Scholar] [CrossRef]
- Caby, M.-P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Zonneveld, M.I.; Brisson, A.R.; van Herwijnen, M.J.C.; Tan, S.; van de Lest, C.H.A.; Redegeld, F.A.; Garssen, J.; Wauben, M.H.M.; Nolte-’t Hoen, E.N.M. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J. Extracell Vesicles 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.R.; Pleet, M.L.; Enose-Akahata, Y.; Erickson, J.; Monaco, M.C.; Akpamagbo, Y.; Velluci, A.; Tanaka, Y.; Azodi, S.; Lepene, B.; et al. Viral antigens detectable in CSF exosomes from patients with retrovirus associated neurologic disease: Functional role of exosomes. Clin. Transl. Med. 2018, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Ebert, B.; Rai, A.J. Isolation and Characterization of Amniotic Fluid-Derived Extracellular Vesicles for Biomarker Discovery. Methods Mol. Biol. 2019, 1885, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Pleet, M.L.; Erickson, J.; DeMarino, C.; Barclay, R.A.; Cowen, M.; Lepene, B.; Liang, J.; Kuhn, J.H.; Prugar, L.; Stonier, S.W.; et al. Ebola Virus VP40 Modulates Cell Cycle and Biogenesis of Extracellular Vesicles. J. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pleet, M.L.; Mathiesen, A.; DeMarino, C.; Akpamagbo, Y.A.; Barclay, R.A.; Schwab, A.; Iordanskiy, S.; Sampey, G.C.; Lepene, B.; Nekhai, S.; et al. Ebola VP40 in Exosomes Can Cause Immune Cell Dysfunction. Front. Microbiol. 2016, 7, 1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleet, M.L.; DeMarino, C.; Lepene, B.; Aman, M.J.; Kashanchi, F. The Role of Exosomal VP40 in Ebola Virus Disease. DNA Cell Biol. 2017, 36, 243–248. [Google Scholar] [CrossRef] [Green Version]
- DeMarino, C.; Pleet, M.L.; Cowen, M.; Barclay, R.A.; Akpamagbo, Y.; Erickson, J.; Ndembi, N.; Charurat, M.; Jumare, J.; Bwala, S.; et al. Antiretroviral Drugs Alter the Content of Extracellular Vesicles from HIV-1-Infected Cells. Sci. Rep. 2018, 8, 7653. [Google Scholar] [CrossRef]
- Jaworski, E.; Narayanan, A.; Van Duyne, R.; Shabbeer-Meyering, S.; Iordanskiy, S.; Saifuddin, M.; Das, R.; Afonso, P.V.; Sampey, G.C.; Chung, M.; et al. Human T-lymphotropic Virus Type 1-infected Cells Secrete Exosomes That Contain Tax Protein. J. Biol. Chem. 2014, 289, 22284–22305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.B.; Bell, C.R.; Bibb, K.E.; Gu, L.; Coats, M.T.; Matthews, Q.L. Pathogens and Their Effect on Exosome Biogenesis and Composition. Biomedicines 2018, 6, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, D.; Zhao, W.-L.; Ye, Y.-Y.; Bai, X.-C.; Liu, R.-Q.; Chang, L.-F.; Zhou, Q.; Sui, S.-F. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010, 11, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhu, Y.-L.; Zhou, Y.-Y.; Liang, G.-F.; Wang, Y.-Y.; Hu, F.-H.; Xiao, Z.-D. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 2014, 289, 22258–22267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Messina, L.; Gutiérrez-Vázquez, C.; Rivas-García, E.; Sánchez-Madrid, F.; de la Fuente, H. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol. Cell 2015, 107, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Luo, F.; Liu, X.; Lu, L.; Xu, H.; Yang, Q.; Xue, J.; Shi, L.; Li, J.; Zhang, A.; et al. NF-kB-regulated exosomal miR-155 promotes the inflammation associated with arsenite carcinogenesis. Cancer Lett. 2017, 388, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, X.; Bao, J.; Wang, Y.; Liu, H.; Tang, L. Exosomes in Pathogen Infections: A Bridge to Deliver Molecules and Link Functions. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Chettimada, S.; Lorenz, D.R.; Misra, V.; Dillon, S.T.; Reeves, R.K.; Manickam, C.; Morgello, S.; Kirk, G.D.; Mehta, S.H.; Gabuzda, D. Exosome markers associated with immune activation and oxidative stress in HIV patients on antiretroviral therapy. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Li, M.; Ramratnam, B. Proteomic Characterization of Exosomes from HIV-1-Infected Cells. Methods Mol. Biol. 2016, 1354, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Barclay, R.A.; Schwab, A.; DeMarino, C.; Akpamagbo, Y.; Lepene, B.; Kassaye, S.; Iordanskiy, S.; Kashanchi, F. Exosomes from uninfected cells activate transcription of latent HIV-1. J. Biol. Chem. 2017, 292, 11683–11701. [Google Scholar] [CrossRef] [Green Version]
- Barclay, R.A.; Mensah, G.A.; Cowen, M.; DeMarino, C.; Kim, Y.; Pinto, D.O.; Erickson, J.; Kashanchi, F. Extracellular Vesicle Activation of Latent HIV-1 Is Driven by EV-Associated c-Src and Cellular SRC-1 via the PI3K/AKT/mTOR Pathway. Viruses 2020, 12, 665. [Google Scholar] [CrossRef]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Gu, L.; Matthews, Q.L. Role of TIM-4 in exosome-dependent entry of HIV-1 into human immune cells. Int. J. Nanomed. 2017, 12, 4823. [Google Scholar] [CrossRef] [Green Version]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Matthews, Q.L. Tetraspanin blockage reduces exosome-mediated HIV-1 entry. Arch. Virol. 2018, 163, 1683–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Rojas, P.P.; Quiroz-García, E.; Monroy-Martínez, V.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Ruiz-Ordaz, B.H. Participation of Extracellular Vesicles from Zika-Virus-Infected Mosquito Cells in the Modification of Naïve Cells’ Behavior by Mediating Cell-to-Cell Transmission of Viral Elements. Cells 2020, 9, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Yuan, M.; Zhang, T.; Zheng, N.; Wu, Z. EVs Containing Host Restriction Factor IFITM3 Inhibited ZIKV Infection of Fetuses in Pregnant Mice through Trans-placenta Delivery. Mol. Ther. 2020, S1525001620304834. [Google Scholar] [CrossRef]
- Zhao, T.; Matsuoka, M. HBZ and its roles in HTLV-1 oncogenesis. Front. Microbiol. 2012, 3, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherian, M.A.; Baydoun, H.H.; Al-Saleem, J.; Shkriabai, N.; Kvaratskhelia, M.; Green, P.; Ratner, L. Akt Pathway Activation by Human T-cell Leukemia Virus Type 1 Tax Oncoprotein. J. Biol. Chem. 2015, 290, 26270–26281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi Ghezeldasht, S.; Shirdel, A.; Assarehzadegan, M.A.; Hassannia, T.; Rahimi, H.; Miri, R.; Rezaee, S.A.R. Human T Lymphotropic Virus Type I (HTLV-I) Oncogenesis: Molecular Aspects of Virus and Host Interactions in Pathogenesis of Adult T cell Leukemia/Lymphoma (ATL). Iran. J. Basic Med. Sci. 2013, 16, 179–195. [Google Scholar]
- El-Saghir, J.; Nassar, F.; Tawil, N.; El-Sabban, M. ATL-derived exosomes modulate mesenchymal stem cells: Potential role in leukemia progression. Retrovirology 2016, 13, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otaguiri, K.K.; Dos Santos, D.F.; Slavov, S.N.; Depieri, L.V.; Palma, P.V.B.; Meirelles, F.V.; Covas, D.T.; da Silveira, J.C.; Kashima, S. TAX-mRNA-Carrying Exosomes from Human T Cell Lymphotropic Virus Type 1-Infected Cells Can Induce Interferon-Gamma Production In Vitro. AIDS Res. Hum. Retrovir. 2018, 34, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Goon, P.K.C.; Hanon, E.; Igakura, T.; Tanaka, Y.; Weber, J.N.; Taylor, G.P.; Bangham, C.R.M. High frequencies of Th1-type CD4+ T cells specific to HTLV-1 Env and Tax proteins in patients with HTLV-1–associated myelopathy/tropical spastic paraparesis. Blood 2002, 99, 3335–3341. [Google Scholar] [CrossRef] [PubMed]
- Jeannin, P.; Chaze, T.; Giai Gianetto, Q.; Matondo, M.; Gout, O.; Gessain, A.; Afonso, P.V. Proteomic analysis of plasma extracellular vesicles reveals mitochondrial stress upon HTLV-1 infection. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, A.R.; Wang, S.E. Cancer-derived extracellular vesicles: The ‘soil conditioner’ in breast cancer metastasis? Cancer Metastasis Rev. 2016, 35, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; De Milito, A.; Lugini, L.; Borghi, M.; Calabrò, L.; Spada, M.; Perdicchio, M.; Marino, M.L.; Federici, C.; Iessi, E.; et al. High Levels of Exosomes Expressing CD63 and Caveolin-1 in Plasma of Melanoma Patients. PLoS ONE 2009, 4, e5219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimondo, S.; Saieva, L.; Corrado, C.; Fontana, S.; Flugy, A.; Rizzo, A.; De Leo, G.; Alessandro, R. Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Commun. Signal. 2015, 13, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Zhang, G.; Kong, L.; Xu, S.; Wang, Y.; Dong, M. Leukemia-derived exosomes induced IL-8 production in bone marrow stromal cells to protect the leukemia cells against chemotherapy. Life Sci. 2019, 221, 187–195. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Kalluri, R. Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer 2019, 18, 52. [Google Scholar] [CrossRef]
- Valenti, R.; Huber, V.; Filipazzi, P.; Pilla, L.; Sovena, G.; Villa, A.; Corbelli, A.; Fais, S.; Parmiani, G.; Rivoltini, L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006, 66, 9290–9298. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Clayton, A.; Mitchell, J.P.; Court, J.; Mason, M.D.; Tabi, Z. Human Tumor-Derived Exosomes Selectively Impair Lymphocyte Responses to Interleukin-2. Cancer Res. 2007, 67, 7458–7466. [Google Scholar] [CrossRef] [Green Version]
- Stefanius, K.; Servage, K.; de Souza Santos, M.; Gray, H.F.; Toombs, J.E.; Chimalapati, S.; Kim, M.S.; Malladi, V.S.; Brekken, R.; Orth, K. Human pancreatic cancer cell exosomes, but not human normal cell exosomes, act as an initiator in cell transformation. eLife 2019, 8, e40226. [Google Scholar] [CrossRef]
- Abd Elmageed, Z.Y.; Yang, Y.; Thomas, R.; Ranjan, M.; Mondal, D.; Moroz, K.; Fang, Z.; Rezk, B.M.; Moparty, K.; Sikka, S.C.; et al. Neoplastic Reprogramming of Patient-Derived Adipose Stem Cells by Prostate Cancer Cell-Associated Exosomes. Stem Cells 2014, 32, 983–997. [Google Scholar] [CrossRef] [Green Version]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016. [Google Scholar] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Ren, R.; Sun, H.; Ma, C.; Liu, J.; Wang, H. Colon cancer cells secrete exosomes to promote self-proliferation by shortening mitosis duration and activation of STAT3 in a hypoxic environment. Cell Biosci. 2019, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.-L.; Qu, X.-J.; Zhao, M.-F.; Teng, Y.-E.; Zhang, Y.; Hou, K.-Z.; Jiang, Y.-H.; Yang, X.-H.; Liu, Y.-P. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig. Liver Dis. 2009, 41, 875–880. [Google Scholar] [CrossRef]
- Hanchard, B.; LaGrenade, L.; Carberry, C.; Fletcher, V.; Williams, E.; Cranston, B.; Blattner, W.A.; Manns, A. Childhood infective dermatitis evolving into adult T-cell leukaemia after 17 years. Lancet 1991, 338, 1593–1594. [Google Scholar] [CrossRef]
- Yamazato, Y.; Miyazato, A.; Kawakami, K.; Yara, S.; Kaneshima, H.; Saito, A. High Expression of p40tax and Pro-inflammatory Cytokines and Chemokines in the Lungs of Human T-Lymphotropic Virus Type 1-Related Bronchopulmonary Disorders. Chest 2003, 124, 2283–2292. [Google Scholar] [CrossRef]
- Matsuyama, W.; Kawabata, M.; Mizoguchi, A.; Iwami, F.; Wakimoto, J.; Osame, M. Influence of human T lymphotrophic virus type I on cryptogenic fibrosing alveolitis—HTLV-I associated fibrosing alveolitis: Proposal of a new clinical entity. Clin. Exp. Immunol. 2003, 133, 397–403. [Google Scholar] [CrossRef]
- Sagawa, K.; Mochizuki, M.; Masuoka, K.; Katagiri, K.; Katayama, T.; Maeda, T.; Tanimoto, A.; Sugita, S.; Watanabe, T.; Itoh, K. Immunopathological mechanisms of human T cell lymphotropic virus type 1 (HTLV-I) uveitis. Detection of HTLV-I-infected T cells in the eye and their constitutive cytokine production. J. Clin. Investig. 1995, 95, 852–858. [Google Scholar] [CrossRef]
- Takatsuki, K.; Uchiyama, T.; Sagawa, K.; Yodoi, J. Adult T cell leukemia in Japan. Topic in Hematology. In The 16th International Congress of Hematology; Seno, S., Takaku, F., Irino, S., Eds.; Excerpta Medica: Amsterdam, The Netherlands, 1977; pp. 73–77. [Google Scholar]
- Yoshida, M.; Miyoshi, I.; Hinuma, Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 1982, 79, 2031–2035. [Google Scholar] [CrossRef] [Green Version]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef]
- Kannagi, M.; Hasegawa, A.; Nagano, Y.; Kimpara, S.; Suehiro, Y. Impact of host immunity on HTLV-1 pathogenesis: Potential of Tax-targeted immunotherapy against ATL. Retrovirology 2019, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Yasunaga, J. Human T-cell leukemia virus type 1: Replication, proliferation and propagation by Tax and HTLV-1 bZIP factor. Curr. Opin. Virol. 2013, 3, 684–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Kehrl, J.H.; Burton, J.; Tendler, C.L.; Jeang, K.T.; Danielpour, D.; Thevenin, C.; Kim, K.Y.; Sporn, M.B.; Roberts, A.B. Transactivation of the transforming growth factor beta 1 (TGF-beta 1) gene by human T lymphotropic virus type 1 tax: A potential mechanism for the increased production of TGF-beta 1 in adult T cell leukemia. J. Exp. Med. 1990, 172, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Satou, Y.; Sugata, K.; Miyazato, P.; Green, P.L.; Imamura, T.; Matsuoka, M. HTLV-1 bZIP factor enhances TGF-β signaling through p300 coactivator. Blood 2011, 118, 1865–1876. [Google Scholar] [CrossRef]
- Sawada, L.; Nagano, Y.; Hasegawa, A.; Kanai, H.; Nogami, K.; Ito, S.; Sato, T.; Yamano, Y.; Tanaka, Y.; Masuda, T.; et al. IL-10-mediated signals act as a switch for lymphoproliferation in Human T-cell leukemia virus type-1 infection by activating the STAT3 and IRF4 pathways. PLoS Pathog. 2017, 13, e1006597. [Google Scholar] [CrossRef] [Green Version]
- Kannagi, M.; Sugamura, K.; Kinoshita, K.; Uchino, H.; Hinuma, Y. Specific cytolysis of fresh tumor cells by an autologous killer T cell line derived from an adult T cell leukemia/lymphoma patient. J. Immunol. 1984, 133, 1037–1041. [Google Scholar]
- Azran, I.; Schavinsky-Khrapunsky, Y.; Aboud, M. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology 2004, 1, 20. [Google Scholar] [CrossRef] [Green Version]
- Currer, R.; Van Duyne, R.; Jaworski, E.; Guendel, I.; Sampey, G.; Das, R.; Narayanan, A.; Kashanchi, F. HTLV Tax: A Fascinating Multifunctional Co-Regulator of Viral and Cellular Pathways. Front. Microbiol. 2012, 3, 406. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.R.; Kashanchi, F.; Jacobson, S. Exosomes in Viral Disease. Neurotherapeutics 2016, 13, 535–546. [Google Scholar] [CrossRef]
- Welle, S. Endocrine, Paracrine, and Autocrine Regulation. In Human Protein Metabolism; Welle, S., Ed.; Springer: New York, NY, USA, 1999; pp. 124–160. ISBN 978-1-4612-1458-8. [Google Scholar]
- Hood, J.L.; San, R.S.; Wickline, S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef] [Green Version]
- Lavorgna, A.; Matsuoka, M.; Harhaj, E.W. A critical role for IL-17RB signaling in HTLV-1 tax-induced NF-κB activation and T-cell transformation. Retrovirology 2015, 12, P52. [Google Scholar] [CrossRef]
- Matsuura, E.; Nozuma, S.; Tashiro, Y.; Kubota, R.; Izumo, S.; Takashima, H. HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP): A comparative study to identify factors that influence disease progression. J. Neurol. Sci. 2016, 371, 112–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozhgani, S.-H.; Piran, M.; Zarei-Ghobadi, M.; Jafari, M.; Jazayeri, S.-M.; Mokhtari-Azad, T.; Teymoori-Rad, M.; Valizadeh, N.; Farajifard, H.; Mirzaie, M.; et al. An insight to HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) pathogenesis; evidence from high-throughput data integration and meta-analysis. Retrovirology 2019, 16, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, M.; Usuku, K.; Matsumoto, W.; Kodama, D.; Takenouchi, N.; Moritoyo, T.; Hashiguchi, S.; Ichinose, M.; Bangham, C.R.; Izumo, S.; et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: High proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 1998, 4, 586–593. [Google Scholar] [CrossRef]
- Furukawa, Y.; Fujisawa, J.; Osame, M.; Toita, M.; Sonoda, S.; Kubota, R.; Ijichi, S.; Yoshida, M. Frequent clonal proliferation of human T-cell leukemia virus type 1 (HTLV-1)-infected T cells in HTLV-1-associated myelopathy (HAM-TSP). Blood 1992, 80, 1012–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, J.A.; Nagai, M.; Brennan, M.B.; Mora, C.A.; Jacobson, S. In vitro spontaneous lymphoproliferation in patients with human T-cell lymphotropic virus type I-associated neurologic disease: Predominant expansion of CD8+ T cells. Blood 2001, 98, 1506–1511. [Google Scholar] [CrossRef] [Green Version]
- Itoyama, Y.; Minato, S.; Kira, J.; Goto, I.; Sato, H.; Okochi, K.; Yamamoto, N. Spontaneous proliferation of peripheral blood lymphocytes increased in patients with HTLV-I-associated myelopathy. Neurology 1988, 38, 1302–1307. [Google Scholar] [CrossRef]
- Tendler, C.L.; Greenberg, S.J.; Blattner, W.A.; Manns, A.; Murphy, E.; Fleisher, T.; Hanchard, B.; Morgan, O.; Burton, J.D.; Nelson, D.L. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: Pathogenic implications and a rationale for immunotherapy. Proc. Natl. Acad. Sci. USA 1990, 87, 5218–5222. [Google Scholar] [CrossRef] [Green Version]
- Azimi, N.; Nagai, M.; Jacobson, S.; Waldmann, T.A. IL-15 plays a major role in the persistence of Tax-specific CD8 cells in HAM/TSP patients. Proc. Natl. Acad. Sci. USA 2001, 98, 14559–14564. [Google Scholar] [CrossRef] [Green Version]
- Ando, H.; Sato, T.; Tomaru, U.; Yoshida, M.; Utsunomiya, A.; Yamauchi, J.; Araya, N.; Yagishita, N.; Coler-Reilly, A.; Shimizu, Y.; et al. Positive feedback loop via astrocytes causes chronic inflammation in virus-associated myelopathy. Brain 2013, 136, 2876–2887. [Google Scholar] [CrossRef] [Green Version]
- Grant, C.; Barmak, K.; Alefantis, T.; Yao, J.; Jacobson, S.; Wigdahl, B. Human T cell leukemia virus type I and neurologic disease: Events in bone marrow, peripheral blood, and central nervous system during normal immune surveillance and neuroinflammation. J. Cell. Physiol. 2002, 190, 133–159. [Google Scholar] [CrossRef] [PubMed]
- Nozuma, S.; Jacobson, S. Neuroimmunology of Human T-Lymphotropic Virus Type 1-Associated Myelopathy/Tropical Spastic Paraparesis. Front. Microbiol. 2019, 10, 885. [Google Scholar] [CrossRef]
- Yamano, Y.; Takenouchi, N.; Li, H.-C.; Tomaru, U.; Yao, K.; Grant, C.W.; Maric, D.A.; Jacobson, S. Virus-induced dysfunction of CD4+CD25+ T cells in patients with HTLV-I–associated neuroimmunological disease. J. Clin. Investig. 2005, 115, 1361–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamano, Y.; Cohen, C.J.; Takenouchi, N.; Yao, K.; Tomaru, U.; Li, H.-C.; Reiter, Y.; Jacobson, S. Increased Expression of Human T Lymphocyte Virus Type I (HTLV-I) Tax11-19 Peptide–Human Histocompatibility Leukocyte Antigen A*201 Complexes on CD4+ CD25+T Cells Detected by Peptide-specific, Major Histocompatibility Complex–restricted Antibodies in Patients with HTLV-I–associated Neurologic Disease. J. Exp. Med. 2004, 199, 1367–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.; Schwartz, R.A. Human T-lymphotrophic virus type 1–associated infective dermatitis: A comprehensive review. J. Am. Acad. Dermatol. 2011, 64, 152–160. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.D.F.S.P.; Fatal, P.L.; Primo, J.R.L.; da Silva, J.L.S.; Batista, E.D.S.; Farré, L.; Bittencourt, A.L. Infective dermatitis associated with human T-cell lymphotropic virus type 1: Evaluation of 42 cases observed in Bahia, Brazil. Clin. Infect. Dis. 2012, 54, 1714–1719. [Google Scholar] [CrossRef] [Green Version]
- Di Martino Ortiz, B.; Riveros, R.; Medina, R.; Morel, M. Infective Dermatitis in an Adult Patient with HTLV-1. Am. J. Derm. 2015, 37, 944–948. [Google Scholar] [CrossRef] [Green Version]
- Grenade, L.L.; Manns, A.; Fletcher, V.; Carberry, C.; Hanchard, B.; Maloney, E.M.; Cranston, B.; Williams, N.P.; Wilks, R.; Kang, E.C.; et al. Clinical, Pathologic, and Immunologic Features of Human T-Lymphotrophic Virus Type I–Associated Infective Dermatitis in Children. Arch. Dermatol. 1998, 134, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Sweet, R.D. A pattern of eczema in Jamaica. Br. J. Dermatol. 1966, 78, 93–100. [Google Scholar] [CrossRef]
- La Grenade, L.; Fletcher, V.; Carberry, C.; Hanchard, B.; Cranston, B.; Williams, N.P.; Chow, M.; Blattner, W.A. Infective dermatitis of Jamaican children 1966–1991—abstract. West. Indian Med. J. 1992, 41, 33. [Google Scholar]
- Tsukasaki, K.; Yamada, Y.; Ikeda, S.; Tomonaga, M. Infective dermatitis among patients with ATL in Japan. Int. J. Cancer 1994, 57, 293. [Google Scholar] [CrossRef]
- LaGrenade, L.; Morgan, C.; Carberry, C.; Hanchard, B.; Fletcher, V.; Gray, R.; Cranston, B.; Rodgers-Johnson, P.; Manns, A. Tropical spastic paraparesis occurring in HTLV-1 associated infective dermatitis. Report of two cases. West. Indian Med. J. 1995, 44, 34–35. [Google Scholar]
- Varandas, C.M.N.; da Silva, J.L.S.; Primo, J.R.L.; de Oliveira, M. de F.S.P.; Moreno-Carvalho, O.; Farre, L.; Bittencourt, A.L. Early Juvenile Human T-cell Lymphotropic Virus Type-1-Associated Myelopathy/Tropical Spastic Paraparesis: Study of 25 Patients. Clin. Infect. Dis. 2018, 67, 1427–1433. [Google Scholar] [CrossRef]
- Maria de Fátima, S.P.; Bittencourt, A.L.; Brites, C.; Soares, G.; Hermes, C.; Almeida, F.O. HTLV-I associated myelopathy/tropical spastic paraparesis in a 7-year-old boy associated with infective dermatitis. J. Neurol. Sci. 2004, 222, 35–38. [Google Scholar] [CrossRef]
- Primo, J.; Siqueira, I.; Nascimento, M.C.F.; Oliveira, M.F.; Farre, L.; Carvalho, E.M.; Bittencourt, A.L. High HTLV-1 proviral load, a marker for HTLV-1 associated myelopathy/tropical spastic paraparesis, is also detected in patients with infective dermatitis associated with HTLV-1. Braz. J. Med. Biol. Res. 2009, 42, 761–764. [Google Scholar] [CrossRef] [Green Version]
- McGill, N.-K.; Vyas, J.; Shimauchi, T.; Tokura, Y.; Piguet, V. HTLV-1-associated infective dermatitis: Updates on the pathogenesis. Exp. Dermatol. 2012, 21, 815–821. [Google Scholar] [CrossRef]
- Ahuja, J.; Lepoutre, V.; Wigdahl, B.; Khan, Z.K.; Jain, P. Induction of proinflammatory cytokines by human T cell leukemia virus type 1 Tax protein as determined by multiplexed cytokine protein array analyses of human dendritic cells. Biomed. Pharm. 2007, 61, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Hlela, C.; Bittencourt, A. Infective Dermatitis Associated with HTLV-1 Mimics Common Eczemas in Children and May Be a Prelude to Severe Systemic Diseases. Dermatol. Clin. 2014, 32, 237–248. [Google Scholar] [CrossRef]
- Hong, S.-W.; Choi, E.-B.; Min, T.-K.; Kim, J.-H.; Kim, M.-H.; Jeon, S.G.; Lee, B.-J.; Gho, Y.S.; Jee, Y.-K.; Pyun, B.-Y.; et al. An Important Role of α-Hemolysin in Extracellular Vesicles on the Development of Atopic Dermatitis Induced by Staphylococcus aureus. PLoS ONE 2014, 9, e100499. [Google Scholar] [CrossRef]
- Jun, S.H.; Lee, J.H.; Kim, S.I.; Choi, C.W.; Park, T.I.; Jung, H.R.; Cho, J.W.; Kim, S.H.; Lee, J.C. Staphylococcus aureus-derived membrane vesicles exacerbate skin inflammation in atopic dermatitis. Clin. Exp. Allergy 2017, 47, 85–96. [Google Scholar] [CrossRef]
- Shao, S.; Fang, H.; Li, Q.; Wang, G. Extracellular vesicles in Inflammatory Skin Disorders: From Pathophysiology to Treatment. Theranostics 2020, 10, 9937–9955. [Google Scholar] [CrossRef]
- Hong, S.-W.; Kim, M.-R.; Lee, E.-Y.; Kim, J.H.; Kim, Y.-S.; Jeon, S.G.; Yang, J.-M.; Lee, B.-J.; Pyun, B.-Y.; Gho, Y.S.; et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy 2011, 66, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Oizumi, A.; Nakayama, H.; Okino, N.; Iwahara, C.; Kina, K.; Matsumoto, R.; Ogawa, H.; Takamori, K.; Ito, M.; Suga, Y.; et al. Pseudomonas-Derived Ceramidase Induces Production of Inflammatory Mediators from Human Keratinocytes via Sphingosine-1-Phosphate. PLoS ONE 2014, 9, e89402. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.-O.; Ha, D.H.; Kim, J.O.; Crumrine, D.A.; Meyer, J.M.; Wakefield, J.S.; Lee, Y.; Kim, B.; Kim, S.; Kim, H.; et al. Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis. Cells 2020, 9, 680. [Google Scholar] [CrossRef] [Green Version]
- Okada, F.; Ando, Y.; Yoshitake, S.; Yotsumoto, S.; Matsumoto, S.; Wakisaka, M.; Maeda, T.; Mori, H. Pulmonary CT Findings in 320 Carriers of Human T-Lymphotropic Virus Type 1. Radiology 2006, 240, 559–564. [Google Scholar] [CrossRef]
- Sugimoto, M.; Nakashima, H.; Watanabe, S.; Uyama, E.; Tanaka, F.; Ando, M.; Araki, S.; Kawasaki, S. T-lymphocyte alveolitis in HTLV-I-associated myelopathy. Lancet 1987, 2, 1220. [Google Scholar] [CrossRef]
- Teruya, H.; Tomita, M.; Senba, M.; Ishikawa, C.; Tamayose, M.; Miyazato, A.; Yara, S.; Tanaka, Y.; Iwakura, Y.; Fujita, J.; et al. Human T-cell leukemia virus type I infects human lung epithelial cells and induces gene expression of cytokines, chemokines and cell adhesion molecules. Retrovirology 2008, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, T.; Higashimoto, I.; Takashima, H.; Izumo, S.; Kubota, R. Human T-lymphotropic virus type I (HTLV-I)-specific CD8+ cells accumulate in the lungs of patients infected with HTLV-I with pulmonary involvement. J. Med. Virol. 2012, 84, 1120–1127. [Google Scholar] [CrossRef]
- Yamakawa, H.; Yoshida, M.; Yabe, M.; Ishikawa, T.; Takagi, M.; Tanoue, S.; Sano, K.; Nishiwaki, K.; Sato, S.; Shimizu, Y.; et al. Human T-cell Lymphotropic Virus Type-1 (HTLV-1)-associated Bronchioloalveolar Disorder Presenting with Mosaic Perfusion. Intern. Med. 2015, 54, 3039–3043. [Google Scholar] [CrossRef] [Green Version]
- Magno Falcão, L.F.; Falcão, A.S.C.; Medeiros Sousa, R.C.; Vieira, W.D.B.; de Oliveira, R.T.M.; Normando, V.M.F.; Dias, G.A.D.S.; Santos, M.C.D.S.; Rocha, R.S.B.; Yoshikawa, G.T.; et al. CT Chest and pulmonary functional changes in patients with HTLV-associated myelopathy in the Eastern Brazilian Amazon. PLoS ONE 2017, 12, e0186055. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.R.N.; Falcão, L.F.M.; Falcão, A.S.C.; Normando, V.M.F.; Quaresma, J.A.S. Human T Lymphotropic Virus and Pulmonary Diseases. Front. Microbiol. 2018, 9, 1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, Y.; Ishikawa, C.; Tamaki, K.; Senba, M.; Fujita, J.; Mori, N. Interleukin-1 alpha produced by human T-cell leukaemia virus type I-infected T cells induces intercellular adhesion molecule-1 expression on lung epithelial cells. J. Med. Microbiol. 2011, 60, 1750–1761. [Google Scholar] [CrossRef]
- Nakayama, Y.; Yamazato, Y.; Tamayose, M.; Atsumi, E.; Yara, S.; Higa, F.; Tateyama, M.; Fujita, J. Increased expression of HBZ and Foxp3 mRNA in bronchoalveolar lavage cells taken from human T-lymphotropic virus type 1-associated lung disorder patients. Intern. Med. 2013, 52, 2599–2609. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y.; Araya, J.; Ito, S.; Kobayashi, K.; Kosaka, N.; Yoshioka, Y.; Kadota, T.; Hara, H.; Kuwano, K.; Ochiya, T. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J. Extracell. Vesicles 2015, 4, 28388. [Google Scholar] [CrossRef]
- Xu, H.; Ling, M.; Xue, J.; Dai, X.; Sun, Q.; Chen, C.; Liu, Y.; Zhou, L.; Liu, J.; Luo, F.; et al. Exosomal microRNA-21 derived from bronchial epithelial cells is involved in aberrant epithelium-fibroblast cross-talk in COPD induced by cigarette smoking. Theranostics 2018, 8, 5419–5433. [Google Scholar] [CrossRef]
- Deng, L.; Blanco, F.J.; Stevens, H.; Lu, R.; Caudrillier, A.; McBride, M.; McClure, J.D.; Grant, J.; Thomas, M.; Frid, M.; et al. miR-143 Activation Regulates Smooth Muscle and Endothelial Cell Crosstalk in Pulmonary Arterial Hypertension. Circ. Res. 2015, 117, 870–883. [Google Scholar] [CrossRef]
- Mattes, J.; Collison, A.; Plank, M.; Phipps, S.; Foster, P.S. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc. Natl. Acad. Sci. USA 2009, 106, 18704–18709. [Google Scholar] [CrossRef] [Green Version]
- Collison, A.; Herbert, C.; Siegle, J.S.; Mattes, J.; Foster, P.S.; Kumar, R.K. Altered expression of microRNA in the airway wall in chronic asthma: miR-126 as a potential therapeutic target. BMC Pulm. Med. 2011, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Halim, T.Y.F.; Krauss, R.H.; Sun, A.C.; Takei, F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 2012, 36, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Maes, T.; Cobos, F.A.; Schleich, F.; Sorbello, V.; Henket, M.; De Preter, K.; Bracke, K.R.; Conickx, G.; Mesnil, C.; Vandesompele, J.; et al. Asthma inflammatory phenotypes show differential microRNA expression in sputum. J. Allergy Clin. Immunol. 2016, 137, 1433–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Xin, W.; Ma, C.; Zhang, H.; Mao, M.; Liu, Y.; Zheng, X.; Zhang, L.; Yu, X.; Li, H.; et al. Exosomal 15-LO2 mediates hypoxia-induced pulmonary artery hypertension in vivo and in vitro. Cell Death Dis. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martin-Medina, A.; Lehmann, M.; Burgy, O.; Hermann, S.; Baarsma, H.A.; Wagner, D.E.; De Santis, M.M.; Ciolek, F.; Hofer, T.P.; Frankenberger, M.; et al. Increased Extracellular Vesicles Mediate WNT5A Signaling in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 198, 1527–1538. [Google Scholar] [CrossRef]
- Narayanan, A.; Jaworski, E.; Van Duyne, R.; Iordanskiy, S.; Guendel, I.; Das, R.; Currer, R.; Sampey, G.; Chung, M.; Kehn-Hall, K.; et al. Exosomes derived from HTLV-1 infected cells contain the viral protein Tax. Retrovirology 2014, 11, O46. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, M.; Watanabe, T.; Yamaguchi, K.; Yoshimura, K.; Nakashima, S.; Shirao, M.; Araki, S.; Takatsuki, K.; Mori, S.; Miyata, N. Uveitis associated with human T-cell lymphotropic virus type I. Am. J. Ophthalmol. 1992, 114, 123–129. [Google Scholar] [CrossRef]
- Kamoi, K.; Mochizuki, M. HTLV-1 uveitis. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Ono, A.; Mochizuki, M.; Yamaguchi, K.; Miyata, N.; Watanabe, T. Increased number of circulating HTLV-1 infected cells in peripheral blood mononuclear cells of HTLV-1 uveitis patients: A quantitative polymerase chain reaction study. Br. J. Ophthalmol. 1995, 79, 270–276. [Google Scholar] [CrossRef]
- Ono, A.; Mochizuki, M.; Yamaguchi, K.; Miyata, N.; Watanabe, T. Immunologic and virologic characterization of the primary infiltrating cells in the aqueous humor of human T-cell leukemia virus type-1 uveitis. Accumulation of the human T-cell leukemia virus type-1-infected cells and constitutive expression of viral and interleukin-6 messenger ribonucleic acids. Invest. Ophthalmol. Vis. Sci. 1997, 38, 676–689. [Google Scholar]
- Yoshimura, K.; Mochizuki, M.; Araki, S.; Miyata, N.; Yamaguchi, K.; Tajima, K.; Watanabe, T. Clinical and immunologic features of human T-cell lymphotropic virus type I uveitis. Am. J. Ophthalmol. 1993, 116, 156–163. [Google Scholar] [CrossRef]
- Holtkamp, G.M.; Kijlstra, A.; Peek, R.; de Vos, A.F. Retinal Pigment Epithelium-immune System Interactions: Cytokine Production and Cytokine-induced Changes. Prog. Retin. Eye Res. 2001, 20, 29–48. [Google Scholar] [CrossRef]
- Liu, B.; Li, Z.; Mahesh, S.P.; Kurup, S.K.; Giam, C.-Z.; Nussenblatt, R.B. HTLV-1 infection of human retinal pigment epithelial cells and inhibition of viral infection by an antibody to ICAM-1. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1510–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamiri, P.; Sugita, S.; Streilein, J.W. Immunosuppressive properties of the pigmented epithelial cells and the subretinal space. Chem. Immunol. Allergy 2007, 92, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Hettich, C.; Wilker, S.; Mentlein, R.; Lucius, R.; Roider, J.; Klettner, A. The retinal pigment epithelium (RPE) induces FasL and reduces iNOS and Cox2 in primary monocytes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Knickelbein, J.E.; Liu, B.; Arakelyan, A.; Zicari, S.; Hannes, S.; Chen, P.; Li, Z.; Grivel, J.-C.; Chaigne-Delalande, B.; Sen, H.N.; et al. Modulation of Immune Responses by Extracellular Vesicles From Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4101–4107. [Google Scholar] [CrossRef] [Green Version]
- Atienzar-Aroca, S.; Flores-Bellver, M.; Serrano-Heras, G.; Martinez-Gil, N.; Barcia, J.M.; Aparicio, S.; Perez-Cremades, D.; Garcia-Verdugo, J.M.; Diaz-Llopis, M.; Romero, F.J.; et al. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J. Cell Mol. Med. 2016, 20, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Fan, X.; Rui, H.; Xinjun, R.; Dejia, W.; Chuanzhen, Z.; Li, X. Exosomes derived from RPE cells under oxidative stress mediate inflammation and apoptosis of normal RPE cells through Apaf1/caspase-9 axis. J. Cell. Biochem. 2020. [Google Scholar] [CrossRef]
- Kinjo, T.; Ham-Terhune, J.; Peloponese, J.-M.; Jeang, K.-T. Induction of Reactive Oxygen Species by Human T-Cell Leukemia Virus Type 1 Tax Correlates with DNA Damage and Expression of Cellular Senescence Marker. J. Virol. 2010, 84, 5431–5437. [Google Scholar] [CrossRef] [Green Version]
- Macchi, B.; Balestrieri, E.; Ascolani, A.; Hilburn, S.; Martin, F.; Mastino, A.; Taylor, G.P. Susceptibility of Primary HTLV-1 Isolates from Patients with HTLV-1-Associated Myelopathy to Reverse Transcriptase Inhibitors. Viruses 2011, 3, 469–483. [Google Scholar] [CrossRef] [Green Version]
- Toyama, M.; Hamasaki, T.; Uto, T.; Aoyama, H.; Okamoto, M.; Hashmoto, Y.; Baba, M. Synergistic Inhibition of HTLV-1-infected Cell Proliferation by Combination of Cepharanthine and a Tetramethylnaphthalene Derivative. Anticancer Res. 2012, 32, 2639–2645. [Google Scholar] [PubMed]
- Nakamura, T.; Matsuo, T.; Fukuda, T.; Yamato, S.; Yamaguchi, K.; Kinoshita, I.; Matsuzaki, T.; Nishiura, Y.; Nagasato, K.; Narita-Masuda, T.; et al. Efficacy of prosultiamine treatment in patients with human T lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis: Results from an open-label clinical trial. BMC Med. 2013, 11, 182. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Coler-Reilly, A.L.G.; Yagishita, N.; Araya, N.; Inoue, E.; Furuta, R.; Watanabe, T.; Uchimaru, K.; Matsuoka, M.; Matsumoto, N.; et al. Mogamulizumab (Anti-CCR4) in HTLV-1–Associated Myelopathy. N. Engl. J. Med. 2018, 378, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Oh, U.; McCormick, M.J.; Datta, D.; Turner, R.V.; Bobb, K.; Monie, D.D.; Sliskovic, D.R.; Tanaka, Y.; Zhang, J.; Meshulam, J.; et al. Inhibition of immune activation by a novel nuclear factor-kappa B inhibitor in HTLV-I–associated neurologic disease. Blood 2011, 117, 3363–3369. [Google Scholar] [CrossRef] [PubMed]
- Enose-Akahata, Y.; Oh, U.; Grant, C.; Jacobson, S. Retrovirally induced CTL degranulation mediated by IL-15 expression and infection of mononuclear phagocytes in patients with HTLV-I–associated neurologic disease. Blood 2008, 112, 2400–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldmann, T.A.; Conlon, K.C.; Stewart, D.M.; Worthy, T.A.; Janik, J.E.; Fleisher, T.A.; Albert, P.S.; Figg, W.D.; Spencer, S.D.; Raffeld, M.; et al. Phase 1 trial of IL-15 trans presentation blockade using humanized Mik-Beta-1 mAb in patients with T-cell large granular lymphocytic leukemia. Blood 2013, 121, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.; Cankovic, M.; Furtado, L.V.; Meier, F.; Gocke, C.D. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? A report of the association for molecular pathology. J. Mol. Diagn. 2015, 17, 209–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef] [Green Version]
- Datta, A.; Kim, H.; McGee, L.; Johnson, A.E.; Talwar, S.; Marugan, J.; Southall, N.; Hu, X.; Lal, M.; Mondal, D.; et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: A drug repurposing strategy for advanced cancer. Sci. Rep. 2018, 8, 8161. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Xu, Y.; Xu, W.; Li, M.; Su, H.; Li, C.; Liu, Z. The exosome secretion inhibitor neticonazole suppresses intestinal dysbacteriosis-induced tumorigenesis of colorectal cancer. Investig. New Drugs 2020, 38, 221–228. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Al-Nedawi, K.; Meehan, B.; Kerbel, R.S.; Allison, A.C.; Rak, J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl. Acad. Sci. USA 2009, 106, 3794–3799. [Google Scholar] [CrossRef] [Green Version]
- Costa Verdera, H.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control. Release 2017, 266, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Barrès, C.; Blanc, L.; Bette-Bobillo, P.; André, S.; Mamoun, R.; Gabius, H.-J.; Vidal, M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood 2010, 115, 696–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH Is a Key Factor for Exosome Traffic in Tumor Cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Somerville, C.C. Modulating Cytokine Production via Select Packaging and Secretion from Extracellular Vesicles. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, S.; Ansa-Addo, E.A.; Kholia, S.; Stratton, D.; Valley, S.; Lange, S.; Inal, J. Inhibition of microvesiculation sensitizes prostate cancer cells to chemotherapy and reduces docetaxel dose required to limit tumor growth in vivo. Sci. Rep. 2015, 5, 13006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Nair, A.M.; Fernandez, S.; Mathes, L.; Lairmore, M.D. Enhancement of LFA-1-Mediated T Cell Adhesion by Human T Lymphotropic Virus Type 1 p12I. J. Immunol. 2006, 176, 5463–5470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Chen, L.; Huang, X.; Wu, K.; Ding, S.; Wang, W.; Wang, B.; Smith, C.; Ren, C.; Ni, H.; et al. Calpain inhibitor MDL28170 improves the transplantation-mediated therapeutic effect of bone marrow-derived mesenchymal stem cells following traumatic brain injury. Stem Cell Res. Ther. 2019, 10, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, A.; Kim, H.; Lal, M.; McGee, L.; Johnson, A.; Moustafa, A.A.; Jones, J.C.; Mondal, D.; Ferrer, M.; Abdel-Mageed, A.B. Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells. Cancer Lett. 2017, 408, 73–81. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [Green Version]
- Kosgodage, U.S.; Trindade, R.P.; Thompson, P.R.; Inal, J.M.; Lange, S. Chloramidine/Bisindolylmaleimide-I-Mediated Inhibition of Exosome and Microvesicle Release and Enhanced Efficacy of Cancer Chemotherapy. Int. J. Mol. Sci. 2017, 18, 1007. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Zhang, H.; Zhao, R.; Jing, R.; Xu, Y.; He, M.; Peer, J.; Kim, Y.C.; Luo, J.; et al. Zika virus propagation and release in human fetal astrocytes can be suppressed by neutral sphingomyelinase-2 inhibitor GW4869. Cell Discov. 2018, 4, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verweij, F.J.; Bebelman, M.P.; Jimenez, C.R.; Garcia-Vallejo, J.J.; Janssen, H.; Neefjes, J.; Knol, J.C.; de Goeij-de Haas, R.; Piersma, S.R.; Baglio, S.R.; et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J. Cell Biol. 2018, 217, 1129–1142. [Google Scholar] [CrossRef]
- Niu, Z.; Pang, R.T.K.; Liu, W.; Li, Q.; Cheng, R.; Yeung, W.S.B. Polymer-based precipitation preserves biological activities of extracellular vesicles from an endometrial cell line. PLoS ONE 2017, 12, e0186534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Peng, Y.; Jiang, Y.; Wu, Y.; Ding, Y.; Wang, Y.; Xu, D.; Fu, Q. Imipramine Protects against Bone Loss by Inhibition of Osteoblast-Derived Microvesicles. Int. J. Mol. Sci. 2017, 18, 1013. [Google Scholar] [CrossRef] [Green Version]
- Horváth, Z.; Vécsei, L. Treatment with Pantethine. J. Evid. Based Complementary Altern. Med. 2011, 16, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Kavian, N.; Marut, W.; Servettaz, A.; Nicco, C.; Chéreau, C.; Lemaréchal, H.; Guilpain, P.; Chimini, G.; Galland, F.; Weill, B.; et al. Pantethine Prevents Murine Systemic Sclerosis Through the Inhibition of Microparticle Shedding. Arthritis Rheumatol. 2015, 67, 1881–1890. [Google Scholar] [CrossRef]
- Lima, L.G.; Chammas, R.; Monteiro, R.Q.; Moreira, M.E.C.; Barcinski, M.A. Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett. 2009, 283, 168–175. [Google Scholar] [CrossRef]
- Menck, K.; Sönmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef]
- Figuera-Losada, M.; Stathis, M.; Dorskind, J.M.; Thomas, A.G.; Bandaru, V.V.R.; Yoo, S.-W.; Westwood, N.J.; Rogers, G.W.; McArthur, J.C.; Haughey, N.J.; et al. Cambinol, a Novel Inhibitor of Neutral Sphingomyelinase 2 Shows Neuroprotective Properties. PLoS ONE 2015, 10, e0124481. [Google Scholar] [CrossRef] [Green Version]
- Rizkallah, G.; Alais, S.; Futsch, N.; Tanaka, Y.; Journo, C.; Mahieux, R.; Dutartre, H. Dendritic cell maturation, but not type I interferon exposure, restricts infection by HTLV-1, and viral transmission to T-cells. PLoS Pathog. 2017, 13, e1006353. [Google Scholar] [CrossRef]
- Nonaka, M.; Uota, S.; Saitoh, Y.; Takahashi, M.; Sugimoto, H.; Amet, T.; Arai, A.; Miura, O.; Yamamoto, N.; Yamaoka, S. Role for protein geranylgeranylation in adult T-cell leukemia cell survival. Exp. Cell Res. 2009, 315, 141–150. [Google Scholar] [CrossRef]
- Pinto, D.O.; DeMarino, C.; Vo, T.T.; Cowen, M.; Kim, Y.; Pleet, M.L.; Barclay, R.A.; Noren Hooten, N.; Evans, M.K.; Heredia, A.; et al. Low-Level Ionizing Radiation Induces Selective Killing of HIV-1-Infected Cells with Reversal of Cytokine Induction Using mTOR Inhibitors. Viruses 2020, 12, 885. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, M.; Berini, C.; Ducasa, N.; Malkovsky, M.; Fisch, P.; Biglione, M. Molecular detection of human T-lymphotropic virus type 1 infection among oncology patients in Germany: A retrospective view. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Fedele, C.; Lu, H.; Nevalainen, M.T.; Keen, J.H.; Languino, L.R. Exosome-mediated Transfer of αvβ3 Integrin from Tumorigenic to Nontumorigenic Cells Promotes a Migratory Phenotype. Mol. Cancer Res. 2016, 14, 1136–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaglia, F.; Krishn, S.R.; Daaboul, G.G.; Sarker, S.; Pippa, R.; Domingo-Domenech, J.; Kumar, G.; Fortina, P.; McCue, P.; Kelly, W.K.; et al. Small extracellular vesicles modulated by αVβ3 integrin induce neuroendocrine differentiation in recipient cancer cells. J. Extracell. Vesicles 2020, 9, 1761072. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Meckes, D.G. Extracellular Vesicle Integrins Distinguish Unique Cancers. Proteomes 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Harden, M.E.; Munger, K. Human papillomavirus 16 E6 and E7 oncoprotein expression alters microRNA expression in extracellular vesicles. Virology 2017, 508, 63–69. [Google Scholar] [CrossRef]
- Sproviero, D.; La Salvia, S.; Giannini, M.; Crippa, V.; Gagliardi, S.; Bernuzzi, S.; Diamanti, L.; Ceroni, M.; Pansarasa, O.; Poletti, A.; et al. Pathological Proteins Are Transported by Extracellular Vesicles of Sporadic Amyotrophic Lateral Sclerosis Patients. Front. Neurosci. 2018, 12, 487. [Google Scholar] [CrossRef]
| HTLV-1 Prevalence Rates | |||
|---|---|---|---|
| Continent | Country/Region | Seroprevalence Rates | References |
| Australia | Certain Indigenous populations of Queensland | >40% | [26,27] |
| Asia | Southwestern isles of Japan including Shikoku, Kyushu, and Okinawa | 37% | [28] |
| Taiwan | 0.1–1.0% | ||
| The Mashhad area of northeastern Iran | up to 3% | [4,29] | |
| China, Iraq, Israel, Lebanon, Saudi Arabia, Turkey, Singapore, South Korea | <0.03% | [7,30,31] | |
| Africa | Morocco | 0.6% | [9,32] |
| Benin, Cameroon, and Guinea-Bissau | >5% | ||
| Côte d’lvoire | 0.5–2% | [6] | |
| Burkina Faso, Chad and Gambia | 1–1.2% | ||
| Senegal | 0.2–1.2% | [33] | |
| Togo | 1–1.6% | ||
| Kenyan | 2.7–19.5% | ||
| Congo | 3.2% | ||
| Nigeria | 5.5% | ||
| Mozambique | 1.5% | ||
| Guinea | 1.05% | ||
| Ghana | 0.5–4.2% | ||
| Malawi | 0.63% | ||
| Seychelles | >15% | ||
| Gabon (rural adult Gabonese populations) | 8.7%. | [7,34] | |
| Central African Republic | 0.6% | [6] | |
| South Africa | 1% | [33] | |
| Europe | France | 0.0039% | [9,35] |
| The United Kingdom | 0.03% | [36] | |
| North America | United States (especially in New York, NY, and Miami, FL) | 0.035% | [9,37] |
| Caribbean islands (Jamaica) | 5% | ||
| South and Central America | Chile | 0.73%, | [9,38] |
| Argentina | 0.07% | [9,38] | |
| Colombia, Venezuela, Guyana, Surinam, Panama, and Honduras | 5–14% | [39] | |
| Brazil (in Bahia) | >15% | [40] | |
| Mechanisms of Action | Drugs | Effect | Block | References |
|---|---|---|---|---|
| EV trafficking/EV release | Calpeptin MDL28170 | Inhibits calpain, cleavage of cortactin, and MVB formation | EV biogenesis/release | [229,231] |
| Manumycin | Blocks farnesyl transferase, hinders Ras from binding to plasma membrane, prevents budding from plasma membrane, stimulates n-SMase 2 activity | EV release | [232,233] | |
| Tipifarnib | Inhibits the activity of farnesyltransferase | EV release | [220] | |
| Y27632 | Inhibits Rho-associated kinase ROCK1 and ROCK2 | EV release | [234] | |
| Neticonazole | Decreases Alix, Rab27a, and nSMase2 | EV release | [220,221] | |
| Lipid metabolism/EV release | GW4869 | Inhibits nSMase 2 and subsequent incorporation of ceramide into EVs | EV release | [235,236] |
| Cambinol | Inhibits nSMase2 | EV release | [237] | |
| Imipramine | Promotes the degradation of aSMase that later detaches from the plasma membrane | EV (i.e., MV) release | [219,238] | |
| Pantethine | Inhibits fatty acid and cholesterol synthesis to block MV biogenesis | EV (i.e., MV) biogenesis | [239,240] | |
| EV uptake | Dynasore | Blocks Dynamin-2-mediated clathrin- and caveolin-dependent endocytosis | EV uptake | [222] |
| Annexin V Diannexin | Blocks EV ligand (e.g., phosphatidylserine EV surface) | EV uptake | [223,241] | |
| Cytochalasin B Cytochalasin D | Blocks actin polymerization and endocytosis | EV uptake | [224,225] | |
| Filipin Simvastatin | Blocks EV uptake via lipid raft-mediated endocytosis | EV uptake | [222] | |
| PPIs | Alters pH and blocks EV uptake via membrane fusion | EV uptake | [222,226] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Sharif, S.; Pinto, D.O.; Mensah, G.A.; Dehbandi, F.; Khatkar, P.; Kim, Y.; Branscome, H.; Kashanchi, F. Extracellular Vesicles in HTLV-1 Communication: The Story of an Invisible Messenger. Viruses 2020, 12, 1422. https://doi.org/10.3390/v12121422
Al Sharif S, Pinto DO, Mensah GA, Dehbandi F, Khatkar P, Kim Y, Branscome H, Kashanchi F. Extracellular Vesicles in HTLV-1 Communication: The Story of an Invisible Messenger. Viruses. 2020; 12(12):1422. https://doi.org/10.3390/v12121422
Chicago/Turabian StyleAl Sharif, Sarah, Daniel O. Pinto, Gifty A. Mensah, Fatemeh Dehbandi, Pooja Khatkar, Yuriy Kim, Heather Branscome, and Fatah Kashanchi. 2020. "Extracellular Vesicles in HTLV-1 Communication: The Story of an Invisible Messenger" Viruses 12, no. 12: 1422. https://doi.org/10.3390/v12121422
APA StyleAl Sharif, S., Pinto, D. O., Mensah, G. A., Dehbandi, F., Khatkar, P., Kim, Y., Branscome, H., & Kashanchi, F. (2020). Extracellular Vesicles in HTLV-1 Communication: The Story of an Invisible Messenger. Viruses, 12(12), 1422. https://doi.org/10.3390/v12121422








