Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans
Abstract
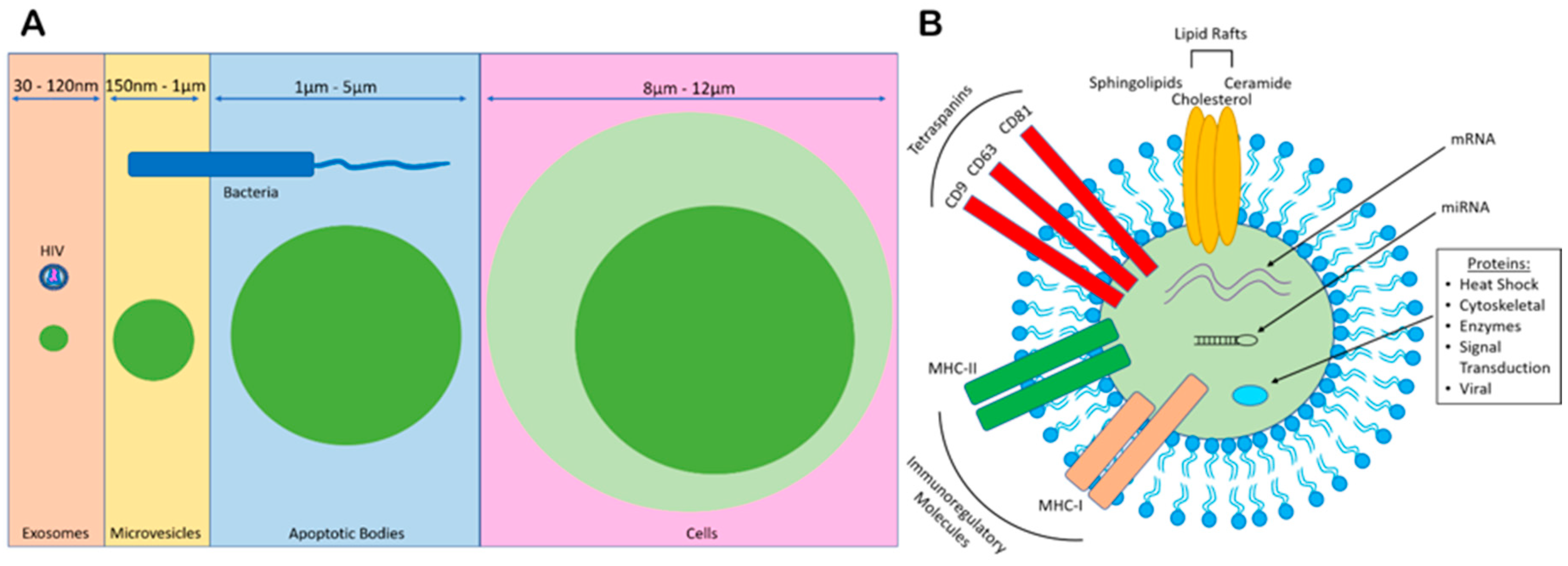
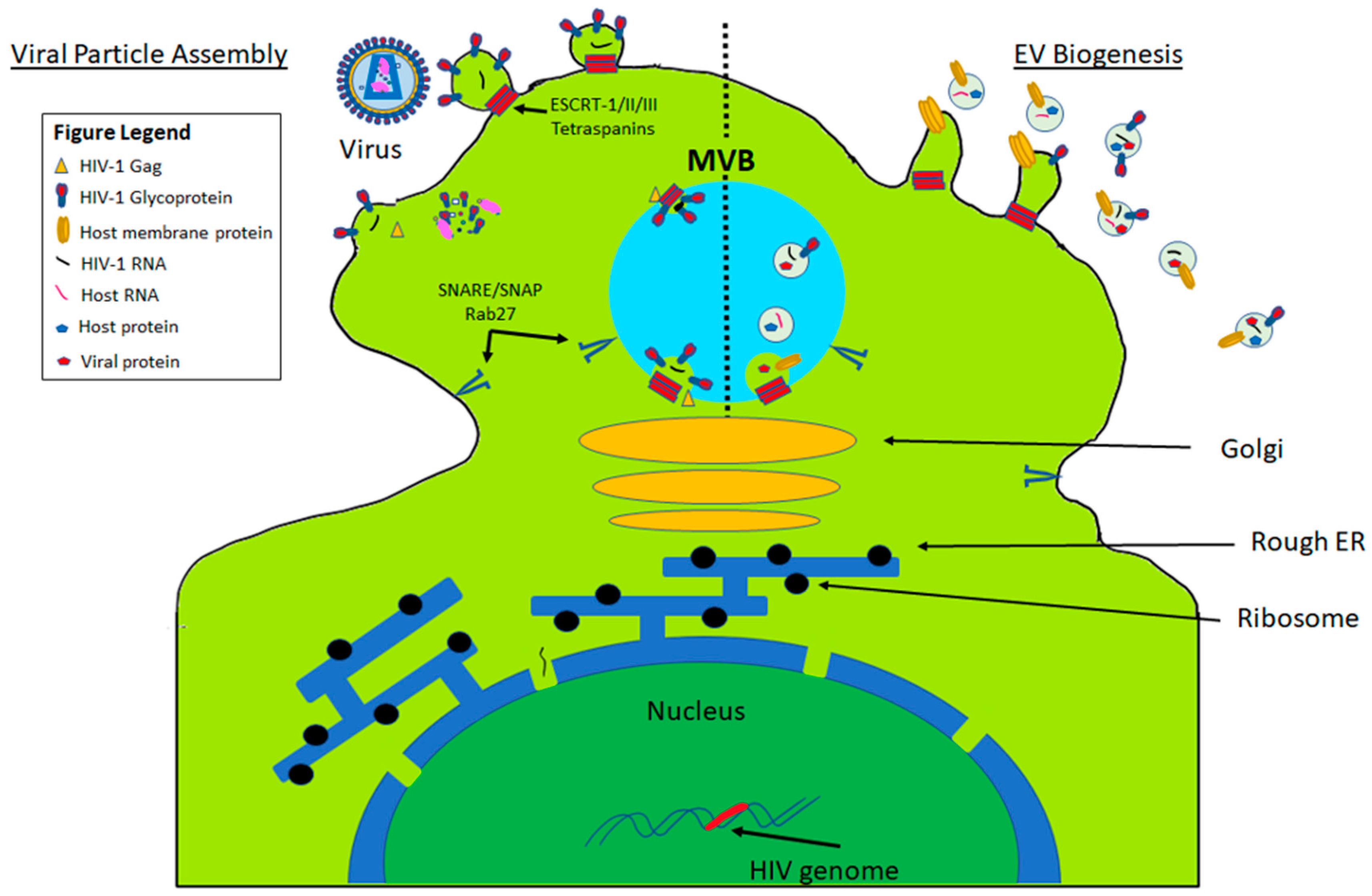
1. Extracellular Vesicle (EV) Biogenesis
2. Isolation of EVs
2.1. Differential Centrifugation—The Gold Standard
2.2. Immunoaffinity
2.3. Density Gradient—OptiPrep™
2.4. Chromatography
2.5. Precipitation
2.6. Ultrafiltration
2.7. Nanoplasmon-Enhanced Scattering (nPES)
2.8. Lab-On-Chip Exosome Isolation
3. Exosomal Content and Characterization
4. Role of EVs in the Pathogenesis of Viral Infections
4.1. Picornaviridae and Togaviridae
4.2. Herpesviridae
4.3. Filoviridae
4.4. Paramyxoviridae
4.5. Orthomyxoviridae
4.6. Hepadnaviridae
4.7. Flaviviridae
4.7.1. ZIKA
4.7.2. EV-Mediated Restriction of ZIKV Pathogenesis
4.7.3. EV-Mediated Enhancement of ZIKV Neuropathology
4.8. Retroviridae
4.8.1. Human Immunodeficiency Virus Type 1 (HIV-1)
4.8.2. EV Interaction with Host Cell Restriction Factors and HIV
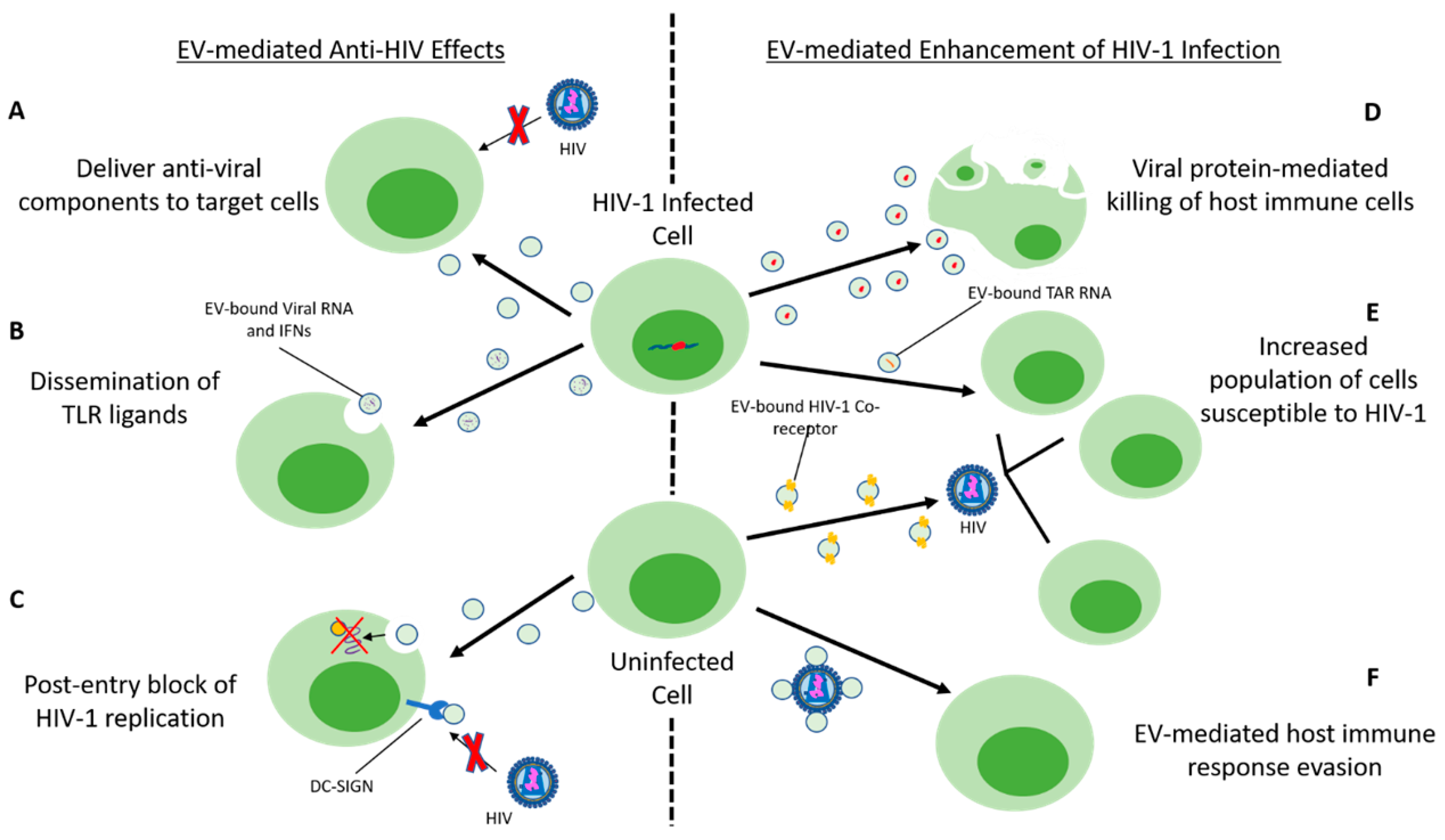
4.8.3. Immune Cell-Derived EVs and Antiviral Effects
4.8.4. EV-Mediated Enhancement of HIV-1 Infection
4.9. Coronaviridae
4.10. Polyomaviridae
5. Therapeutic Potential of EVs as Antiviral Agents
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [PubMed]
- Caruso, S.; Poon, I.K.H. Apoptotic Cell-Derived extracellular vesicles: More Than Just Debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- MacKenzie, A.; Wilson, H.L.; Kiss-Toth, E.; Dower, S.K.; North, R.A.; Surprenant, A. Rapid Secretion of Interleukin-1β by Microvesicle Shedding. Immunity 2001, 15, 825–835. [Google Scholar] [CrossRef]
- Alenquer, M.; Amorim, M.J. Exosome Biogenesis, Regulation, and Function in Viral Infection. Viruses 2015, 7, 5066–5083. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Grange, C.; Fonsato, V.; Tetta, C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am. J. Cancer Res. 2011, 1, 98–110. [Google Scholar]
- Eitan, E.; Suire, C.; Zhang, S.; Mattson, M.P. Impact of lysosome status on extracellular vesicle content and release. Ageing Res. Rev. 2016, 32, 65–74. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Menck, K.; Sonmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef]
- Andreu, Z.; Yanez-Mo, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Boussac, M.; Veron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.; Manrique-Hoyos, N.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Gronborg, M.; Mobius, W.; Rhee, J.; et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 2010, 189, 223–232. [Google Scholar] [CrossRef]
- Savina, A.; Fader, C.M.; Damiani, M.T.; Colombo, M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 2005, 6, 131–143. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Dias, M.V.S.; Costa, C.S.; daSilva, L.L.P. The Ambiguous Roles of Extracellular Vesicles in HIV Replication and Pathogenesis. Front. Microbiol. 2018, 9, 2411. [Google Scholar] [CrossRef]
- Baixauli, F.; Lopez-Otin, C.; Mittelbrunn, M. Exosomes and autophagy: Coordinated mechanisms for the maintenance of cellular fitness. Front. Immunol. 2014, 5, 403. [Google Scholar] [CrossRef]
- Bretz, N.P.; Ridinger, J.; Rupp, A.K.; Rimbach, K.; Keller, S.; Rupp, C.; Marme, F.; Umansky, L.; Umansky, V.; Eigenbrod, T.; et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J. Biol. Chem. 2013, 288, 36691–36702. [Google Scholar] [CrossRef]
- Buzas, E.I.; Gyorgy, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.; Court, J.; Navabi, H.; Adams, M.; Mason, M.D.; Hobot, J.A.; Newman, G.R.; Jasani, B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J. Immunol. Methods 2001, 247, 163–174. [Google Scholar] [CrossRef]
- Kahlert, C.; Kalluri, R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. 2013, 91, 431–437. [Google Scholar] [CrossRef]
- Melo, S.A.; Sugimoto, H.; O’Connell, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Chahar, H.S.; Bao, X.Y.; Casola, A. Exosomes and Their Role in the Life Cycle and Pathogenesis of RNA Viruses. Viruses 2015, 7, 3204–3225. [Google Scholar] [CrossRef]
- Ramakrishnaiah, V.; Thumann, C.; Fofana, I.; Habersetzer, F.; Pan, Q.W.; de Ruiter, P.E.; Willemsen, R.; Demmers, J.A.A.; Raj, V.S.; Jenster, G.; et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13109–13113. [Google Scholar] [CrossRef]
- Altan-Bonnet, N. Extracellular vesicles are the Trojan horses of viral infection. Curr. Opin. Microbiol. 2016, 32, 77–81. [Google Scholar] [CrossRef]
- Chen, Y.H.; Du, W.; Hagemeijer, M.C.; Takvorian, P.M.; Pau, C.; Cali, A.; Brantner, C.A.; Stempinski, E.S.; Connelly, P.S.; Ma, H.C.; et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell 2015, 160, 619–630. [Google Scholar] [CrossRef]
- Inal, J.M.; Jorfi, S. Coxsackievirus B transmission and possible new roles for extracellular vesicles. Biochem. Soc. Trans. 2013, 41, 299–302. [Google Scholar] [CrossRef]
- Kalamvoki, M.; Du, T.; Roizman, B. Cells infected with herpes simplex virus 1 export to uninfected cells exosomes containing STING, viral mRNAs, and microRNAs. Proc. Natl. Acad. Sci. USA 2014, 111, E4991–E4996. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, C.; Nagy, N.; Gentile, M.; Lyberg, K.; Gumz, J.; Vallhov, H.; Puga, I.; Klein, E.; Gabrielsson, S.; Cerutti, A.; et al. Exosomes derived from Burkitt’s lymphoma cell lines induce proliferation, differentiation, and class-switch recombination in B cells. J. Immunol. 2014, 192, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
- Keryer-Bibens, C.; Pioche-Durieu, C.; Villemant, C.; Souquere, S.; Nishi, N.; Hirashima, M.; Middeldorp, J.; Busson, P. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer 2006, 6, 283. [Google Scholar] [CrossRef] [PubMed]
- Klibi, J.; Niki, T.; Riedel, A.; Pioche-Durieu, C.; Souquere, S.; Rubinstein, E.; Le Moulec, S.; Guigay, J.; Hirashima, M.; Guemira, F.; et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 2009, 113, 1957–1966. [Google Scholar] [CrossRef]
- Dukers, D.F.; Meij, P.; Vervoort, M.B.; Vos, W.; Scheper, R.J.; Meijer, C.J.; Bloemena, E.; Middeldorp, J.M. Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J. Immunol. 2000, 165, 663–670. [Google Scholar] [CrossRef]
- Flanagan, J.; Middeldorp, J.; Sculley, T. Localization of the Epstein-Barr virus protein LMP 1 to exosomes. J. Gen. Virol. 2003, 84, 1871–1879. [Google Scholar] [CrossRef]
- Baseler, L.; Chertow, D.S.; Johnson, K.M.; Feldmann, H.; Morens, D.M. The Pathogenesis of Ebola Virus Disease. Ann. Rev. Pathol. Mech. Dis. 2017, 12, 387–418. [Google Scholar] [CrossRef]
- Nehls, J.; Businger, R.; Hoffmann, M.; Brinkmann, C.; Fehrenbacher, B.; Schaller, M.; Maurer, B.; Schonfeld, C.; Kramer, D.; Hailfinger, S.; et al. Release of Immunomodulatory Ebola Virus Glycoprotein-Containing Microvesicles Is Suppressed by Tetherin in a Species-Specific Manner. Cell Rep. 2019, 26, 1841–1853.e6. [Google Scholar] [CrossRef]
- Pleet, M.L.; Erickson, J.; DeMarino, C.; Barclay, R.A.; Cowen, M.; Lepene, B.; Liang, J.; Kuhn, J.H.; Prugar, L.; Stonier, S.W.; et al. Ebola Virus VP40 Modulates Cell Cycle and Biogenesis of Extracellular Vesicles. J. Infect. Dis. 2018, 218, S365–S387. [Google Scholar] [CrossRef]
- Messaoudi, I.; Amarasinghe, G.K.; Basler, C.F. Filovirus pathogenesis and immune evasion: Insights from Ebola virus and Marburg virus. Nat. Rev. Microbiol. 2015, 13, 663–676. [Google Scholar] [CrossRef]
- Pleet, M.L.; Mathiesen, A.; DeMarino, C.; Akpamagbo, Y.A.; Barclay, R.A.; Schwab, A.; Iordanskiy, S.; Sampey, G.C.; Lepene, B.; Ilinykh, P.A.; et al. Ebola VP40 in Exosomes Can Cause Immune Cell Dysfunction (vol 7, 1765, 2016). Front. Microbiol. 2018, 9, 692. [Google Scholar] [CrossRef] [PubMed]
- Chahar, H.S.; Corsello, T.; Kudlicki, A.S.; Komaravelli, N.; Casola, A. Respiratory Syncytial Virus Infection Changes Cargo Composition of Exosome Released from Airway Epithelial Cells. Sci. Rep. 2018, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Bomberger, J.; Lashua, L.; Fischer, D.; Hendricks, M. Exosome-Associated Iron Release during Respiratory Virus Co-Infection Enhances Pseudomonas aeruginosa Biofilm Growth. FASEB J. 2016, 30, 1223. [Google Scholar] [CrossRef]
- Kesimer, M.; Scull, M.; Brighton, B.; DeMaria, G.; Burns, K.; O’Neal, W.; Pickles, R.J.; Sheehan, J.K. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: A possible role in innate defense. FASEB J. 2009, 23, 1858–1868. [Google Scholar] [CrossRef]
- Nadmdari, H.; Keshavarz, M.; Mokhtari-Azad, T.; Rezaei, F. Evaluation of Antibody and Cytokines Responses in Intranasally and Intramuscularly Administrated BALB/C Mice with Influenza Virus-Like Particle. Acta Med. Iran. 2017, 55, 604–611. [Google Scholar] [PubMed]
- Deriu, E.; Boxx, G.M.; He, X.; Pan, C.; Benavidez, S.D.; Cen, L.; Rozengurt, N.; Shi, W.; Cheng, G. Influenza Virus Affects Intestinal Microbiota and Secondary Salmonella Infection in the Gut through Type I Interferons. PLoS Pathog. 2016, 12, e1005572. [Google Scholar] [CrossRef]
- Cheng, L.; Sharples, R.A.; Scicluna, B.J.; Hill, A.F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef]
- Keshavarz, M.; Dianat-Moghadam, H.; Sofiani, V.H.; Karimzadeh, M.; Zargar, M.; Moghoofei, M.; Biglari, H.; Ghorbani, S.; Nahand, J.S.; Mirzaei, H. miRNA-based strategy for modulation of influenza A virus infection. Epigenomics 2018, 10, 829–844. [Google Scholar] [CrossRef]
- Loveday, E.-K.; Svinti, V.; Diederich, S.; Pasick, J.; Jean, F. Temporal- and strain-specific host microRNA molecular signatures associated with swine-origin H1N1 and avian-origin H7N7 influenza A virus infection. J. Virol. 2012, 86, 6109–6122. [Google Scholar] [CrossRef]
- Tambyah, P.A.; Sepramaniam, S.; Mohamed Ali, J.; Chai, S.C.; Swaminathan, P.; Armugam, A.; Jeyaseelan, K. microRNAs in circulation are altered in response to influenza A virus infection in humans. PLoS ONE 2013, 8, e76811. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Tseng, C.H.; Chen, Y.C.; Yu, W.Y.; Ho, M.Y.; Ho, C.Y.; Lai, M.M.C.; Su, W.C. Exosome-delivered and Y RNA-derived small RNA suppresses influenza virus replication. J. Biomed. Sci. 2019, 26, 58. [Google Scholar] [CrossRef]
- Cypryk, W.; Lorey, M.; Puustinen, A.; Nyman, T.A.; Matikainen, S. Proteomic and Bioinformatic Characterization of Extracellular Vesicles Released from Human Macrophages upon Influenza A Virus Infection. J. Proteome Res. 2017, 16, 217–227. [Google Scholar] [CrossRef]
- Bruce, E.A.; Digard, P.; Stuart, A.D. The Rab11 pathway is required for influenza a virus budding and filament formation. J. Virol. 2010, 84, 5848–5859. [Google Scholar] [CrossRef]
- Hutchinson, E.C.; Charles, P.D.; Hester, S.S.; Thomas, B.; Trudgian, D.; Martínez-Alonso, M.; Fodor, E. Conserved and host-specific features of influenza virion architecture. Nat. Commun. 2014, 5, 4816. [Google Scholar] [CrossRef]
- Kapoor, N.R.; Chadha, R.; Kumar, S.; Choedon, T.; Reddy, V.S.; Kumar, V. The HBx gene of hepatitis B virus can influence hepatic microenvironment via exosomes by transferring its mRNA and protein. Virus Res. 2017, 240, 166–174. [Google Scholar] [CrossRef]
- Kouwaki, T.; Fukushima, Y.; Daito, T.; Sanada, T.; Yamamoto, N.; Mifsud, E.J.; Leong, C.R.; Tsukiyama-Kohara, K.; Kohara, M.; Matsumoto, M.; et al. Extracellular Vesicles Including Exosomes Regulate Innate Immune Responses to Hepatitis B Virus Infection. Front. Immunol. 2016, 7, 335. [Google Scholar] [CrossRef]
- Spear, P.; Wu, M.-R.; Sentman, M.-L.; Sentman, C.L. NKG2D ligands as therapeutic targets. Cancer Immun. 2013, 13, 8. [Google Scholar]
- Jia, X.; Chen, J.; Megger, D.A.; Zhang, X.; Kozlowski, M.; Zhang, L.; Fang, Z.; Li, J.; Chu, Q.; Wu, M.; et al. Label-free Proteomic Analysis of Exosomes Derived from Inducible Hepatitis B Virus-Replicating HepAD38 Cell Line. Mol. Cell. Proteom. 2017, 16, S144–S160. [Google Scholar] [CrossRef]
- Yang, Y.; Han, Q.; Hou, Z.; Zhang, C.; Tian, Z.; Zhang, J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell. Mol. Immunol. 2017, 14, 465–475. [Google Scholar] [CrossRef]
- Yao, Z.; Qiao, Y.; Li, X.; Chen, J.; Ding, J.; Bai, L.; Shen, F.; Shi, B.; Liu, J.; Peng, L.; et al. Exosomes Exploit the Virus Entry Machinery and Pathway to Transmit Alpha Interferon-Induced Antiviral Activity. J. Virol. 2018, 92, e01578-18. [Google Scholar] [CrossRef]
- Bukong, T.N.; Momen-Heravi, F.; Kodys, K.; Bala, S.; Szabo, G. Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90. PLoS Pathog. 2014, 10, e1004424. [Google Scholar] [CrossRef]
- Cosset, F.L.; Dreux, M. HCV transmission by hepatic exosomes establishes a productive infection. J. Hepatol. 2014, 60, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A.; Zhang, C.; Huys, A.; Richardson, C.D. Human Ago2 Is Required for Efficient MicroRNA 122 Regulation of Hepatitis C Virus RNA Accumulation and Translation. J. Virol. 2011, 85, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.S.; Woodson, M.; Neupane, B.; Bai, F.W.; Sherman, M.B.; Choi, K.H.; Neelakanta, G.; Sultana, H. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog. 2018, 14, e1006764. [Google Scholar] [CrossRef]
- Anderson, M.R.; Kashanchi, F.; Jacobson, S. Exosomes in Viral Disease. Neurotherapeutics 2016, 13, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Li, Z.L.; Yuan, S. The Role of Secretory Autophagy in Zika Virus Transfer through the Placental Barrier. Front. Cell. Infect. Microbiol. 2017, 6, 206. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Valderramos, S.G.; Wu, A.; Ouyang, S.; Li, C.; Brasil, P.; Bonaldo, M.; Coates, T.; Nielsen-Saines, K.; Jiang, T.; et al. From Mosquitos to Humans: Genetic Evolution of Zika Virus. Cell Host Microbe 2016, 19, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Klase, Z.A.; Khakhina, S.; Schneider Ade, B.; Callahan, M.V.; Glasspool-Malone, J.; Malone, R. Zika Fetal Neuropathogenesis: Etiology of a Viral Syndrome. PLoS Negl. Trop. Dis. 2016, 10, e0004877. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Lennemann, N.J.; Ouyang, Y.; Bramley, J.C.; Morosky, S.; Marques, E.T., Jr.; Cherry, S.; Sadovsky, Y.; Coyne, C.B. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe 2016, 19, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Chiramel, A.I.; Best, S.M. Role of autophagy in Zika virus infection and pathogenesis. Virus Res. 2018, 254, 34–40. [Google Scholar] [CrossRef]
- Delorme-Axford, E.; Donker, R.B.; Mouillet, J.F.; Chu, T.; Bayer, A.; Ouyang, Y.; Wang, T.; Stolz, D.B.; Sarkar, S.N.; Morelli, A.E.; et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12048–12053. [Google Scholar] [CrossRef]
- Bayer, A.; Lennemann, N.J.; Ouyang, Y.; Sadovsky, E.; Sheridan, M.A.; Roberts, R.M.; Coyne, C.B.; Sadovsky, Y. Chromosome 19 microRNAs exert antiviral activity independent from type III interferon signaling. Placenta 2018, 61, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Li, Y.J.; Zhang, H.N.; Zhao, R.Z.; Jing, R.; Xu, Y.H.; He, M.; Peer, J.; Kim, Y.C.; Luo, J.T.; et al. Zika virus propagation and release in human fetal astrocytes can be suppressed by neutral sphingomyelinase-2 inhibitor GW4869. Cell Discov. 2018, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.M.; Lahon, A.; Suter, M.A.; Arya, R.P.; Seferovic, M.D.; Vogt, M.B.; Hu, M.; Stossi, F.; Mancini, M.A.; Harris, R.A.; et al. Primary Human Placental Trophoblasts are Permissive for Zika Virus (ZIKV) Replication. Sci. Rep. 2017, 7, 41389. [Google Scholar] [CrossRef]
- Yelamanchili, S.V.; Lamberty, B.G.; Rennard, D.A.; Morsey, B.M.; Hochfelder, C.G.; Meays, B.M.; Levy, E.; Fox, H.S. MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease. PLoS Pathog. 2015, 11, e1005032. [Google Scholar] [CrossRef]
- Muller, J.A.; Harms, M.; Kruger, F.; Gross, R.; Joas, S.; Hayn, M.; Dietz, A.N.; Lippold, S.; von Einem, J.; Schubert, A.; et al. Semen inhibits Zika virus infection of cells and tissues from the anogenital region. Nat. Commun. 2018, 9, 2207. [Google Scholar] [CrossRef]
- Ngono, A.E.; Shresta, S. Immune Response to Dengue and Zika. Annu. Rev. Immunol. 2018, 36, 279–308. [Google Scholar] [CrossRef]
- Holder, B.; Jones, T.; Sancho Shimizu, V.; Rice, T.F.; Donaldson, B.; Bouqueau, M.; Forbes, K.; Kampmann, B. Macrophage Exosomes Induce Placental Inflammatory Cytokines: A Novel Mode of Maternal-Placental Messaging. Traffic 2016, 17, 168–178. [Google Scholar] [CrossRef]
- Wang, W.B.; Li, G.; Wu, D.; Luo, Z.; Pan, P.; Tian, M.F.; Wang, Y.C.; Xiao, F.; Li, A.X.; Wu, K.L.; et al. Zika virus infection induces host inflammatory responses by facilitating NLRP3 inflammasome assembly and interleukin-1 beta secretion. Nat. Commun. 2018, 9, 106. [Google Scholar] [CrossRef]
- Zhou, W.S.; Woodson, M.; Sherman, M.B.; Neelakanta, G.; Sultana, H. Exosomes mediate Zika virus transmission through SMPD3 neutral Sphingomyelinase in cortical neurons. Emerg. Microbes Infect. 2019, 8, 307–326. [Google Scholar] [CrossRef]
- Chun, T.W.; Fauci, A.S. HIV reservoirs: Pathogenesis and obstacles to viral eradication and cure. AIDS 2012, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef] [PubMed]
- Madison, M.N.; Okeoma, C.M. Exosomes: Implications in HIV-1 Pathogenesis. Viruses 2015, 7, 4093–4118. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, D.; Richards, C.M.; Carpenter, M.A.; Wang, J.; Ikeda, T.; Becker, J.T.; Cheng, A.Z.; McCann, J.L.; Shaban, N.M.; Salamango, D.J.; et al. Genetic and mechanistic basis for APOBEC3H alternative splicing, retrovirus restriction, and counteraction by HIV-1 protease. Nat. Commun. 2018, 9, 4137. [Google Scholar] [CrossRef]
- Miyagi, E.; Opi, S.; Takeuchi, H.; Khan, M.; Goila-Gaur, R.; Kao, S.; Strebel, K. Enzymatically active APOBEC3G is required for efficient inhibition of human immunodeficiency virus type 1. J. Virol. 2007, 81, 13346–13353. [Google Scholar] [CrossRef]
- Holmes, M.; Zhang, F.W.; Bieniasz, P.D. Single-Cell and Single-Cycle Analysis of HIV-1 Replication. PLoS Pathog. 2015, 11, e1004961. [Google Scholar] [CrossRef]
- de Carvalho, J.V.; de Castro, R.O.; da Silva, E.Z.; Silveira, P.P.; da Silva-Januario, M.E.; Arruda, E.; Jamur, M.C.; Oliver, C.; Aguiar, R.S.; daSilva, L.L. Nef neutralizes the ability of exosomes from CD4+ T cells to act as decoys during HIV-1 infection. PLoS ONE 2014, 9, e113691. [Google Scholar] [CrossRef]
- Tumne, A.; Prasad, V.S.; Chen, Y.; Stolz, D.B.; Saha, K.; Ratner, D.M.; Ding, M.; Watkins, S.C.; Gupta, P. Noncytotoxic suppression of human immunodeficiency virus type 1 transcription by exosomes secreted from CD8+ T cells. J. Virol. 2009, 83, 4354–4364. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Zhou, Y.; Zhou, R.H.; Ho, W.Z.; Li, J.L. Exosomes contribute to the transmission of anti-HIV activity from TLR3-activated brain microvascular endothelial cells to macrophages. Antivir. Res. 2016, 134, 167–171. [Google Scholar] [CrossRef]
- Guo, L.; Xu, X.Q.; Zhou, L.; Zhou, R.H.; Wang, X.; Li, J.L.; Liu, J.B.; Liu, H.; Zhang, B.; Ho, W.Z. Human Intestinal Epithelial Cells Release Antiviral Factors That Inhibit HIV Infection of Macrophages. Front. Immunol. 2018, 9, 247. [Google Scholar] [CrossRef]
- Smith, J.A.; Daniel, R. Human vaginal fluid contains exosomes that have an inhibitory effect on an early step of the HIV-1 life cycle. AIDS 2016, 30, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Madison, M.N.; Jones, P.H.; Okeoma, C.M. Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology 2015, 482, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Madison, M.N.; Roller, R.J.; Okeoma, C.M. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 2014, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Naslund, T.I.; Paquin-Proulx, D.; Paredes, P.T.; Vallhov, H.; Sandberg, J.K.; Gabrielsson, S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS 2014, 28, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filen, J.J.; Lahesmaa, R.; Norman, M.; Neve, E.P.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef]
- Mack, M.; Kleinschmidt, A.; Bruhl, H.; Klier, C.; Nelson, P.J.; Cihak, J.; Plachy, J.; Stangassinger, M.; Erfle, V.; Schlondorff, D. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 2000, 6, 769–775. [Google Scholar] [CrossRef]
- Rozmyslowicz, T.; Majka, M.; Kijowski, J.; Murphy, S.L.; Conover, D.O.; Poncz, M.; Ratajczak, J.; Gaulton, G.N.; Ratajczak, M.Z. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS 2003, 17, 33–42. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Mairuhu, A.T.; Italiano, J.E. Platelet- and megakaryocyte-derived microparticles. Semin. Thromb. Hemost. 2010, 36, 881–887. [Google Scholar] [CrossRef]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Matthews, Q.L. Tetraspanin blockage reduces exosome-mediated HIV-1 entry. Arch. Virol. 2018, 163, 1683–1689. [Google Scholar] [CrossRef]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Gu, L.; Matthews, Q.L. Role of TIM-4 in exosome-dependent entry of HIV-1 into human immune cells. Int. J. Nanomed. 2017, 12, 4823–4833. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, W.L.; Ye, Y.Y.; Bai, X.C.; Liu, R.Q.; Chang, L.F.; Zhou, Q.; Sui, S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010, 11, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Miyanishi, M.; Tada, K.; Koike, M.; Uchiyama, Y.; Kitamura, T.; Nagata, S. Identification of Tim4 as a phosphatidylserine receptor. Nature 2007, 450, 435–439. [Google Scholar] [CrossRef]
- Kadiu, I.; Narayanasamy, P.; Dash, P.K.; Zhang, W.; Gendelman, H.E. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J. Immunol. 2012, 189, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Sampey, G.C.; Saifuddin, M.; Schwab, A.; Barclay, R.; Punya, S.; Chung, M.C.; Hakami, R.M.; Zadeh, M.A.; Lepene, B.; Klase, Z.A.; et al. Exosomes from HIV-1-infected Cells Stimulate Production of Pro-inflammatory Cytokines through Trans-activating Response (TAR) RNA. J. Biol. Chem. 2016, 291, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Birge, R.B.; Boeltz, S.; Kumar, S.; Carlson, J.; Wanderley, J.; Calianese, D.; Barcinski, M.; Brekken, R.A.; Huang, X.; Hutchins, J.T.; et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016, 23, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Arakelyan, A.; Fitzgerald, W.; Zicari, S.; Vanpouille, C.; Margolis, L. Extracellular Vesicles Carry HIV Env and Facilitate Hiv Infection of Human Lymphoid Tissue. Sci. Rep. 2017, 7, 1695. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.M.; Fang, Y.; Fallon, J.K.; Yang, J.M.; Hildreth, J.E.; Gould, S.J. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J. Cell Biol. 2006, 172, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, S.C.; Federico, M. Sequences within RNA coding for HIV-1 Gag p17 are efficiently targeted to exosomes. Cell. Microbiol. 2013, 15, 412–429. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wu, N.; Gan, X.; Yan, W.; Morrell, J.C.; Gould, S.J. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007, 5, e158. [Google Scholar] [CrossRef]
- Raymond, A.D.; Campbell-Sims, T.C.; Khan, M.; Lang, M.; Huang, M.B.; Bond, V.C.; Powell, M.D. HIV Type 1 Nef Is Released from Infected Cells in CD45(+) Microvesicles and Is Present in the Plasma of HIV-Infected Individuals. AIDS Res. Hum. Retrovir. 2011, 27, 167–178. [Google Scholar] [CrossRef]
- Campbell, T.D.; Khan, M.; Huang, M.B.; Bond, V.C.; Powell, M.D. HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions. Ethn. Dis. 2008, 18, S2-14-19. [Google Scholar]
- Lenassi, M.; Cagney, G.; Liao, M.F.; Vaupotic, T.; Bartholomeeusen, K.; Cheng, Y.F.; Krogan, N.J.; Plemenitas, A.; Peterlin, B.M. HIV Nef is Secreted in Exosomes and Triggers Apoptosis in Bystander CD4(+) T Cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef]
- Stumptner-Cuvelette, P.; Jouve, M.; Helft, J.; Dugast, M.; Glouzman, A.S.; Jooss, K.; Raposo, G.; Benaroch, P. Human immunodeficiency virus-1 Nef expression induces intracellular accumulation of multivesicular bodies and major histocompatibility complex class II complexes: Potential role of phosphatidylinositol 3-kinase. Mol. Biol. Cell 2003, 14, 4857–4870. [Google Scholar] [CrossRef] [PubMed]
- Sevilya, Z.; Chorin, E.; Gal-Garber, O.; Zelinger, E.; Turner, D.; Avidor, B.; Berke, G.; Hassin, D. Killing of Latently HIV-Infected CD4 T Cells by Autologous CD8 T Cells Is Modulated by Nef. Front. Immunol. 2018, 9, 2068. [Google Scholar] [CrossRef]
- Jacob, R.A.; Johnson, A.L.; Pawlak, E.N.; Dirk, B.S.; Van Nynatten, L.R.; Haeryfar, S.M.M.; Dikeakos, J.D. The interaction between HIV-1 Nef and adaptor protein-2 reduces Nefmediated CD4+ T cell apoptosis. Virology 2017, 509, 1–10. [Google Scholar] [CrossRef] [PubMed]
- James, C.O.; Huang, M.B.; Khan, M.; Garcia-Barrio, M.; Powell, M.D.; Bond, V.C. Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors. J. Virol. 2004, 78, 3099–3109. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Schierer, S.; Blume, K.; Dindorf, J.; Wittki, S.; Xiang, W.; Ostalecki, C.; Koliha, N.; Wild, S.; Schuler, G.; et al. HIV-Nef and ADAM17-Containing Plasma Extracellular Vesicles Induce and Correlate with Immune Pathogenesis in Chronic HIV Infection. Ebiomedicine 2016, 6, 103–113. [Google Scholar] [CrossRef]
- Arenaccio, C.; Chiozzini, C.; Columba-Cabezas, S.; Manfredi, F.; Affabris, E.; Baur, A.; Federico, M. Exosomes from Human Immunodeficiency Virus Type 1 (HIV-1)-Infected Cells License Quiescent CD4(+) T Lymphocytes to Replicate HIV-1 through a Nef- and ADAM17-Dependent Mechanism. J. Virol. 2014, 88, 11529–11539. [Google Scholar] [CrossRef]
- Roth, W.W.; Huang, M.B.; Addae Konadu, K.; Powell, M.D.; Bond, V.C. Micro RNA in Exosomes from HIV-Infected Macrophages. Int. J. Environ. Res. Public Health 2015, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- De Vries, W.; Berkhout, B. RNAi suppressors encoded by pathogenic human viruses. Int. J. Biochem. Cell Biol. 2008, 40, 2007–2012. [Google Scholar] [CrossRef]
- Bernard, M.A.; Zhao, H.; Yue, S.C.; Anandaiah, A.; Koziel, H.; Tachado, S.D. Novel HIV-1 MiRNAs Stimulate TNF alpha Release in Human Macrophages via TLR8 Signaling Pathway. PLoS ONE 2014, 9, e106006. [Google Scholar] [CrossRef] [PubMed]
- Aqil, M.; Naqvi, A.R.; Mallik, S.; Bandyopadhyay, S.; Maulik, U.; Jameel, S. The HIV Nef protein modulates cellular and exosomal miRNA profiles in human monocytic cells. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef]
- Boisse, L.; Gill, M.J.; Power, C. HIV infection of the central nervous system: Clinical features and neuropathogenesis. Neurol. Clin. 2008, 26, 799–819. [Google Scholar] [CrossRef] [PubMed]
- Raymond, A.D.; Diaz, P.; Chevelon, S.; Agudelo, M.; Yndart-Arias, A.; Ding, H.; Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Roy, U.; et al. Microglia-derived HIV Nef plus exosome impairment of the blood-brain barrier is treatable by nanomedicine-based delivery of Nef peptides. J. Neurovirol. 2016, 22, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kuate, S.; Cinatl, J.; Doerr, H.W.; Überla, K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology 2007, 362, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.M. Coronavirus: Organization, replication and expression of genome. Annu. Rev. Microbiol. 1990, 44, 303–333. [Google Scholar] [CrossRef]
- Spaan, W.; Cavanagh, D.; Horzinek, M.C. Coronaviruses: Structure and Genome Expression. J. Gen. Virol. 1988, 69, 2939–2952. [Google Scholar] [CrossRef]
- Kwon, Y.; Nukala, S.B.; Srivastava, S.; Miyamoto, H.; Ismail, N.I.; Ong, S.-B.; Lee, W.H.; Ong, S.-G. Exosomes Facilitate Transmission of SARS-CoV-2 Genome into Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Giannessi, F.; Aiello, A.; Franchi, F.; Percario, Z.A.; Affabris, E. The Role of Extracellular Vesicles as Allies of HIV, HCV and SARS Viruses. Viruses 2020, 12, 571. [Google Scholar] [CrossRef]
- Kang, J.-S. Chapter 20—The potential of exosomes as theragnostics in various clinical situations. In Exosomes; Edelstein, L., Smythies, J., Quesenberry, P., Noble, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 467–486. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Viscidi, R.P.; Shah, K.V. Chapter 157—Polyomaviruses. In Infectious Diseases, 3rd ed.; Cohen, J., Opal, S.M., Powderly, W.G., Eds.; Content Repository Only: London, UK, 2010; pp. 1570–1572. [Google Scholar]
- Morris-Love, J.; Gee, G.V.; O’Hara, B.A.; Assetta, B.; Atkinson, A.L.; Dugan, A.S.; Haley, S.A.; Atwood, W.J. JC Polyomavirus Uses Extracellular Vesicles to Infect Target Cells. mBio 2019, 10. [Google Scholar] [CrossRef]
- O’Hara, B.A.; Morris-Love, J.; Gee, G.V.; Haley, S.A.; Atwood, W.J. JC Virus infected choroid plexus epithelial cells produce extracellular vesicles that infect glial cells independently of the virus attachment receptor. PLoS Pathog. 2020, 16, e1008371. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, I.; Clausi, V.; Nukuzuma, S.; Della Malva, N.; Nosi, D.; Giannecchini, S. Polyomavirus JC microRNA expression after infection in vitro. Virus Res. 2016, 213, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Handala, L.; Blanchard, E.; Raynal, P.I.; Roingeard, P.; Morel, V.; Descamps, V.; Castelain, S.; Francois, C.; Duverlie, G.; Brochot, E.; et al. BK Polyomavirus Hijacks Extracellular Vesicles for En Bloc Transmission. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- McMillan, J.; Batrakova, E.; Gendelman, H.E. Cell delivery of therapeutic nanoparticles. Prog. Mol. Biol. Transl. Sci. 2011, 104, 563–601. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V.; Mitra, A.K. Nanocarrier fabrication and macromolecule drug delivery: Challenges and opportunities. Ther. Deliv. 2016, 7, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Wahid, B.; Ali, A.; Rafique, S.; Idrees, M. Current status of therapeutic and vaccine approaches against Zika virus. Eur. J. Intern. Med. 2017, 44, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.K.; Campbell, J.P. Placental structure, function and drug transfer. BJA Educ. 2015, 15, 84–89. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Kinch, M.S.; Yunus, A.S.; Lear, C.; Mao, H.W.; Chen, H.S.; Fesseha, Z.; Luo, G.X.; Nelson, E.A.; Li, L.M.; Huang, Z.H.; et al. FGI-104: A broad-spectrum small molecule inhibitor of viral infection. Am. J. Transl. Res. 2009, 1, 87–98. [Google Scholar] [CrossRef] [PubMed]

| Patdogenesis | Effect on Patdogenesis | Virus | Component | Outcome | Reference |
| Picornaviridae and Togaviridae | |||||
| Viral packaging within vesicles. | Enhancement | Picornaviridae | Phosphatidylserine (PS) lipid-enriched vesicles | Increased viral replication | [41,42] |
| Depolymerization of the host’s actin cytoskeleton | Enhancement | Coxsackievirus B1 | Increased intracellular calcium concentration | Increased non-lytic viral spread | [43] |
| Increased EV biogenesis | Enhancement | Coxsackievirus B1 | Replication competent genome within EVs | Increased viral spread | [43] |
| Infectious virions hijacking apoptotic bodies | Enhancement | Chikungunya virus | Apoptotic bodies | Increased viral spread | [3] |
| Herpesviridae | |||||
| miRNA and mRNA are transported via exosomes | Enhancement | HSV-1 | Exosome-bound miRNA and mRNA | Suppressed viral reactivation, facilitating viral transmission to new host | [44] |
| EV-bound MHC-II activates CD4+ T-cells | Inhibition | EBV | EVs derived from EBV infected B-lymphocytes | Potentially activate CD4+ T- lymphocytes | [6,38] |
| Transport of immunoregulator protein galectin-9, a CD4+ T-cell apoptosis inducer | Enhancement | EBV | EBV-infected nasopharyngeal carcinoma cell-derived exosome-bound immunoregulator protein galectin-9 | Increased evasion of host immune response | [6,46,47] |
| Inhibition of Natural Killer (NK) cell cytotoxicity, IFN-γ production, and T-lymphocyte activation and proliferation by LMP1 | Enhancement | EBV | EV-bound LMP1 | Increased evasion of host immune response | [6,46,48,49] |
| Filoviridae | |||||
| Transportation of VP40 into the cell nucleus and subsequent binding of VP40 to cyclin D1′s promoter | Enhancement | EBOV | VP40-laden exosomes | Facilitates the regulation of EV synthesis via over-transcription of cyclin D1, dysregulating the cell cycle. | [52] |
| Transportation of VP40 into the cell nucleus | Enhancement | EBOV | VP40-laden exosomes | Exerts a dose-dependent decrease in cellular viability of recipient monocytes and T-cells | [52] |
| Modulation of RNAi machinery, such as Dicer and Ago 1 | Enhancement | EBOV | VP40-laden exosomes | Inducing cell death of recipient naïve cells while upregulating exosome biogenesis. | [54] |
| Paramyxoviridae | |||||
| RSV infection upregulated expression of select exosome-bound miRNA and piRNA content | Enhancement | RSV | Exosomes generated from RSV infected A549 cells | Increased exosomal miRNA and piRNA content | [55] |
| Exposure of PBMC-isolated human monocytes to exosomes derived from RSV infected cells | Enhancement | RSV | Exosomes generated from RSV infected A549 cells | Induced the secretion of proinflammatory mediators, such as IP-10, RANTES, and MCP-1 | [55] |
| Orthomyxoviridae | |||||
| Intercellular communication via exosomal miRNAs | Enhancement | IAVs | Exosomes generated from IAV infected cells | Modulate cell function, alter recipient cell pathways, facilitate viral persistence, and alter circulating miRNAs | [58,59,60,61,62,63] |
| Exosomes containing miRNA hsa-miR-1975 | Enhancement | IAVs | IAV-infected human lung adenocarcinoma epithelial A549 cell-derived exosomes | inhibit IAV replication by inducing interferon production | [64] |
| The transportation to the apical side of the membrane of IAV progeny RNA by attaching to Rab11 vesicles | Enhancement | IAVs | Exosomes generated from IAV infected cells | Facilitating late stage IAV budding and infection | [6,66] |
| IAVs integrate exosomal proteins or markers such as Annexin A3, CD9, CD81, and ICAM1 | Enhancement | IAVs | Exosomes generated from IAV infected cells | Contribution to the influenza virion structure, viral spread | [67] |
| Hepnaviridae | |||||
| HBV HBx protein-mediated host gene stimulation, cell cycle interference, and mitogenic signaling | Enhancement | HBV | HBx protein and mRNA encapsulated within exosomes | Permits horizontal transfer of its gene products, expression of viral protein, and facilitates oncogenic activities | [68] |
| Inducing proliferative signaling and enhancing exosome biogenesis via increasing neutral sphingomyelinase 2 activity | Enhancement | HBV | HBx protein and mRNA encapsulated within exosomes | Altered exosomal cargo (quantitatively and qualitatively) and promote HBV-associated liver diseases | [68] |
| Induce mRNA expression of the NKG2D ligand in macrophages | Inhibition | HBV | Exosomes generated from HBV infected cells and which contain viral RNA | NK cell activation, confirmed by CD69 upregulation, and induction of IFN-γ production promoting innate immunity and lymphocyte activation to defend the host from infections | [69,70] |
| Infection with HBV | Enhancement | HBV | Exosomes generated from HBV infected cells | An increase in immunosuppressive miRNAs: miR-21 and miR-29a, within CD81+ exosomes, transferred from hepatocytes to macrophages | [69] |
| Downregulation of IL-12p35 and IL-12p40 | Enhancement | HBV | Exosomes generated from HBV infected cells and containing immunosuppressive miRNAs: miR-21 and miR-29a | Potential inhibition of NK cell activity and facilitation of viral evasion of the host immune response | [69] |
| HBV modulation of exosome-bound proteins, including the increase of 5 proteasome subunit proteins: PSMD1, PSMD7, PSMD14, PSMC1, and PSMC2, enhancing proteolytic activity | Enhancement | HBV | 35 exosome-bound proteins quantitatively altered as a result of HBV infection in HBV-infected HepAD38 hepatoblastoma cell line-derived exosomes | Significant reducing monocyte IL-6 production and modulation of proinflammatory molecules | [71] |
| Uptake of these HBV-laden exosomes by cells | Enhancement | HBV | HBV-laden exosomes | Impairment of NK cell production of IFN-γ, NK cell survival and proliferation, cytolytic activity, and NK cell responsiveness to stimulation from poly (I:C) | [72] |
| Antiviral activity has been observed to be transferred from liver nonparenchymal cells (LNPCs) to hepatocytes via exosomes | Inhibition | HBV | LNPC-derived exosomes | IFN-α induced HBV antiviral activity | [73] |
| Flaviviridae | |||||
| Viral packaging within vesicles. | Enhancement | HCV | Exosome-bound viral particles | Increased viral spread. Activate immune cells and establish infection | [74,75] |
| Transportation of viral regulatory elements: Human Ago2 and miR-122 | Enhancement | HCV | Exosome-bound Ago2 and miR-122 | Increased viral spread | [74,76] |
| Infected-tick cell-derived EVs mediate transmission of viral RNA and NS1 protein | Enhancement | LGTV | Exosome-bound viral RNA and NS1 | Increased transmission from arthropod vectors to humans. Disseminate virus within host neuronal cells. | [31,77] |
| Transfer of antiviral properties from EVs carrying C19MC miRNAs | Inhibition | ZIKV | EV-bound C19MC miRNAs | Increased autophagy and viral resistance. Decreased ZIKV viral replication. | [83,84] |
| Downregulation of miR-21 after exposure to EVs | Inhibition | ZIKV | Infected HPT cell-derived EVs | Decreased TLR7-mediated neurotoxicity | [87,88] |
| Exposure of placental cells to EVs | Enhancement | ZIKV | Macrophage-derived exosomes | Induction of placental proinflammatory cytokine production. | [91] |
| Stimulation of human macrophage IL-1β secretion | Enhancement | ZIKV | ZIKV NS5-mediated activation of NLRP3 | Activation of host inflammatory response and macrophage recruitment promotes inflammation | [92] |
| EVs transmitted across neurons | Enhancement | ZIKV | EV-bound ZIKV-RNA and E-protein | Increased ZIKV transmission across neurons | [93] |
| Modulation of SMPD3 activity as a result of ZIKV cortical neuron infection | Enhancement | ZIKV | EV-bound SMPD3 | Increased EV biogenesis, viral burden, and viral transmission | [93] |
| Retrovirdae | |||||
| Release of HIV-1 infected cell-derived EVs | Enhancement | HIV | gp120 laden HIV-1 envelope (Env) protein | Increased HIV-1 infectivity in lymphoid tissues | [119,120,121,122] |
| Increased EV-mediated Nef egress | Enhancement | HIV | EV-bound Nef protein | Increased EV secretion, presence of MVBs within cells, decay of CD4+ T-cell populations | [123,124,125,126] |
| Transport of Nef via exosomes to target cells | Enhancement | HIV | EV-bound Nef protein | Promote decay of CD4+ T-cell populations, promote CD8+ T-cell activity, CXCR4-mediated apoptosis, and ADAM17 activation increasing CD4+ T-cell permissiveness to HIV-1 | [127,128,129,130] |
| Differential miRNA content relative to uninfected cells | Enhancement | HIV | HIV-1 co-evolution with the host | Facilitating suppression of host RNA interference (RNAi) | [131,137] |
| Release of HIV-1 infected plasma and macrophage derived EVs | Enhancement | HIV | HIV-1-derived miRNAs, vmiR88 and vmiR99 | Promoting macrophage release of TNF-α, thus supporting chronic immune activation | [138] |
| Modulation of exosomal and cellular miRNA profiles | Enhancement | HIV | EV-bound Nef protein | Modulation of HIV-1 pathogenesis and viral replication | [139] |
| Reduced ZO-1 TJ protein expression in HBMECs and increasing TLR-induced chemokines and cytokines in microglia | Enhancement | HIV | Microglia-derived EV-bound Nef | Disruption of BBB permeability and integrity | [141] |
| Coronaviridae | |||||
| Uptake of these SARS-CoV-2 exosomes by human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) | Enhancement | SARS-CoV-2 | SARS-CoV-2 infected cell-derived exosomes | Upregulation of genes associated with inflammation in hiPSC-CM | [133] |
| Delivery of viral RNA packaged within exosomes | Enhancement | SARS-CoV-2 | SARS-CoV-2 infected cell-derived exosomes | Indirect infection of target cardiomyocytes, exacerbating pathology, and altering the inflammatory state | [134] |
| Incorporation of spike S protein into exosomes and priming with the S-protein exosome vaccine with subsequent boosting via addition of adenoviral vector vaccine | Inhibition | SARS-CoV-2 | Vaccine: Exosomes incorporated with spike S proteins | Generation of neutralizing antibodies titers exceeding those of a SARS-convalescent patient serum | [136,142] |
| Pathogenesis | Component | Outcome | Component | Outcome | Reference |
| Retrovirdae | |||||
| CD8+ T-cell derived exosome transport | Inhibition | HIV | Membrane bound anti-HIV protein moiety | Decreased HIV-1 replication | [101] |
| Transport of antiviral factors at both the protein and mRNA level | Inhibition | HIV | TLR3-activated HBMEC-derived exosomes-bound antiviral factors | Block HIV-1 infection to the CNS. Transferring anti-HIV protection to macrophages | [102] |
| Release of TLR3-activated IEC-derived exosomes containing anti-HIV-1 factors | Inhibition | HIV | HIV-restriction miRNAs (miRNA-20 and miRNA125b), and IFN-stimulated genes (ISGs: ISG15, OAS-1, and Viperin) | Increased Anti-HIV GI innate immunity | [103] |
| Blocking viral reverse transcription | Inhibition | HIV | Vaginal fluid-derived EVs | Post-entry block of HIV-1 replication | [105] |
| Deleterious effects upon HIV-1 reverse transcriptase activity | Inhibition | HIV | Non-infected semen-derived EVs | Post-entry block of HIV-1 replication | [105,106] |
| Viral packaging within vesicles. | Enhancement | HIV | Semen-derived EV-bound functional viral mRNA | Increased viral spread | [105] |
| Binding to DC-SIGN receptor, competing with HIV-1 | Inhibition | HIV | Uninfected donor breast milk-derived EVs | Decreased HIV-1 infection of DC and viral transfer to CD4+ T-lymphocytes | [107] |
| Transport of PBMC-derived EVs to neighboring cells deficient in CCR5 | Enhancement | HIV | PBMCs-derived EVs containing CCR5 | Enhanced cellular susceptibility to HIV-1 | [109] |
| Delivering the HIV-1 co-receptor to nearby tissues lacking CXCR4 expression | Enhancement | HIV | Megakaryocyte-derived EVs containing CXCR4 | Facilitates viral spread | [110,111] |
| Transport of EVs to endothelial cells and PBMCs deficient in CCR5 | Enhancement | HIV | PBMC-derived EV-encapsulated CCR5 chemokine receptors | Enhancing HIV-1 infection | [109] |
| Transport of EVs to cells deficient in CXCR4 | Enhancement | HIV | Megakaryocyte and platelet-derived EV-encapsulated CXXR4 receptors | Enhancing HIV-1 infection | [110,111] |
| Binding of EV-bound TIM-4 to HIV-1 PS surface-bound moieties | Enhancement | HIV | EV-bound TIM4 receptor | Increased exosome-mediated trafficking of HIV-1 to human immune cells | [112,113] |
| HIV-1 Entrapping itself with exosome aggregates via exploitation of exosomal surface properties | Enhancement | HIV | Exosomal surface properties | Host-immune system evasion via camouflage. Increased viral spread | [116] |
| Exposure of EVs to macrophages yield a significant rise in proinflammatory cytokines, TNF-β, and IL-6 | Enhancement | HIV | HIV-1 infected primary cell-derived EV-bound TAR | Enhance undifferentiated naïve cell susceptibility to HIV-1 infection | [117] |
| DC-CD44 receptor binding of apoptotic microvesicles | Enhancement | HIV | Apoptotic body-bound DC-CD44 receptor | Decreased DC-dependent cytokine production and inhibition of DC-mediated T/NK-cell priming | [3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caobi, A.; Nair, M.; Raymond, A.D. Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans. Viruses 2020, 12, 1200. https://doi.org/10.3390/v12101200
Caobi A, Nair M, Raymond AD. Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans. Viruses. 2020; 12(10):1200. https://doi.org/10.3390/v12101200
Chicago/Turabian StyleCaobi, Allen, Madhavan Nair, and Andrea D. Raymond. 2020. "Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans" Viruses 12, no. 10: 1200. https://doi.org/10.3390/v12101200
APA StyleCaobi, A., Nair, M., & Raymond, A. D. (2020). Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans. Viruses, 12(10), 1200. https://doi.org/10.3390/v12101200





