Removal of the C6 Vaccinia Virus Interferon-β Inhibitor in the Hepatitis C Vaccine Candidate MVA-HCV Elicited in Mice High Immunogenicity in Spite of Reduced Host Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells and Viruses
2.3. Plasmid Transfer Vector pGem-RG-C6L wm
2.4. Construction of MVA-HCV ΔC6L
2.5. PCR Analysis
2.6. Expression of HCV Proteins
2.7. Analysis of Virus Growth
2.8. RNA Analysis by Quantitative Real-Time PCR
2.9. Microarray Analysis
2.10. Recruitment of Immune Cells in the Peritoneal Cavity of C57BL/6 Mice
2.11. Peptides
2.12. C57BL/6 Mice Immunization Schedule
2.13. ICS Assay
2.14. Enzyme-Linked Immunosorbent Assay (ELISA)
2.15. Statistical Procedures
3. Results
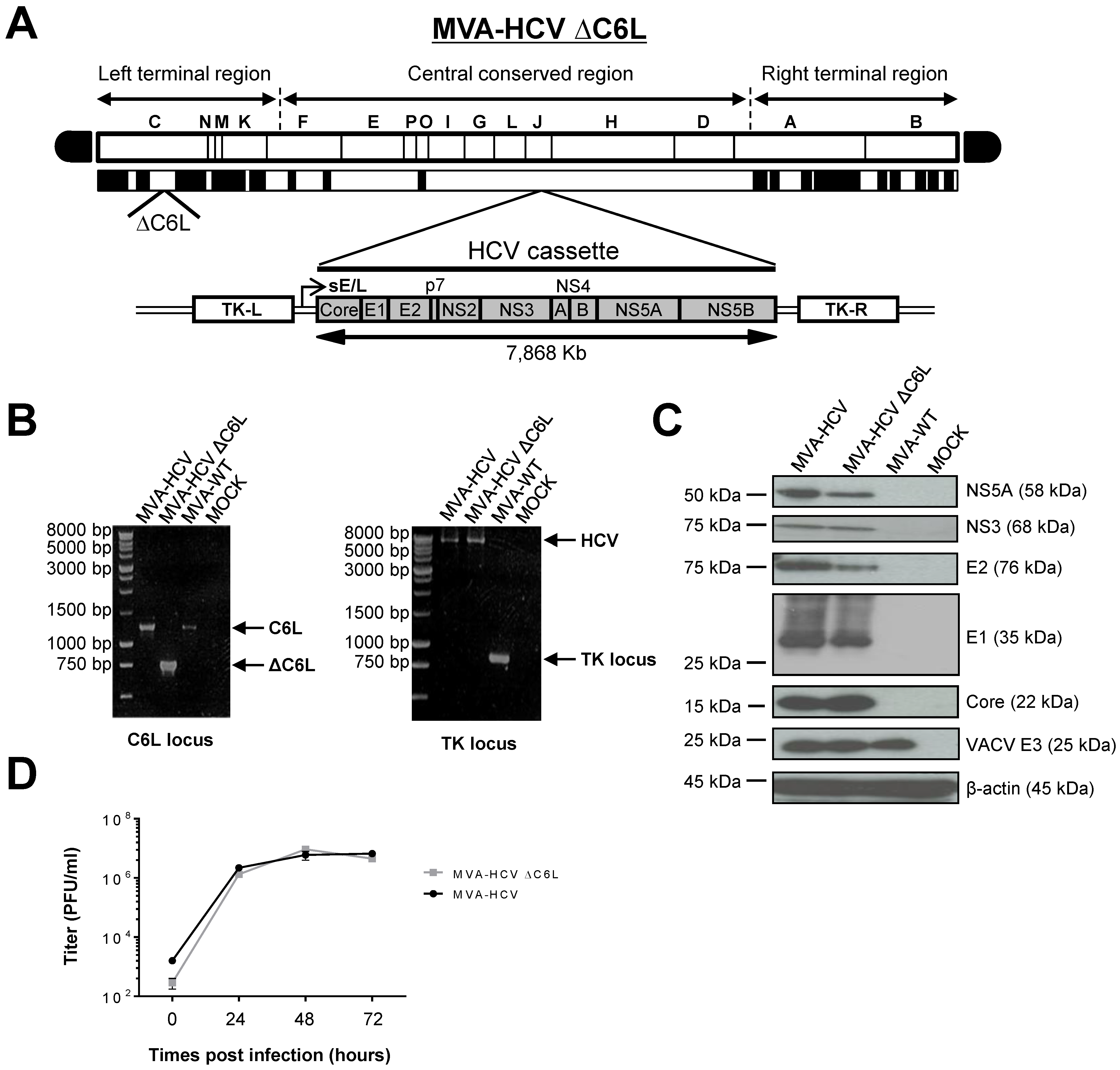
3.1. Generation of MVA-HCV ΔC6L Deletion Mutant
3.2. Expression of HCV Proteins by MVA-HCV ΔC6L
3.3. VACV C6L Gene Is Non-Essential for MVA-HCV Growth
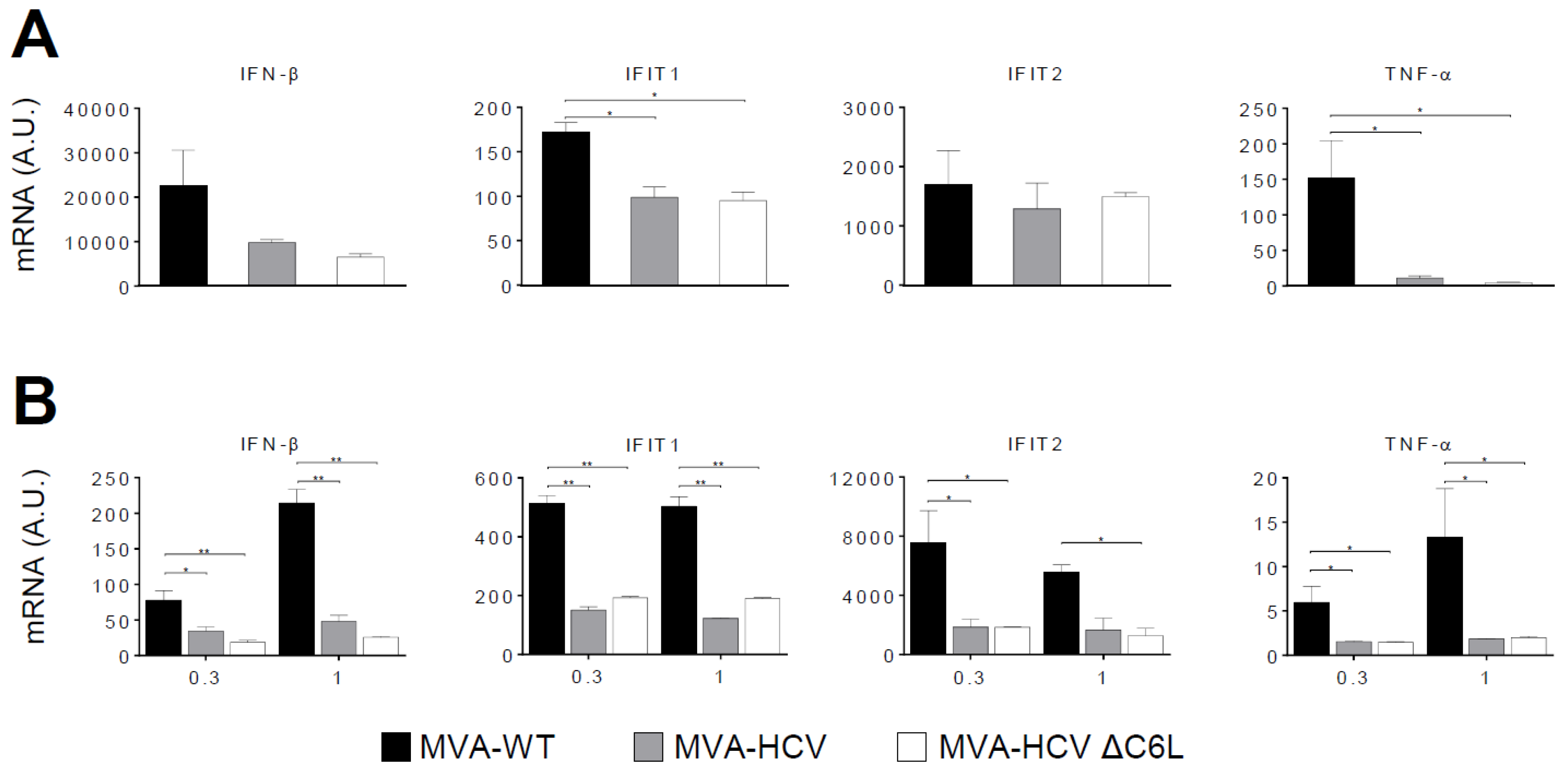
3.4. RT-PCR Showed that MVA-HCV and MVA-HCV ΔC6L Downregulate Expression of Genes Involved in Innate Immunity
3.5. Microarray Analysis Revealed a Severe Reduction of Host Gene Expression by MVA-HCV and MVA-HCV ΔC6L
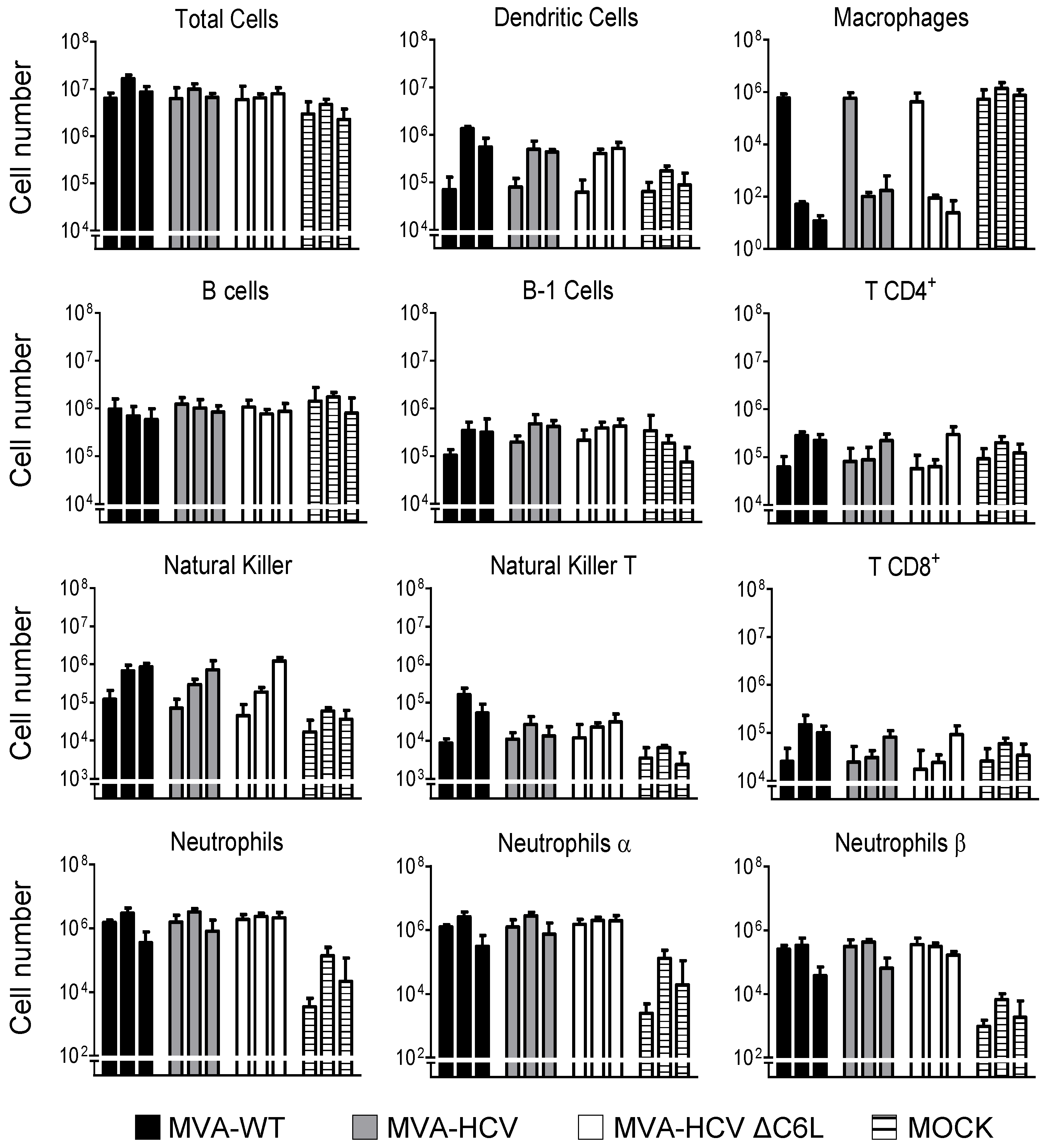
3.6. MVA-HCV and MVA-HCV ΔC6L Triggered Differential Cell Recruitment than MVA-WT in the Peritoneal Cavity of Infected Mice
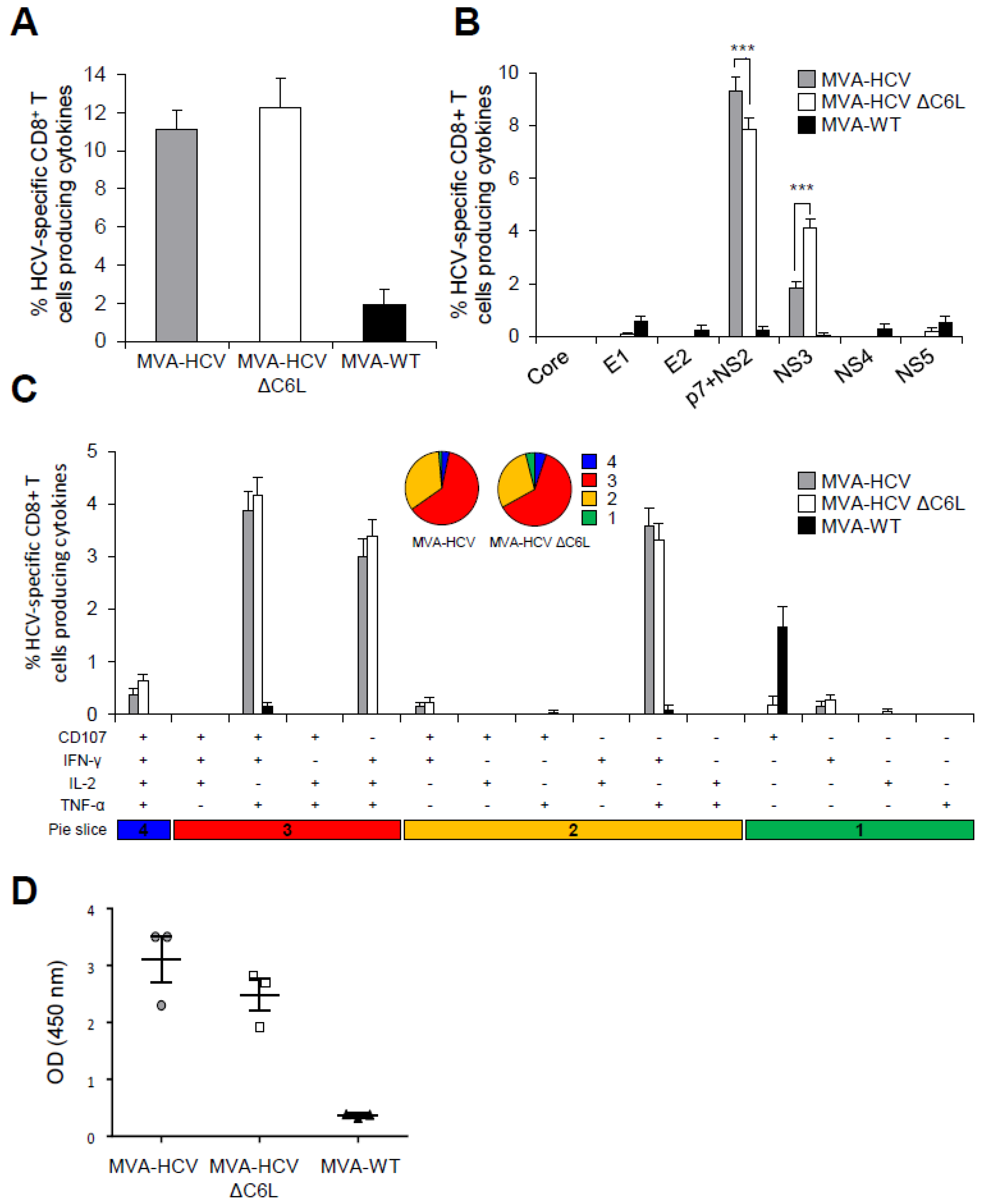
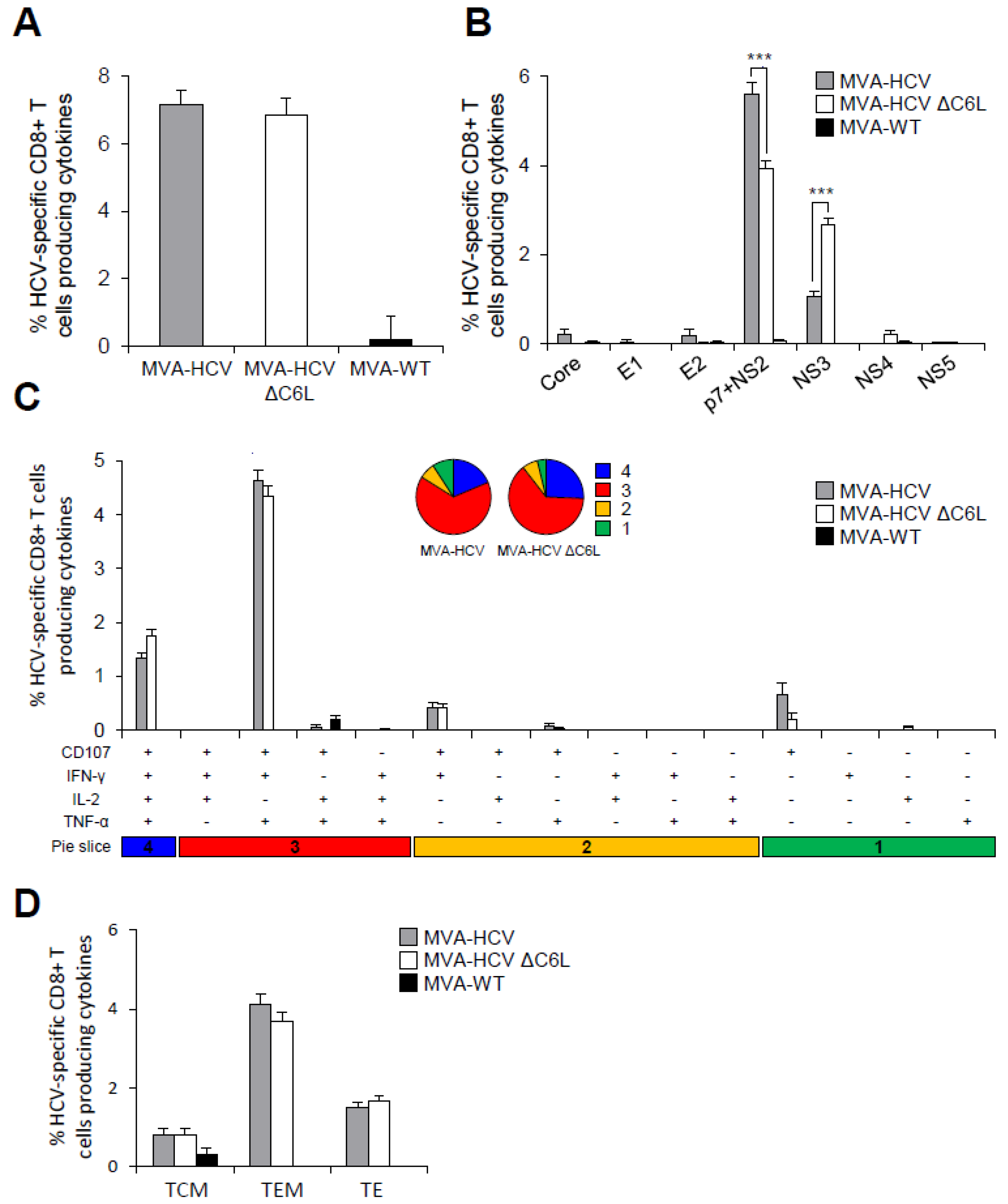
3.7. MVA-HCV and MVA-HCV ΔC6L Induced HCV-Specific T Cell and Humoral Adaptive Immune Responses in Immunized Mice
3.8. MVA-HCV and MVA-HCV ΔC6L Induced HCV-Specific T Cell Memory Immune Responses
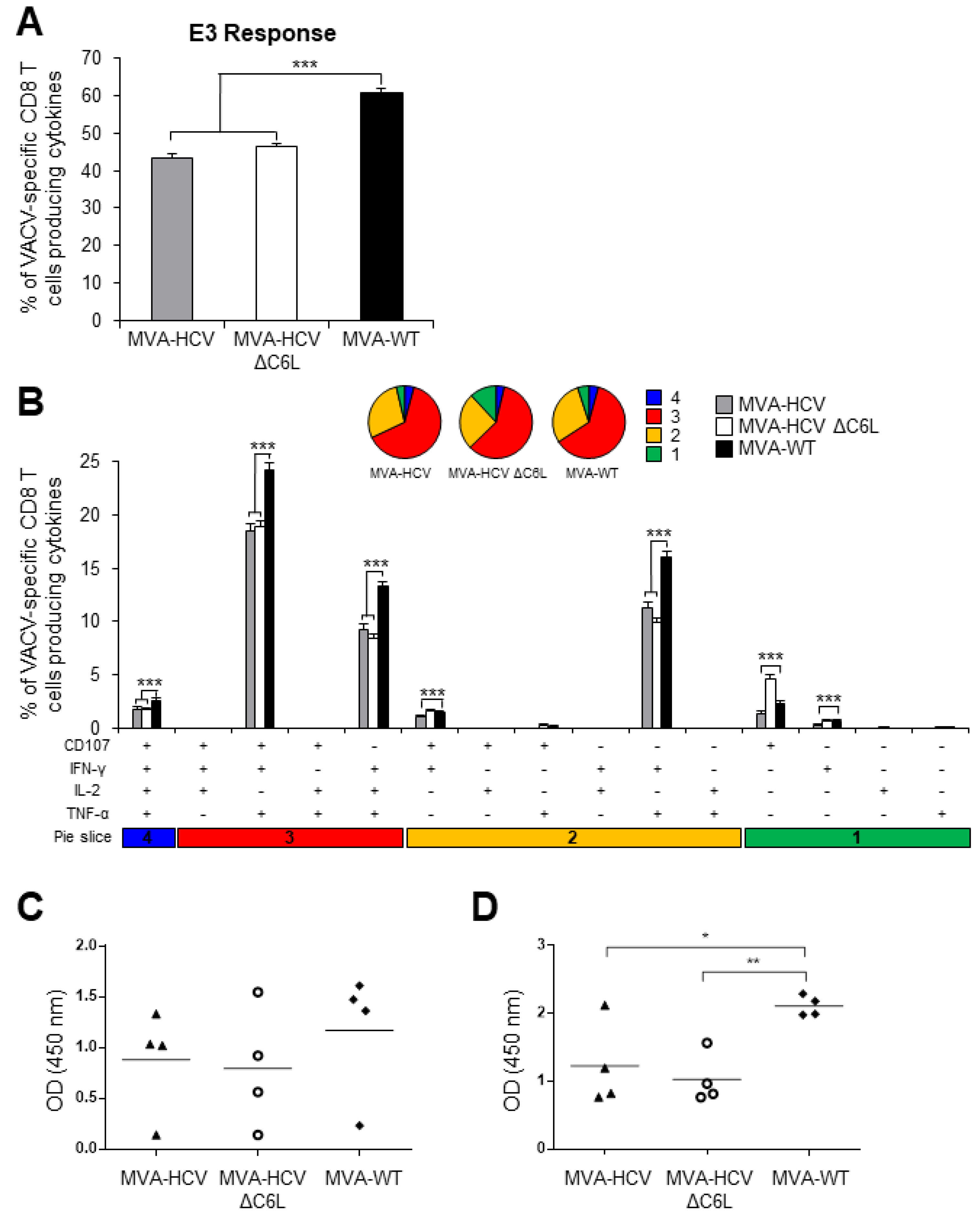
3.9. MVA-HCV and MVA-HCV ΔC6L Induced Similar VACV-Specific T Cell and Humoral Immune Responses
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdelwahab, K.S.; Ahmed Said, Z.N. Status of hepatitis C virus vaccination: Recent update. World J. Gastroenterol. 2016, 22, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.G.; Keck, Z.Y.; Foung, S.K. Viral evasion and challenges of hepatitis C virus vaccine development. Curr. Opin. Virol. 2016, 20, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lontok, E.; Harrington, P.; Howe, A.; Kieffer, T.; Lennerstrand, J.; Lenz, O.; McPhee, F.; Mo, H.; Parkin, N.; Pilot-Matias, T.; et al. Hepatitis C virus drug resistance-associated substitutions: State of the art summary. Hepatology 2015, 62, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C.; Isakov, V.; Svarovskaia, E.S.; Hedskog, C.; Martin, R.; Chodavarapu, K.; Brainard, D.M.; Miller, M.D.; Mo, H.; Molina, J.M.; et al. Late relapse versus hepatitis c virus reinfection in patients with sustained virologic response after sofosbuvir-based therapies. Clin. Infect. Dis. 2017, 64, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cornejo, A.; Lauer, G.M. Hurdles to the development of effective HBV immunotherapies and HCV vaccines. Pathog. Immun. 2017, 2, 102–125. [Google Scholar] [CrossRef] [PubMed]
- Grebely, J.; Prins, M.; Hellard, M.; Cox, A.L.; Osburn, W.O.; Lauer, G.; Page, K.; Lloyd, A.R.; Dore, G.J. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: Towards a vaccine. Lancet Infect. Dis. 2012, 12, 408–414. [Google Scholar] [CrossRef]
- Osburn, W.O.; Fisher, B.E.; Dowd, K.A.; Urban, G.; Liu, L.; Ray, S.C.; Thomas, D.L.; Cox, A.L. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 2010, 138, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Dahari, H.; Feinstone, S.M.; Major, M.E. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology 2010, 139, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Freeman, Z.T.; Cox, A.L. Lessons from nature: Understanding immunity to HCV to guide vaccine design. PLoS Pathog. 2016, 12, e1005632. [Google Scholar] [CrossRef] [PubMed]
- Holz, L.; Rehermann, B. T cell responses in hepatitis C virus infection: Historical overview and goals for future research. Antivir. Res. 2015, 114, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.M. Designing an HCV vaccine: A unique convergence of prevention and therapy? Curr. Opin. Virol. 2017, 23, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.K.; Tarr, A.W.; McKeating, J.A. The past, present and future of neutralizing antibodies for hepatitis c virus. Antivir. Res. 2014, 105, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Rostami, S.; Meshkat, Z. Progress in the development of vaccines for hepatitis C virus infection. World J. Gastroenterol. 2015, 21, 11984–12002. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.E.; Perdiguero, B.; García-Arriaza, J.; Esteban, M. Clinical applications of attenuated MVA poxvirus strain. Expert Rev. Vaccines 2013, 12, 1395–1416. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sampedro, L.; Perdiguero, B.; Mejías-Pérez, E.; García-Arriaza, J.; Di Pilato, M.; Esteban, M. The evolution of poxvirus vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.E.; Najera, J.L.; Krupa, M.; Perdiguero, B.; Esteban, M. MVA and NYVAC as vaccines against emergent infectious diseases and cancer. Curr. Gene Ther. 2011, 11, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Volz, A.; Sutter, G. Modified vaccinia virus ankara: History, value in basic research, and current perspectives for vaccine development. Adv. Virus Res. 2017, 97, 187–243. [Google Scholar] [PubMed]
- Heim, M.H. Interferons and hepatitis C virus. Swiss Med. Wkly. 2012, 142, w13586. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Jiang, J.D.; Peng, Z. Recent advances in the anti-HCV mechanisms of interferon. Acta Pharm. Sin. B 2014, 4, 241–247. [Google Scholar] [CrossRef] [PubMed]
- García-Arriaza, J.; Esteban, M. Enhancing poxvirus vectors vaccine immunogenicity. Hum. Vaccines Immunother. 2014, 10, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Unterholzner, L.; Sumner, R.P.; Baran, M.; Ren, H.; Mansur, D.S.; Bourke, N.M.; Randow, F.; Smith, G.L.; Bowie, A.G. Vaccinia virus protein c6 is a virulence factor that binds tbk-1 adaptor proteins and inhibits activation of irf3 and irf7. PLoS Pathog. 2011, 7, e1002247. [Google Scholar] [CrossRef] [PubMed]
- García-Arriaza, J.; Nájera, J.L.; Gómez, C.E.; Tewabe, N.; Sorzano, C.O.; Calandra, T.; Roger, T.; Esteban, M. A candidate HIV/aids vaccine (MVA-b) lacking vaccinia virus gene c6l enhances memory hiv-1-specific T-cell responses. PLoS ONE 2011, 6, e24244. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.H.; Sumner, R.P.; Lu, Y.; Snowden, J.S.; Smith, G.L. Vaccinia virus protein c6 inhibits type I IFN signalling in the nucleus and binds to the transactivation domain of stat2. PLoS Pathog. 2016, 12, e1005955. [Google Scholar] [CrossRef] [PubMed]
- García-Arriaza, J.; Arnáez, P.; Gómez, C.E.; Sorzano, C.; Esteban, M. Improving adaptive and memory immune responses of an HIV/aids vaccine candidate MVA-b by deletion of vaccinia virus genes (c6l and k7r) blocking interferon signaling pathways. PLoS ONE 2013, 8, e66894. [Google Scholar] [CrossRef] [PubMed]
- Sumner, R.P.; Ren, H.; Smith, G.L. Deletion of immunomodulator c6 from vaccinia virus strain western reserve enhances virus immunogenicity and vaccine efficacy. J. Gen. Virol. 2013, 94, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.E.; Perdiguero, B.; Cepeda, M.V.; Mingorance, L.; García-Arriaza, J.; Vandermeeren, A.; Sorzano, C.; Esteban, M. High, broad, polyfunctional, and durable T cell immune responses induced in mice by a novel hepatitis C virus (HCV) vaccine candidate (MVA-HCV) based on modified vaccinia virus ankara expressing the nearly full-length HCV genome. J. Virol. 2013, 87, 7282–7300. [Google Scholar] [CrossRef] [PubMed]
- Delaloye, J.; Roger, T.; Steiner-Tardivel, Q.G.; Le Roy, D.; Knaup Reymond, M.; Akira, S.; Petrilli, V.; Gomez, C.E.; Perdiguero, B.; Tschopp, J.; et al. Innate immune sensing of modified vaccinia virus ankara (MVA) is mediated by tlr2-tlr6, mda-5 and the nalp3 inflammasome. PLoS Pathog. 2009, 5, e1000480. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.E.; Nájera, J.L.; Jiménez, E.P.; Jiménez, V.; Wagner, R.; Graf, M.; Frachette, M.J.; Liljeström, P.; Pantaleo, G.; Esteban, M. Head-to-head comparison on the immunogenicity of two HIV/aids vaccine candidates based on the attenuated poxvirus strains MVA and NYVAC co-expressing in a single locus the HIV-1bx08 gp120 and HIV-1(IIIB) Gag-Pol-Nef proteins of clade B. Vaccine 2007, 25, 2863–2885. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.C.; Gherardi, M.M.; Esteban, M. Biology of attenuated modified vaccinia virus ankara recombinant vector in mice: Virus fate and activation of B- and T-cell immune responses in comparison with the western reserve strain and advantages as a vaccine. J. Virol. 2000, 74, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arriaza, J.; Najera, J.L.; Gomez, C.E.; Sorzano, C.O.; Esteban, M. Immunogenic profiling in mice of a HIV/aids vaccine candidate (MVA-b) expressing four HIV-1 antigens and potentiation by specific gene deletions. PLoS ONE 2010, 5, e12395. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K.; Speed, T. Normalization of cDNA microarray data. Methods 2003, 31, 265–273. [Google Scholar] [CrossRef]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ihaka, R.; Gentleman, R. R: A language for dataanalysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- Breitling, R.; Armengaud, P.; Amtmann, A.; Herzyk, P. Rank products: A simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004, 573, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Breitling, R.; McEntee, C.W.; Wittner, B.S.; Nemhauser, J.L.; Chory, J. Rankprod: A bioconductor package for detecting differentially expressed genes in meta-analysis. Bioinformatics 2006, 22, 2825–2827. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, M.; Mejías-Pérez, E.; Zonca, M.; Perdiguero, B.; Gómez, C.E.; Trakala, M.; Nieto, J.; Nájera, J.L.; Sorzano, C.O.; Combadière, C.; et al. Nfκb activation by modified vaccinia virus as a novel strategy to enhance neutrophil migration and HIV-specific T-cell responses. Proc. Natl. Acad. Sci. USA 2015, 112, E1333–E1342. [Google Scholar] [CrossRef] [PubMed]
- García-Arriaza, J.; Gómez, C.E.; Sorzano, C.; Esteban, M. Deletion of the vaccinia virus N2L gene encoding an inhibitor of IRF3 improves the immunogenicity of modified vaccinia virus ankara expressing hiv-1 antigens. J. Virol. 2014, 88, 3392–3410. [Google Scholar] [CrossRef] [PubMed]
- Najera, J.L.; Gomez, C.E.; Garcia-Arriaza, J.; Sorzano, C.O.; Esteban, M. Insertion of vaccinia virus C7L host range gene into NYVAC-b genome potentiates immune responses against HIV-1 antigens. PLoS ONE 2010, 5, e11406. [Google Scholar] [CrossRef] [PubMed]
- Shoukry, N.H.; Grakoui, A.; Houghton, M.; Chien, D.Y.; Ghrayeb, J.; Reimann, K.A.; Walker, C.M. Memory cd8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 2003, 197, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Man John Law, L.; Landi, A.; Magee, W.C.; Lorne Tyrrell, D.; Houghton, M. Progress towards a hepatitis C virus vaccine. Emerg. Microbes Infect. 2013, 2, e79. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.; Folgori, A.; Capone, S.; Swadling, L.; Aston, S.; Kurioka, A.; Meyer, J.; Huddart, R.; Smith, K.; Townsend, R.; et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 2012, 4, 115ra1. [Google Scholar] [CrossRef] [PubMed]
- Swadling, L.; Capone, S.; Antrobus, R.D.; Brown, A.; Richardson, R.; Newell, E.W.; Halliday, J.; Kelly, C.; Bowen, D.; Fergusson, J.; et al. A human vaccine strategy based on chimpanzee adenoviral and mva vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci. Transl. Med. 2014, 6, 261ra153. [Google Scholar] [CrossRef] [PubMed]
- Habersetzer, F.; Honnet, G.; Bain, C.; Maynard-Muet, M.; Leroy, V.; Zarski, J.P.; Feray, C.; Baumert, T.F.; Bronowicki, J.P.; Doffoel, M.; et al. A poxvirus vaccine is safe, induces T-cell responses, and decreases viral load in patients with chronic hepatitis C. Gastroenterology 2011, 141, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.G.; Zubkova, I.; Kachko, A.; Wells, F.; Adler, H.; Sutter, G.; Major, M.E. Qualitative differences in cellular immunogenicity elicited by hepatitis c virus T-cell vaccines employing prime-boost regimens. PLoS ONE 2017, 12, e0181578. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, B.; Esteban, M. The interferon system and vaccinia virus evasion mechanisms. J. Interferon Cytokine Res. 2009, 29, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Benfield, C.T.; Maluquer de Motes, C.; Mazzon, M.; Ember, S.W.; Ferguson, B.J.; Sumner, R.P. Vaccinia virus immune evasion: Mechanisms, virulence and immunogenicity. J. Gen. Virol. 2013, 94, 2367–2392. [Google Scholar] [CrossRef] [PubMed]
- Albarnaz, J.D.; Torres, A.A.; Smith, G.L. Modulating vaccinia virus immunomodulators to improve immunological memory. Viruses 2018, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Dolatimehr, F.; Karimi-Sari, H.; Rezaee-Zavareh, M.S.; Alavian, S.M.; Behnava, B.; Gholami-Fesharaki, M.; Sharafi, H. Combination of sofosbuvir, pegylated-interferon and ribavirin for treatment of hepatitis c virus genotype 1 infection: A systematic review and meta-analysis. Daru 2017, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Torres, M.; Lawitz, E.; Kowdley, K.V.; Nelson, D.R.; Dejesus, E.; McHutchison, J.G.; Cornpropst, M.T.; Mader, M.; Albanis, E.; Jiang, D.; et al. Sofosbuvir (gs-7977) plus peginterferon/ribavirin in treatment-naïve patients with HCV genotype 1: A randomized, 28-day, dose-ranging trial. J. Hepatol. 2013, 58, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Lawitz, E.; Poordad, F.; Brainard, D.M.; Hyland, R.H.; An, D.; Dvory-Sobol, H.; Symonds, W.T.; McHutchison, J.G.; Membreno, F.E. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis c genotype 2 or 3 and cirrhosis. Hepatology 2015, 61, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.R.; Pianko, S.; Brown, A.; Forton, D.; Nahass, R.G.; George, J.; Barnes, E.; Brainard, D.M.; Massetto, B.; Lin, M.; et al. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology 2015, 149, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Meylan, E.; Curran, J.; Hofmann, K.; Moradpour, D.; Binder, M.; Bartenschlager, R.; Tschopp, J. Cardif is an adaptor protein in the rig-i antiviral pathway and is targeted by hepatitis C virus. Nature 2005, 437, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Sun, L.; Seth, R.B.; Pineda, G.; Chen, Z.J. Hepatitis C virus protease ns3/4a cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl. Acad. Sci. USA 2005, 102, 17717–17722. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.E.; Vandermeeren, A.M.; Garcia, M.A.; Domingo-Gil, E.; Esteban, M. Involvement of PKR and RNASE l in translational control and induction of apoptosis after hepatitis c polyprotein expression from a vaccinia virus recombinant. Virol. J. 2005, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Vandermeeren, A.M.; Gomez, C.E.; Patino, C.; Domingo-Gil, E.; Guerra, S.; Gonzalez, J.M.; Esteban, M. Subcellular forms and biochemical events triggered in human cells by HCV polyprotein expression from a viral vector. Virol. J. 2008, 5, 102. [Google Scholar] [CrossRef] [PubMed]
- Duong, F.H.; Christen, V.; Lin, S.; Heim, M.H. Hepatitis C virus-induced up-regulation of protein phosphatase 2a inhibits histone modification and DNA damage repair. Hepatology 2010, 51, 741–751. [Google Scholar] [PubMed]
- Chen, S.; Wu, Z.; Wang, M.; Cheng, A. Innate immune evasion mediated by flaviviridae non-structural proteins. Viruses 2017, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Von dem Bussche, A.; Machida, R.; Li, K.; Loevinsohn, G.; Khander, A.; Wang, J.; Wakita, T.; Wands, J.R.; Li, J. Hepatitis C virus ns2 protein triggers endoplasmic reticulum stress and suppresses its own viral replication. J. Hepatol. 2010, 53, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Foy, E.; Li, K.; Wang, C.; Sumpter, R.; Ikeda, M.; Lemon, S.M.; Gale, M. Regulation of interferon regulatory factor-3 by the hepatitis c virus serine protease. Science 2003, 300, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Wen, Y.; Shu, C.; Han, Q.; Konan, K.V.; Li, P.; Kao, C.C. Hepatitis C virus ns4b can suppress sting accumulation to evade innate immune responses. J. Virol. 2015, 90, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kaname, Y.; Hamamoto, I.; Tsuda, Y.; Wen, X.; Taguwa, S.; Moriishi, K.; Takeuchi, O.; Kawai, T.; Kanto, T.; et al. Hepatitis C virus nonstructural protein 5a modulates the toll-like receptor-myd88-dependent signaling pathway in macrophage cell lines. J. Virol. 2007, 81, 8953–8966. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, J.B.; Kim, H.; Ray, R.; Ray, R.B. Hepatitis C virus ns5a protein modulates irf-7-mediated interferon-α signaling. J. Interferon Cytokine Res. 2014, 34, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Kumthip, K.; Chusri, P.; Jilg, N.; Zhao, L.; Fusco, D.N.; Zhao, H.; Goto, K.; Cheng, D.; Schaefer, E.A.; Zhang, L.; et al. Hepatitis c virus ns5a disrupts stat1 phosphorylation and suppresses type i interferon signaling. J. Virol. 2012, 86, 8581–8591. [Google Scholar] [CrossRef] [PubMed]
- Gale, M.J.; Korth, M.J.; Tang, N.M.; Tan, S.L.; Hopkins, D.A.; Dever, T.E.; Polyak, S.J.; Gretch, D.R.; Katze, M.G. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the pkr protein kinase by the nonstructural 5a protein. Virology 1997, 230, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Neumann-Haefelin, C.; Thimme, R. Adaptive immune responses in hepatitis C virus infection. Curr. Top. Microbiol. Immunol. 2013, 369, 243–262. [Google Scholar] [PubMed]
- Tester, I.; Smyk-Pearson, S.; Wang, P.; Wertheimer, A.; Yao, E.; Lewinsohn, D.M.; Tavis, J.E.; Rosen, H.R. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J. Exp. Med. 2005, 201, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.; Lauer, G.; Isba, R.; Walker, B.; Klenerman, P. Cellular immune responses against hepatitis C virus: The evidence base 2002. Clin. Exp. Immunol. 2002, 128, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Thimme, R.; Oldach, D.; Chang, K.M.; Steiger, C.; Ray, S.C.; Chisari, F.V. Determinants of viral clearance and persistence during acute hepatitis c virus infection. J. Exp. Med. 2001, 194, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, H.; Xiang, J.; Babiuk, L.A.; van Drunen Littel-van den Hurk, S. Dendritic cells pulsed with hepatitis C virus ns3 protein induce immune responses and protection from infection with recombinant vaccinia virus expressing ns3. J. Gen. Virol. 2006, 87, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, B.E.; Verschoor, E.J.; Fagrouch, Z.C.; Mooij, P.; de Groot, N.G.; Bontrop, R.E.; Bogers, W.M.; Heeney, J.L.; Koopman, G. Strong vaccine-induced cd8 T-cell responses have cytolytic function in a chimpanzee clearing hcv infection. PLoS ONE 2014, 9, e95103. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hammond, T.; Watson, M.W.; Flexman, J.P.; Cheng, W.; Fernandez, S.; Price, P. Could a loss of memory T cells limit responses to hepatitis C virus (HCV) antigens in blood leucocytes from patients chronically infected with HCV before and during pegylated interferon-alpha and ribavirin therapy? Clin. Exp. Immunol. 2010, 161, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Maruyama, T.; Lewis, J.; Giang, E.; Tarr, A.W.; Stamataki, Z.; Gastaminza, P.; Chisari, F.V.; Jones, I.M.; Fox, R.I.; et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat. Med. 2008, 14, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Farci, P.; Shimoda, A.; Wong, D.; Cabezon, T.; De Gioannis, D.; Strazzera, A.; Shimizu, Y.; Shapiro, M.; Alter, H.J.; Purcell, R.H. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc. Natl. Acad. Sci. USA 1996, 93, 15394–15399. [Google Scholar] [CrossRef] [PubMed]
- Vanwolleghem, T.; Bukh, J.; Meuleman, P.; Desombere, I.; Meunier, J.C.; Alter, H.; Purcell, R.H.; Leroux-Roels, G. Polyclonal immunoglobulins from a chronic hepatitis C virus patient protect human liver-chimeric mice from infection with a homologous hepatitis C virus strain. Hepatology 2008, 47, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Drummer, H.E. Editorial on “broadly neutralizing antibodies abrogate established hepatitis C virus infection” published in science translational medicine on 17th September 2014. Ann. Transl. Med. 2015, 3, S6. [Google Scholar] [PubMed]

| Gene Name (Probe identity (ID)) a | Accession Number | Fold Change b |
|---|---|---|
| Transmembrane protein 11 (TMEM11) | NM_003876 | +2.05 |
| KIAA0586 (KIAA0586) | NM_014749 | +2.00 |
| CD28 molecule (CD28) | NM_006139 | −2.00 |
| FLJ30313 (lnc-GATA5-2) | lnc-GATA5-2:2 | −2.01 |
| Aquaporin 1 (AQP1) | NM_198098 | −2.03 |
| Gessler Wilms tumor Homo sapiens (SMCR2) | AI821758 | −2.05 |
| Oogenesis homeobox (NOBOX) | NM_001080413 | −2.05 |
| RAS oncogene family (RAB6A) | NM_002869 | −2.08 |
| Ribosomal protein S28 (RPS28) | NM_001031 | −2.08 |
| Major histocompatibility complex, class II, DR beta 5 (HLA-DRB5) | NM_002125 | −2.09 |
| Chimerin 2 (ENST00000409922) | ENST00000409922 | −2.10 |
| Nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1 (NFATC1) | NM_172390 | −2.13 |
| RAS guanyl releasing protein 2 (RASGRP2) | ENST00000377494 | −2.14 |
| Glycosyltransferase 3 family (UGT3A1) | NM_152404 | −2.16 |
| Myotubularin related protein 3 (MTMR3) | NM_021090 | −2.17 |
| Keratin associated protein 2-1 (KRTAP2-1) | NM_001123387 | −2.18 |
| keratin associated protein 5-4 (KRTAP5-4) | NM_001012709 | −2.19 |
| G protein-coupled receptor 88 (GPR88) | NM_022049 | −2.19 |
| G protein pathway suppressor 2 pseudogene (ENST00000430745) | ENST00000430745 | −2.19 |
| Secretory trafficking family member B (MON1B) | NM_014940 | −2.21 |
| Solute carrier family 16, member 11 (SLC16A11) | NM_153357 | −2.24 |
| Vacuolar protein sorting 18 homolog (VPS18) | NM_020857 | −2.26 |
| Synapse defective 1, Rho GTPase, homolog 2 (SYDE2) | NM_032184 | −2.27 |
| Neuron-derived neurotrophic factor (NDNF) | NM_024574 | −2.30 |
| Homeobox 1 (EMX1) | BC037242 | −2.32 |
| Actin-like protein 3 (THC2499666) | THC2499666 | −2.32 |
| Interleukin 15 receptor (IL15RA) | ENST00000379971 | −2.39 |
| Chemokine (C-C motif) ligand 18 (CCL18) | NM_002988 | −2.40 |
| cDNA clone NT2RI2025693 (ENST00000587777) | ENST00000587777 | −2.42 |
| Cellular repressor of E1A-stimulated genes 1 (CREG1) | NM_003851 | −2.46 |
| NYN domain and retroviral integrase containing (NYNRIN) | NM_025081 | −2.49 |
| Uncoupling protein 3 (UCP3) | NM_022803 | −2.53 |
| Phospholipase C (PLCH2) | NM_014638 | −2.57 |
| Long intergenic non-protein coding RNA 687 (LINC00687) | NR_110635 | −3.05 |
| Synuclein, beta (SNCB) | NM_001001502 | −3.05 |
| Arylformamidase (AFMID) | NM_001145526 | −3.20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Q. Marín, M.; Pérez, P.; Gómez, C.E.; S. Sorzano, C.Ó.; Esteban, M.; García-Arriaza, J. Removal of the C6 Vaccinia Virus Interferon-β Inhibitor in the Hepatitis C Vaccine Candidate MVA-HCV Elicited in Mice High Immunogenicity in Spite of Reduced Host Gene Expression. Viruses 2018, 10, 414. https://doi.org/10.3390/v10080414
Q. Marín M, Pérez P, Gómez CE, S. Sorzano CÓ, Esteban M, García-Arriaza J. Removal of the C6 Vaccinia Virus Interferon-β Inhibitor in the Hepatitis C Vaccine Candidate MVA-HCV Elicited in Mice High Immunogenicity in Spite of Reduced Host Gene Expression. Viruses. 2018; 10(8):414. https://doi.org/10.3390/v10080414
Chicago/Turabian StyleQ. Marín, María, Patricia Pérez, Carmen E. Gómez, Carlos Óscar S. Sorzano, Mariano Esteban, and Juan García-Arriaza. 2018. "Removal of the C6 Vaccinia Virus Interferon-β Inhibitor in the Hepatitis C Vaccine Candidate MVA-HCV Elicited in Mice High Immunogenicity in Spite of Reduced Host Gene Expression" Viruses 10, no. 8: 414. https://doi.org/10.3390/v10080414
APA StyleQ. Marín, M., Pérez, P., Gómez, C. E., S. Sorzano, C. Ó., Esteban, M., & García-Arriaza, J. (2018). Removal of the C6 Vaccinia Virus Interferon-β Inhibitor in the Hepatitis C Vaccine Candidate MVA-HCV Elicited in Mice High Immunogenicity in Spite of Reduced Host Gene Expression. Viruses, 10(8), 414. https://doi.org/10.3390/v10080414






