Successful Establishment of Hepatitis E Virus Infection in Pregnant BALB/c Mice
Abstract
1. Instruction
2. Materials and Methods
2.1. Ethics Statement
2.2. Virus
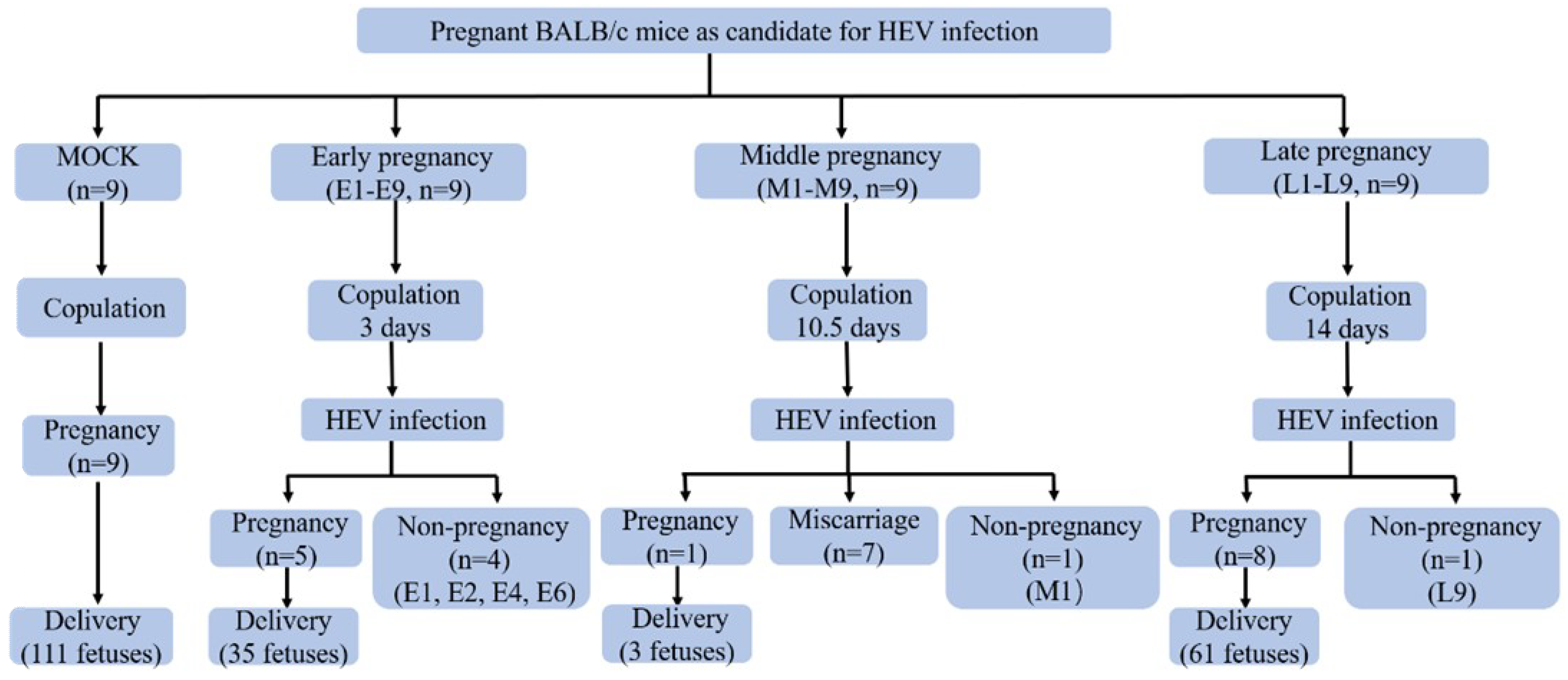
2.3. Experimental Design
2.4. Determination of Pregnancy-Related Hormones
2.5. HEV RNA Detection and Quantification
2.6. Detection of Cytokine Levels
2.7. Immunohistochemistry
2.8. Histopathology
2.9. Statistical Analysis
3. Results
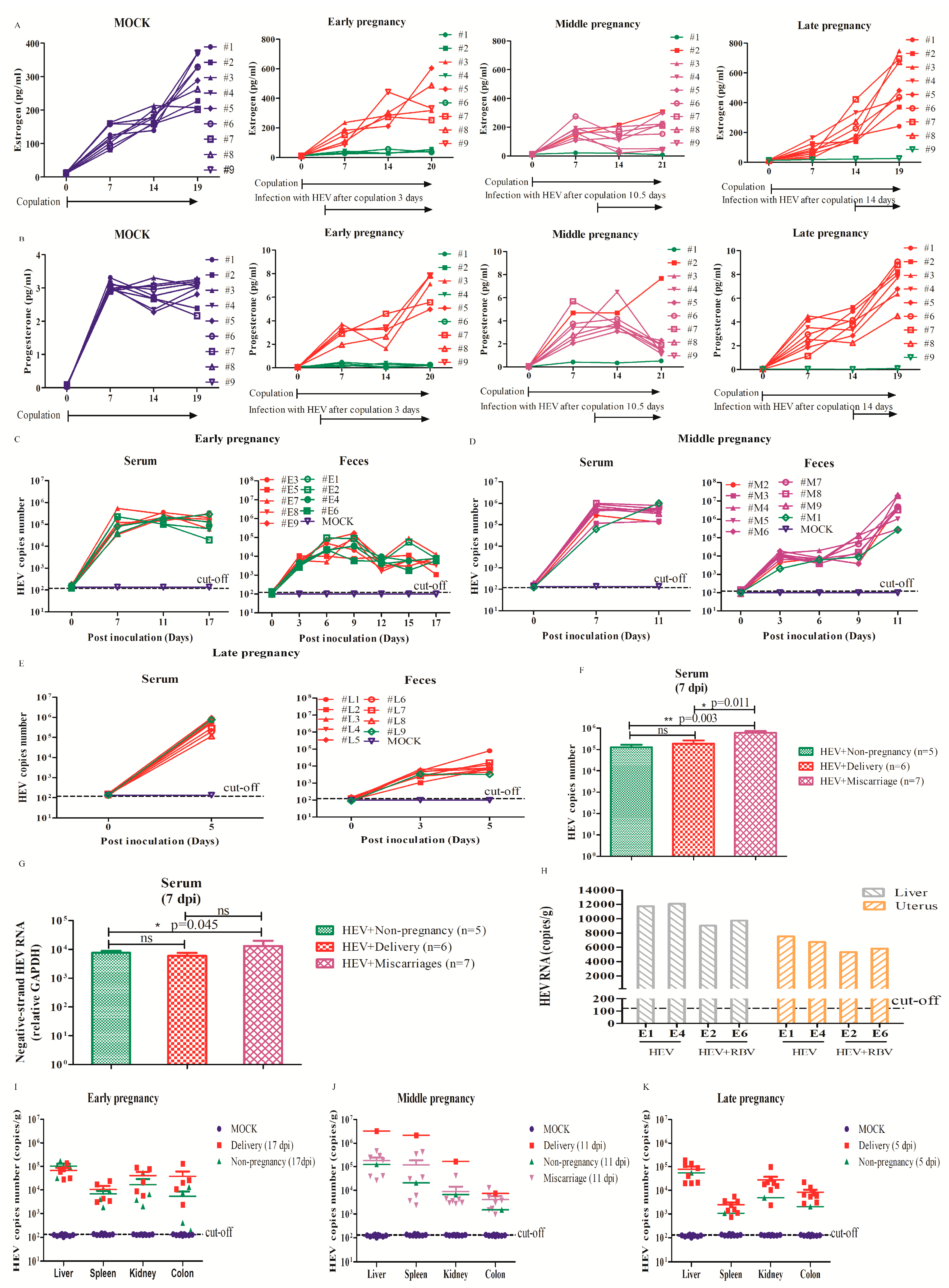
3.1. HEV Successfully Infected Pregnant BALB/c Mice
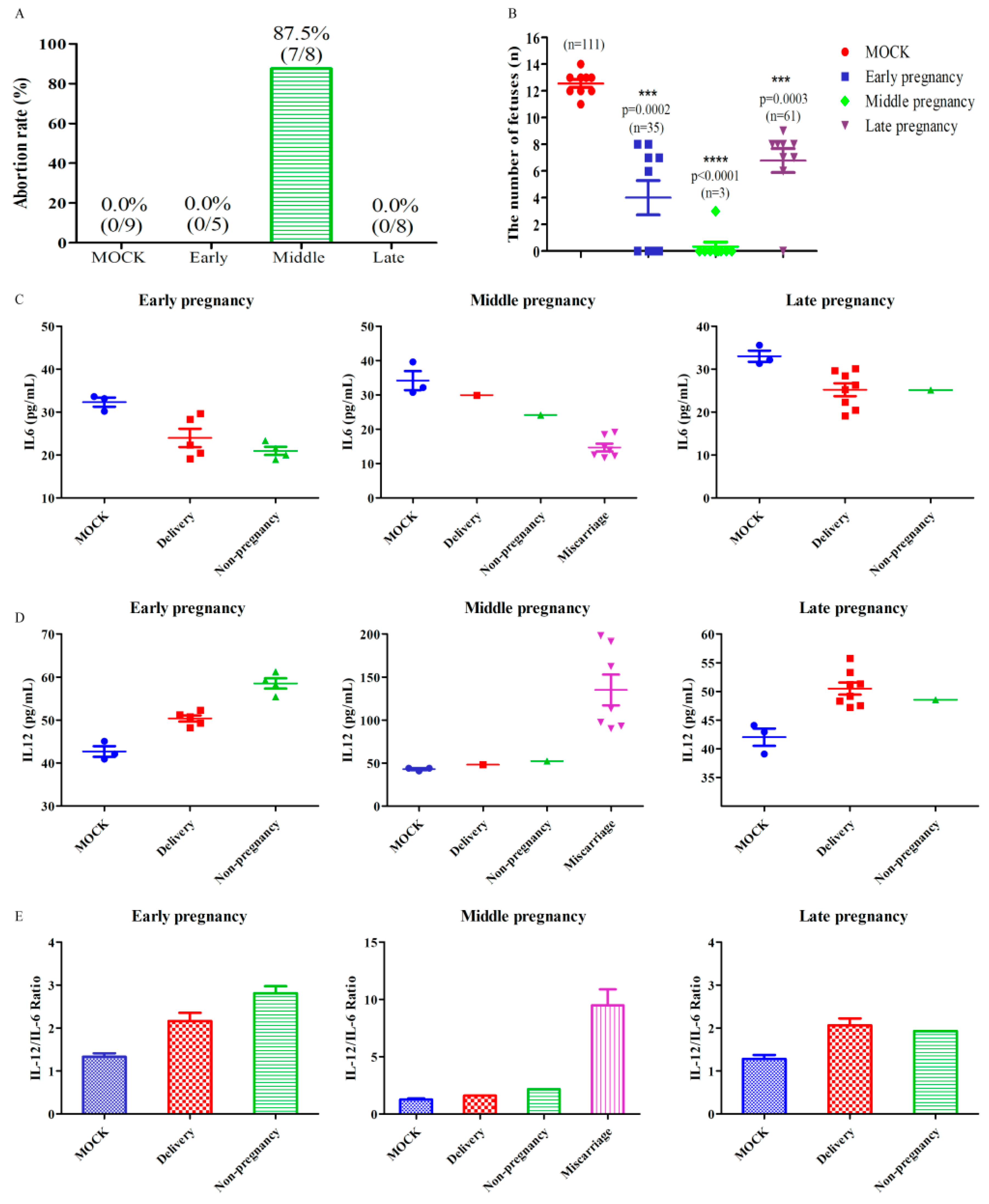
3.2. HEV Infection Leads to Adverse Pregnancy Outcome
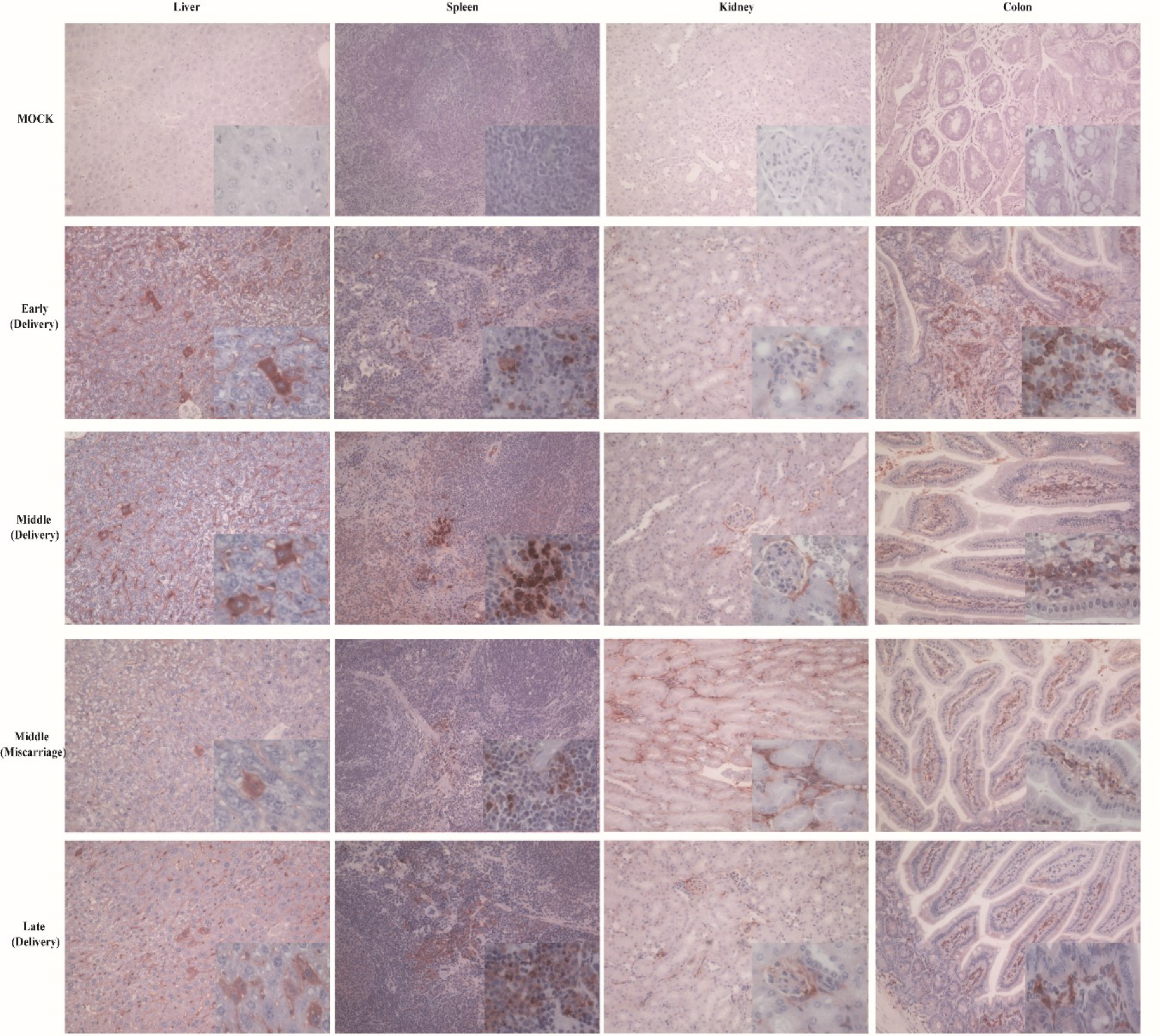
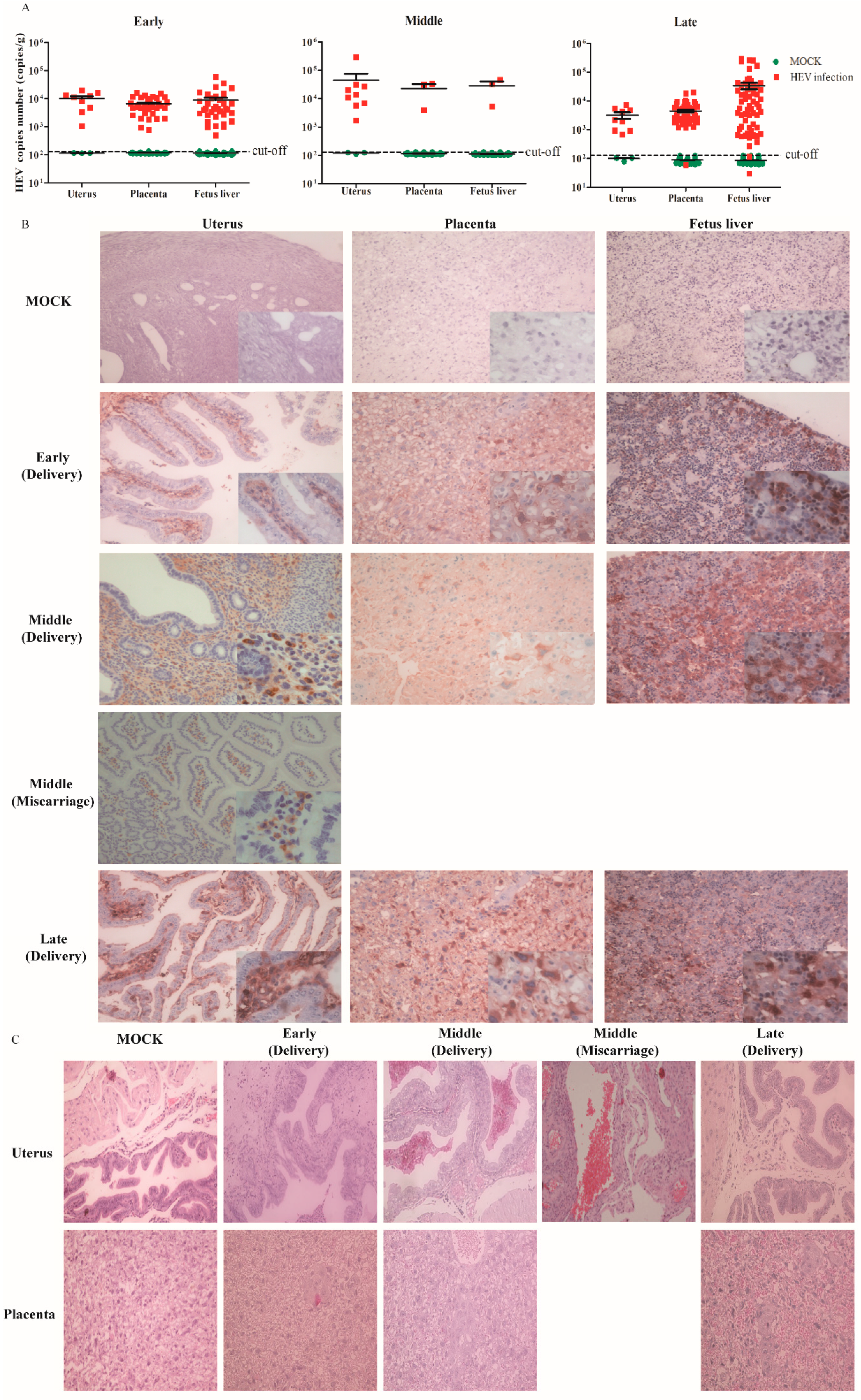
3.3. HEV Vertically Transmits from Mother to Fetus
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rein, D.B.; Stevens, G.A.; Theaker, J.; Wittenborn, J.S.; Wiersma, S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012, 55, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Jameel, S. Hepatitis E. Hepatology 2011, 54, 2218–2226. [Google Scholar] [CrossRef]
- Emerson, S.U.; Purcell, R.H. Hepatitis E virus. Rev. Med. Virol. 2003, 13, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Beniwal, M.; Kar, P.; Sharma, J.B.; Murthy, N.S. Hepatitis E in pregnancy. Int. J. Gynaecol. Obs. 2004, 85, 240–244. [Google Scholar] [CrossRef]
- Sultana, R.; Humayun, S. Fetomaternal outcome in acute hepatitis e. J. Coll Physicians Surg. Pak. 2014, 24, 127–130. [Google Scholar]
- Rayis, D.A.; Jumaa, A.M.; Gasim, G.I.; Karsany, M.S.; Adam, I. An outbreak of hepatitis E and high maternal mortality at Port Sudan, Eastern Sudan. Pathog Glob. Health 2013, 107, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Gouilly, J.; Chen, Q.; Siewiera, J.; Cartron, G.; Levy, C.; Dubois, M.; Al-Daccak, R.; Izopet, J.; Jabrane-Ferrat, N.; El Costa, H. Genotype specific pathogenicity of hepatitis E virus at the human maternal-fetal interface. Nat. Commun. 2018, 9, 4748. [Google Scholar] [CrossRef]
- Perez-Gracia, M.T.; Suay-Garcia, B.; Mateos-Lindemann, M.L. Hepatitis E and pregnancy: Current state. Rev. Med. Virol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Al Mohajer, M.; Shata, M.T. Hepatitis E and pregnancy: Understanding the pathogenesis. Liver Int. Off. J. Int. Assoc. Study Liver 2008, 28, 1190–1199. [Google Scholar] [CrossRef]
- Li, M.; Bu, Q.; Gong, W.; Li, H.; Wang, L.; Li, S.; Sridhar, S.; Cy Woo, P.; Wang, L. Hepatitis E virus infection and its associated adverse feto-maternal outcomes among pregnant women in Qinhuangdao, China. J. Matern. -Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2019. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, H. Perinatal nursing care of the pregnant women with Viral hepatitis E. Mod. Dig. Interv. 2016, 21, 57–60. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J. Nursing care of 20 cases pregnant women infected with Hepatitis E virus. Yiyao Qianyan 2015, 14, 238–239. (In Chinese) [Google Scholar] [CrossRef]
- Jilani, N.; Das, B.C.; Husain, S.A.; Baweja, U.K.; Chattopadhya, D.; Gupta, R.K.; Sardana, S.; Kar, P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J. Gastroenterol. Hepatol. 2007, 22, 676–682. [Google Scholar] [CrossRef]
- Bi, Y.; Yang, C.; Yu, W.; Zhao, X.; Zhao, C.; He, Z.; Jing, S.; Wang, H.; Huang, F. Pregnancy serum facilitates hepatitis E virus replication in vitro. J. Gen. Virol. 2015, 96, 1055–1061. [Google Scholar] [CrossRef]
- Knegendorf, L.; Drave, S.A.; Dao Thi, V.L.; Debing, Y.; Brown, R.J.P.; Vondran, F.W.R.; Resner, K.; Friesland, M.; Khera, T.; Engelmann, M.; et al. Hepatitis E virus replication and interferon responses in human placental cells. Hepatol. Commun. 2018, 2, 173–187. [Google Scholar] [CrossRef]
- Xia, J.; Liu, L.; Wang, L.; Zhang, Y.; Zeng, H.; Liu, P.; Zou, Q.; Wang, L.; Zhuang, H. Experimental infection of pregnant rabbits with hepatitis E virus demonstrating high mortality and vertical transmission. J. Viral Hepat. 2015, 22, 850–857. [Google Scholar] [CrossRef]
- Liu, P.; Bu, Q.N.; Wang, L.; Han, J.; Du, R.J.; Lei, Y.X.; Ouyang, Y.Q.; Li, J.; Zhu, Y.H.; Lu, F.M.; et al. Transmission of hepatitis E virus from rabbits to cynomolgus macaques. Emerg. Infect. Dis. 2013, 19, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Izopet, J.; Dubois, M.; Bertagnoli, S.; Lhomme, S.; Marchandeau, S.; Boucher, S.; Kamar, N.; Abravanel, F.; Guerin, J.L. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg. Infect. Dis. 2012, 18, 1274–1281. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, Z.; Harrison, T.J.; Feng, R.; Zhang, C.; Qiao, Z.; Fan, J.; Ma, H.; Li, M.; Song, A.; et al. A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J. Med. Virol. 2009, 81, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, W.; Gong, G.; Yuan, C.; Yan, Y.; Yang, S.; Cui, L.; Zhu, J.; Yang, Z.; Hua, X. Experimental infection of Balb/c nude mice with Hepatitis E virus. Bmc Infect. Dis. 2009, 9, 93. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Q.; Liu, B.; Sheng, Y.; Du, T.; Hiscox, J.A.; Zhou, E.M.; Zhao, Q. Cross-species infection of mice by rabbit hepatitis E virus. Vet. Microbiol. 2018, 225, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.F.; Haqshenas, G.; Guenette, D.K.; Halbur, P.G.; Schommer, S.K.; Pierson, F.W.; Toth, T.E.; Meng, X.J. Detection by reverse transcription-PCR and genetic characterization of field isolates of swine hepatitis E virus from pigs in different geographic regions of the United States. J. Clin. Microbiol. 2002, 40, 1326–1332. [Google Scholar] [CrossRef]
- Nanda, S.K.; Panda, S.K.; Durgapal, H.; Jameel, S. Detection of the negative strand of hepatitis E virus RNA in the livers of experimentally infected rhesus monkeys: Evidence for viral replication. J. Med. Virol. 1994, 42, 237–240. [Google Scholar] [CrossRef]
- Baylis, S.A.; Blumel, J.; Mizusawa, S.; Matsubayashi, K.; Sakata, H.; Okada, Y.; Nubling, C.M.; Hanschmann, K.M. World Health Organization International Standard to harmonize assays for detection of hepatitis E virus RNA. Emerg. Infect. Dis. 2013, 19, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, Y.; Yu, W.; Jing, S.; Wang, J.; Long, F.; He, Z.; Yang, C.; Bi, Y.; Cao, W.; et al. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology 2016, 64, 350–359. [Google Scholar] [CrossRef]
- Huang, F.; Wang, J.; Yang, C.; Long, F.; Li, Y.; Li, L.; Jing, S.; Wang, H. Chinese pregnant women in their third trimester are more susceptible to HEV infection. Braz J. Infect. Dis 2015, 19, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.D.; Das, B.C.; Kumar, A.; Gondal, R.; Kumar, D.; Kar, P. High viral load and deregulation of the progesterone receptor signaling pathway: Association with hepatitis E-related poor pregnancy outcome. J. Hepatol. 2011, 54, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Makhseed, M.; Raghupathy, R.; Azizieh, F.; Omu, A.; Al-Shamali, E.; Ashkanani, L. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum. Reprod 2001, 16, 2219–2226. [Google Scholar] [CrossRef]
- Salek Farrokhi, A.; Zarnani, A.H.; Moazzeni, S.M. Mesenchymal stem cells therapy protects fetuses from resorption and induces Th2 type cytokines profile in abortion prone mouse model. Transpl. Immunol. 2018, 47, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, L.; Guilbert, L.J.; Wegmann, T.G.; Belosevic, M.; Mosmann, T.R. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions. Correlation with increased IFN-gamma and TNF and reduced IL-10 production by placental cells. J. Immunol. 1996, 156, 653–662. [Google Scholar]
- Domino, M.; Domino, K.; Gajewski, Z. An application of higher order multivariate cumulants in modelling of myoelectrical activity of porcine uterus during early pregnancy. Biosystems 2018, 175, 30–38. [Google Scholar] [CrossRef]
- Enders, A.C. Reasons for diversity of placental structure. Placenta 2009, 30, S15–S18. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, M.A.; Rahman, S.; Khan, M.; Karim, M.F. Hepatitis E virus is a leading cause of acute-on-chronic liver disease: Experience from a tertiary centre in Bangladesh. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 50–52. [Google Scholar] [PubMed]
- Labrique, A.B.; Sikder, S.S.; Krain, L.J.; West, K.P., Jr.; Christian, P.; Rashid, M.; Nelson, K.E. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg. Infect. Dis. 2012, 18, 1401–1404. [Google Scholar] [CrossRef]
- Lhomme, S.; Marion, O.; Abravanel, F.; Chapuy-Regaud, S.; Kamar, N.; Izopet, J. Hepatitis E Pathogenesis. Viruses 2016, 8, 212. [Google Scholar] [CrossRef]
- Kamar, N.; Dalton, H.R.; Abravanel, F.; Izopet, J. Hepatitis E virus infection. Clin. Microbiol. Rev. 2014, 27, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Jeblaoui, A.; Haim-Boukobza, S.; Marchadier, E.; Mokhtari, C.; Roque-Afonso, A.M. Genotype 4 hepatitis e virus in france: An autochthonous infection with a more severe presentation. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2013, 57, e122–e126. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Chadha, M.S.; Banerjee, K.; Srinivasan, M.A.; Chobe, L.p. p. Hepatitis E virus infection in pregnant rhesus monkeys. Indian J. Med. Res. 1993, 97, 4–8. [Google Scholar]
- Kasorndorkbua, C.; Thacker, B.J.; Halbur, P.G.; Guenette, D.K.; Buitenwerf, R.M.; Royer, R.L.; Meng, X.J. Experimental infection of pregnant gilts with swine hepatitis E virus. Can. J. Vet. Res. 2003, 67, 303–306. [Google Scholar]
- Soomro, M.H.; Shi, R.; She, R.; Yang, Y.; Wang, T.; Wu, Q.; Li, H.; Hao, W. Molecular and structural changes related to hepatitis E virus antigen and its expression in testis inducing apoptosis in Mongolian gerbil model. J. Viral. Hepat. 2017, 24, 696–707. [Google Scholar] [CrossRef]
- Li, T.C.; Suzaki, Y.; Ami, Y.; Tsunemitsu, H.; Miyamura, T.; Takeda, N. Mice are not susceptible to hepatitis E virus infection. J. Vet. Med. Sci 2008, 70, 1359–1362. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Wakita, T. Small Animal Models of Hepatitis E Virus Infection. Cold Spring Harb. Perspect. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, J.; Dähnert, L.; Dremsek, P.; Tauscher, K.; Fast, C.; Ziegler, U.; Gröner, A.; Ulrich, R.G.; Groschup, M.H.; Eiden, M. Different Outcomes of Experimental Hepatitis E Virus Infection in Diverse Mouse Strains, Wistar Rats, and Rabbits. Viruses 2019, 11, 1. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Kamili, S.; Jameel, S. Vertical transmission of hepatitis E virus. Lancet 1995, 345, 1025–1026. [Google Scholar] [CrossRef]
- Naidu, S.S.; Viswanathan, R. Infectious hepatitis in pregnancy during Delhi epidemic. Indian J. Med. Res. 1957, 45, 71–76. [Google Scholar]
- Kumar, R.M.; Uduman, S.; Rana, S.; Kochiyil, J.K.; Usmani, A.; Thomas, L. Sero-prevalence and mother-to-infant transmission of hepatitis E virus among pregnant women in the United Arab Emirates. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 100, 9–15. [Google Scholar] [CrossRef]
- El Sayed Zaki, M.; El Aal, A.A.; Badawy, A.; El-Deeb, D.R.; El-Kheir, N.Y. Clinicolaboratory study of mother-to-neonate transmission of hepatitis E virus in Egypt. Am. J. Clin. Pathol. 2013, 140, 721–726. [Google Scholar] [CrossRef]
- Bose, P.D.; Das, B.C.; Hazam, R.K.; Kumar, A.; Medhi, S.; Kar, P. Evidence of extrahepatic replication of hepatitis E virus in human placenta. J. Gen. Virol. 2014, 95, 1266–1271. [Google Scholar] [CrossRef]
- Debing, Y.; Gisa, A.; Dallmeier, K.; Pischke, S.; Bremer, B.; Manns, M.; Wedemeyer, H.; Suneetha, P.V.; Neyts, J. A mutation in the hepatitis E virus RNA polymerase promotes its replication and associates with ribavirin treatment failure in organ transplant recipients. Gastroenterology 2014, 147, 1008–1011. [Google Scholar] [CrossRef]
| Sample | Strands | Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MOCK | Early Pregnancy | Middle Pregnancy | Late Pregnancy | ||||||||
| Del | NP | Mis | Del | NP | Mis | Del | NP | Mis | |||
| Feces | Positive | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 |
| Negative | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 | |
| serum | Positive | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 |
| Negative | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 | |
| Liver | Positive | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 |
| Negative | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 | |
| Spleen | Positive | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 |
| Negative | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 | |
| Kidney | Positive | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 |
| Negative | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 | |
| Colon | Positive | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 |
| Negative | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 | |
| Uterus | Positive | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 |
| Negative | 0/9 | 5/5 | 4/4 | 0/0 | 1/1 | 1/1 | 7/7 | 8/8 | 1/1 | 0/0 | |
| Placenta | Positive | 0/111 | 35/35 | 0/0 | 0/0 | 3/3 | 0/0 | 0/0 | 60/61 | 0/0 | 0/0 |
| Negative | 0/111 | 35/35 | 0/0 | 0/0 | 3/3 | 0/0 | 0/0 | 60/61 | 0/0 | 0/0 | |
| Fetus liver | Positive | 0/111 | 35/35 | 0/0 | 0/0 | 3/3 | 0/0 | 0/0 | 59/61 | 0/0 | 0/0 |
| Negative | 0/111 | 35/35 | 0/0 | 0/0 | 3/3 | 0/0 | 0/0 | 59/61 | 0/0 | 0/0 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Hao, X.; Li, Y.; Long, F.; He, Q.; Huang, F.; Yu, W. Successful Establishment of Hepatitis E Virus Infection in Pregnant BALB/c Mice. Viruses 2019, 11, 451. https://doi.org/10.3390/v11050451
Yang C, Hao X, Li Y, Long F, He Q, Huang F, Yu W. Successful Establishment of Hepatitis E Virus Infection in Pregnant BALB/c Mice. Viruses. 2019; 11(5):451. https://doi.org/10.3390/v11050451
Chicago/Turabian StyleYang, Chenchen, Xianhui Hao, Yunlong Li, Feiyan Long, Qiuxia He, Fen Huang, and Wenhai Yu. 2019. "Successful Establishment of Hepatitis E Virus Infection in Pregnant BALB/c Mice" Viruses 11, no. 5: 451. https://doi.org/10.3390/v11050451
APA StyleYang, C., Hao, X., Li, Y., Long, F., He, Q., Huang, F., & Yu, W. (2019). Successful Establishment of Hepatitis E Virus Infection in Pregnant BALB/c Mice. Viruses, 11(5), 451. https://doi.org/10.3390/v11050451





