Safety of an Oncolytic Myxoma Virus in Dogs with Soft Tissue Sarcoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Recombinant MYXVΔserp2
2.2. Treatment with MYXVΔserp2
2.2.1. Patient Enrollment
2.2.2. Intratumoral Injection of MYXVΔserp2
2.2.3. Post-Operative Injection of MYXVΔserp2
2.3. Patient Monitoring
2.4. Detection of Viral DNA
2.5. Evaluation of the Immune Response to MYXVΔserp2
2.5.1. Tumor Histopathology
2.5.2. Flow Cytometry of Peripheral Blood Leukocytes
2.5.3. Anti-MYXV Antibody Response
3. Results
3.1. Patient Demographics
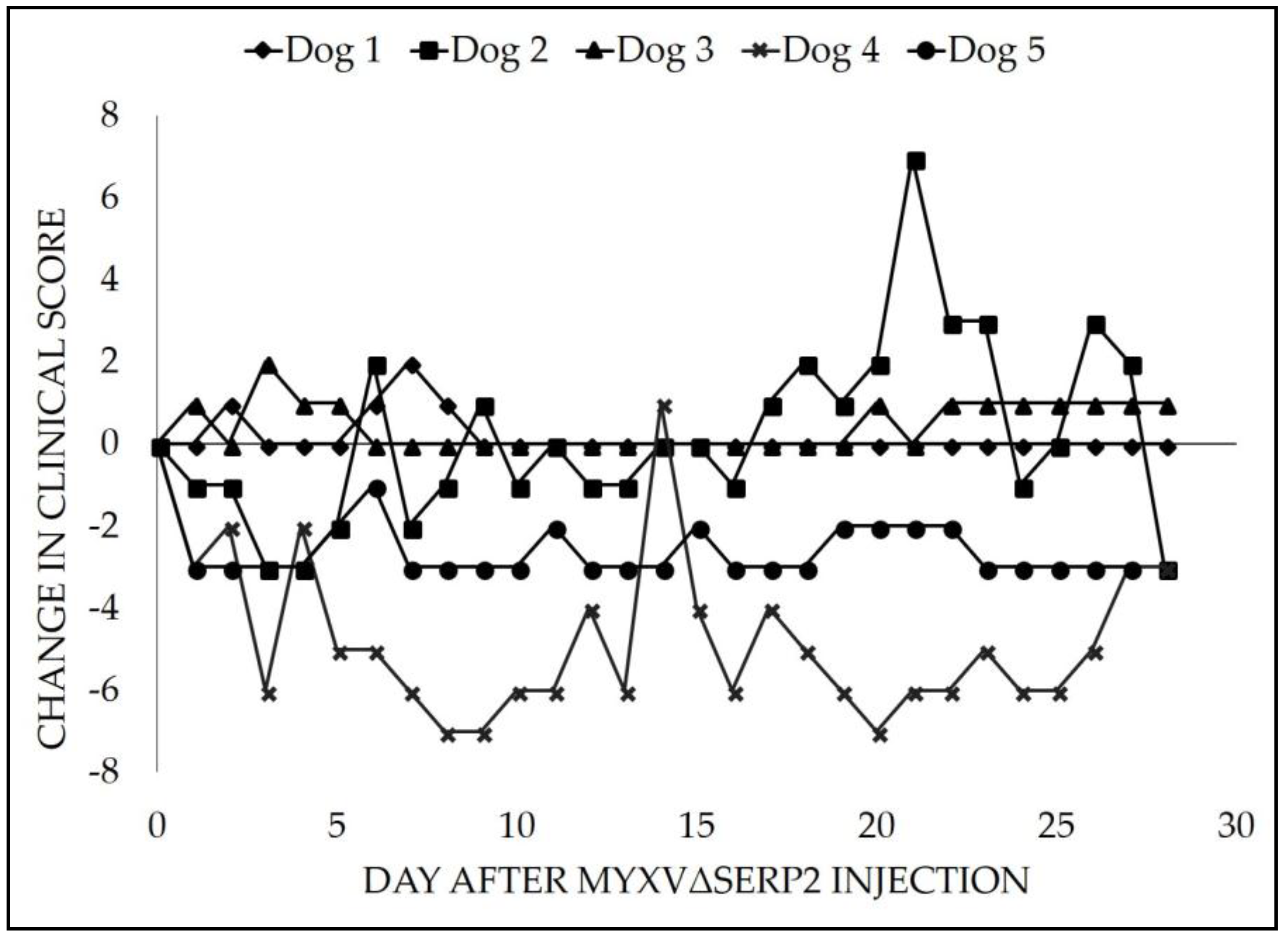
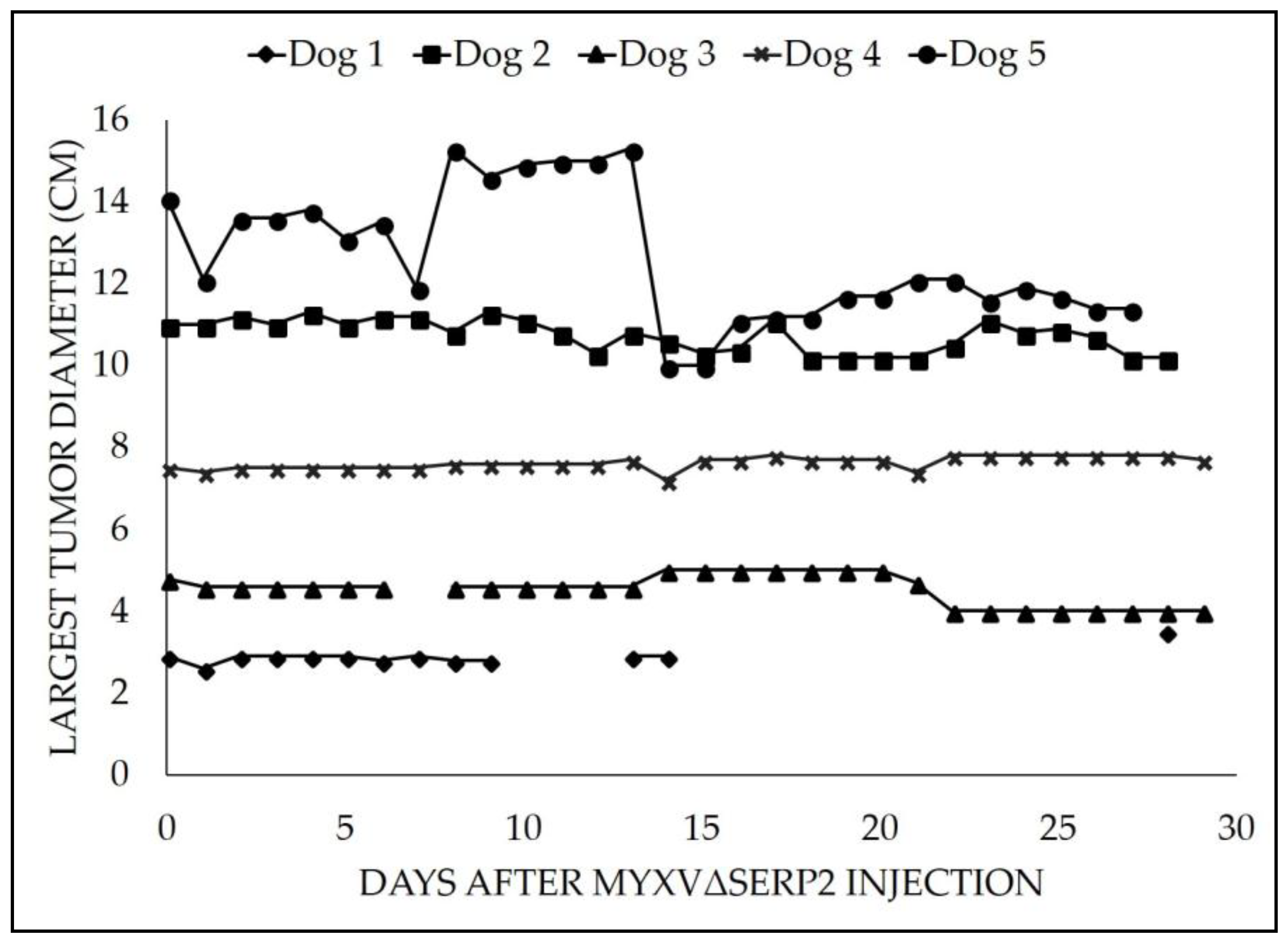
3.2. Clinical Data
3.3. Virus Distribution
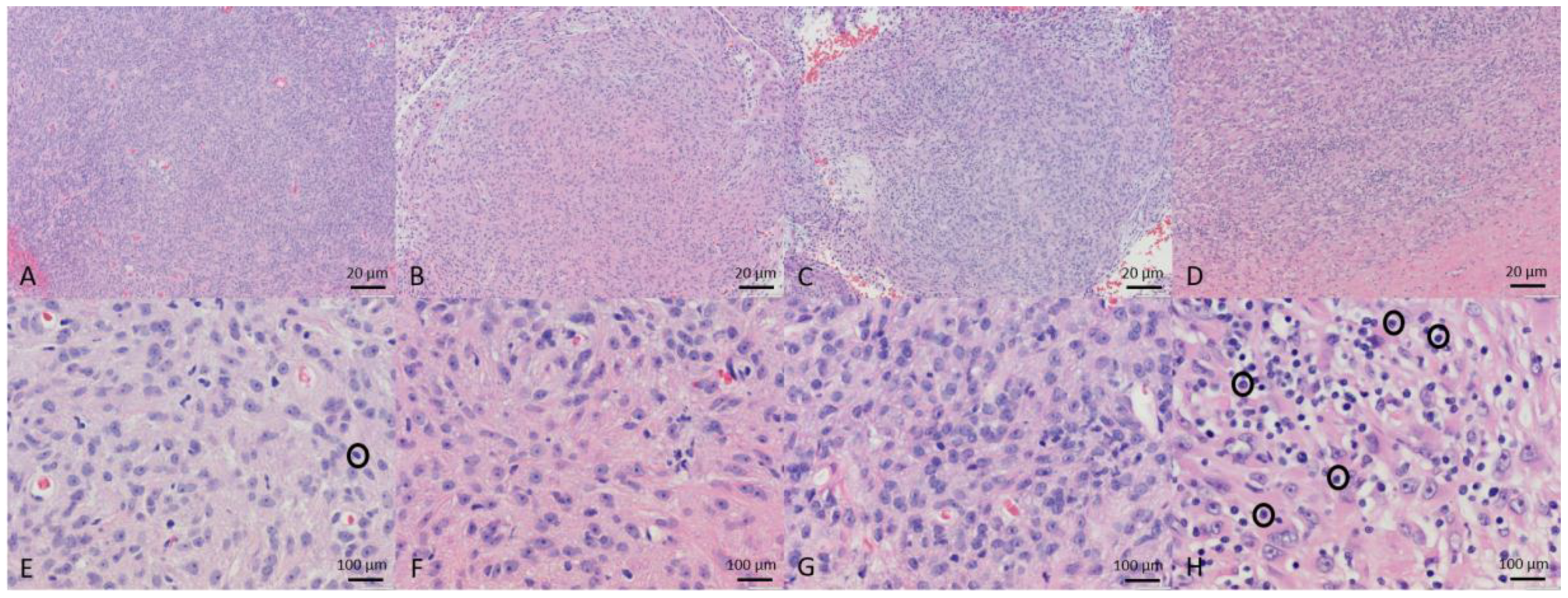
3.4. Tumor Histopathology
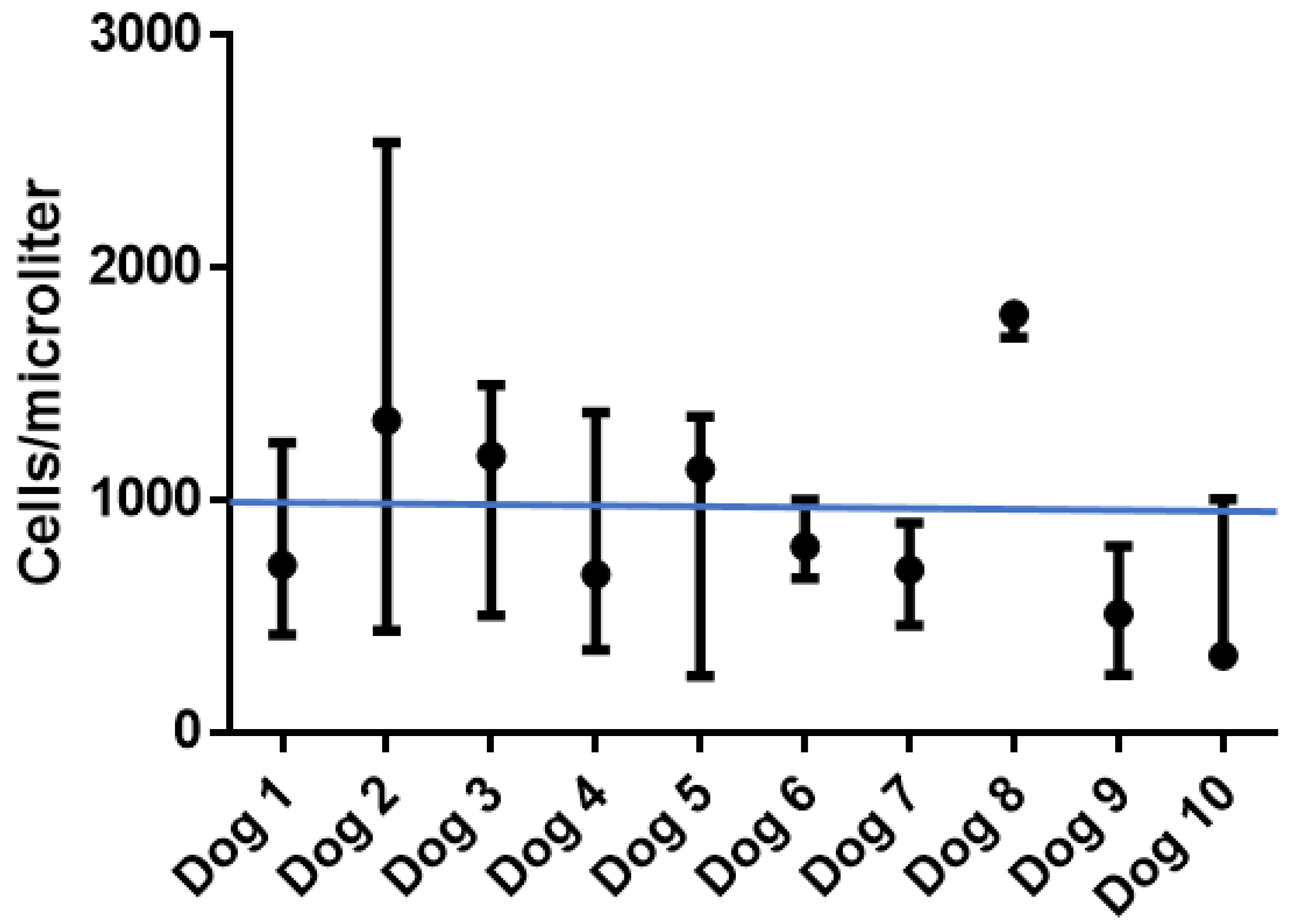
3.5. Peripheral Blood Leukocyte Subsets
3.6. Neutralizing Antibody
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Patient | Treatment | Decreased Hematocrit | Decreased Automated Platelet Count | Increased Urea Nitrogen | Increased Creatinine | Increased Phosphorus | Increased Potassium | Increased Calcium | Increased Alkaline Phosphatase | Increased Creatinine Kinase |
|---|---|---|---|---|---|---|---|---|---|---|
| Dog 1 | 106 pfu MYXVΔserp2 Day 0 | Grade 2 Days 0, 2 | ||||||||
| Dog 2 | 106 pfu MYXVΔserp2 Day 0 | Grade 2 Days 2, 6, 14, 21, 28 | Grade 2 Day 21 | Grade 2 Day 21 | Grade 2 Days 0–7, 14, 21 Grade 3 Day 28 | Grade 2 Days 21, 28 | ||||
| Dog 3 | 106 pfu MYXVΔserp2 Day 0 | Grade 2 Day 7 | ||||||||
| Dog 4 | 106 pfu MYXVΔserp2 Day 0 | Grade 2 Day 1 | ||||||||
| Dog 5 | 106 pfu MYXVΔserp2 Day 0 | Grade 2 Day 28 | ||||||||
| Dog 6 | 107 pfu MYXVΔserp2 Days 0 and 28 | Grade 2 Days −2, 14, 28 | ||||||||
| Dog 7 * | 5 × 106 pfu MYXVΔserp2 Days 0 and 14 | |||||||||
| Dog 8 * | 107 pfu MYXVΔserp2 Days 0 and 18 | |||||||||
| Dog 9 | 8 × 106 pfu MYXVΔserp2 Days 0 and 14 | Grade 3 Days 0, 14, 28 | ||||||||
| Dog 10 * | 107 pfu MYXVΔserp2 Days 0 and 14 |
| Date: | Largest Tumor Diameter (cm): | Weight (pounds): | |||
| Circle a Score for each Clinical Sign | 0 = did not occur. 1 = mild, slightly > normal, occurred once, and no treatment needed. 2 = moderate, 2× > normal, occurred more than once, call 970-297-5000 to discuss signs with a veterinarian. 3 = severe, 3× > normal, treatment needed, call 970-297-5000 to make an appointment to see a veterinarian. | ||||
| Discharge from tumor site | 0 | 1 | 2 | 3 | |
| Pain or licking at tumor site * | 0 | 1 | 2 | 3 | |
| New masses or wounds detected | 0 | 1 | 2 | 3 | |
| Excessive panting | 0 | 1 | 2 | 3 | |
| Vomiting | 0 | 1 | 2 | 3 | |
| Diarrhea | 0 | 1 | 2 | 3 | |
| Water intake > 200 mL per pound | 0 | 1 | 2 | 3 | |
| Increased urination | 0 | 1 | 2 | 3 | |
| Decreased urination | 0 | 1 | 2 | 3 | |
| Decreased interest in food | 0 | 1 | 2 | 3 | |
| Lethargy/Malaise | 0 | 1 | 2 | 3 | |
| Anxiousness/Anxiety | 0 | 1 | 2 | 3 | |
| Increased vocalization | 0 | 1 | 2 | 3 | |
| Other observations: | |||||
References
- Pol, J.; Kroemer, G.; Galluzzi, L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology 2015, 5, e1115641. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Gentschev, I.; Nolte, I.; Ogilvie, G.; Szalay, A.A. Oncolytic virotherapy in veterinary medicine: Current status and future prospects for canine patients. J. Transl. Med. 2012, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, A.; Kanerva, A.; Kremer, E.J.; Bauerschmitz, G.J.; Smith, B.F.; Liu, B.; Wang, M.; Desmond, R.A.; Keriel, A.; Barnett, B.; et al. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol. Ther. 2003, 7, 163–173. [Google Scholar] [CrossRef]
- Suter, S.E.; Chein, M.B.; von Messling, V.; Yip, B.; Cattaneo, R.; Vernau, W.; Madewell, B.R.; London, C.A. In vitro canine distemper virus infection of canine lymphoid cells: A prelude to oncolytic therapy for lymphoma. Clin. Cancer Res. 2005, 11, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.N.; Paielli, D.; Zhang, Y.; Koul, S.; Brown, S.L.; Lu, M.; Seely, J.; Kim, J.H.; Freytag, S.O. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol. Ther. 2006, 13, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Gentschev, I.; Stritzker, J.; Hofmann, E.; Weibel, S.; Yu, Y.A.; Chen, N.; Zhang, Q.; Bullerdiek, J.; Nolte, I.; Szalay, A.A. Use of an oncolytic vaccinia virus for the treatment of canine breast cancer in nude mice: Preclinical development of a therapeutic agent. Cancer Gene Ther. 2009, 16, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Gentschev, I.; Adelfinger, M.; Donat, U.; Hess, M.; Weibel, S.; Nolte, I.; Frentzen, A.; Szalay, A.A. Virotherapy of canine tumors with oncolytic vaccinia virus GLV-1h109 expressing an anti-VEGF single-chain antibody. PLoS ONE 2012, 7, e47472. [Google Scholar] [CrossRef] [PubMed]
- Urbasic, A.S.; Hynes, S.; Somrak, A.; Contakos, S.; Rahman, M.M.; Liu, J.; MacNeill, A.L. Oncolysis of canine tumor cells by myxoma virus lacking the serp2 gene. Am. J. Vet. Res. 2012, 73, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Mihailescu, R.; Vitzu, M.; Brestoiu, S. Treatment and prophylaxis of recurrent genital herpes using vaccinia immunostimulant (Antiherpin). Arch. Roum. Pathol. Exp. Microbiol. 1990, 49, 315–321. [Google Scholar] [PubMed]
- Gentschev, I.; Adelfinger, M.; Josupeit, R.; Rudolph, S.; Ehrig, K.; Donat, U.; Weibel, S.; Chen, N.G.; Yu, Y.A.; Zhang, Q.; et al. Preclinical evaluation of oncolytic vaccinia virus for therapy of canine soft tissue sarcoma. PLoS ONE 2012, 7, e37239. [Google Scholar] [CrossRef] [PubMed]
- Laborda, E.; Puig-Saus, C.; Rodriguez-García, A.; Moreno, R.; Cascalló, M.; Pastor, J.; Alemany, R. A pRb-responsive, RGD-modified, and hyaluronidase-armed canine oncolytic adenovirus for application in veterinary oncology. Mol. Ther. 2014, 22, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Gentschev, I.; Patil, S.S.; Adelfinger, M.; Weibel, S.; Geissinger, U.; Frentzen, A.; Nanhai, G.; Yu, Y.A.; Zhang, Q.; Ogilvie, G.; et al. Characterization and evaluation of a new oncolytic vaccinia virus strain LIVP6.1.1 for canine cancer therapy. Bioengineered 2013, 4, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Autio, K.; Knuuttila, A.; Kipar, A.; Ahonen, M.; Parviainen, S.; Diaconu, I.; Kanerva, A.; Hakonen, T.; Vähä-Koskela, M.; Hemminki, A. Anti-tumour activity of oncolytic Western Reserve vaccinia viruses in canine tumour cell lines, xenografts, and fresh tumour biopsies. Vet. Comp. Oncol. 2016, 14, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Breitbach, C.J.; Moon, A.; Heo, J.; Lee, Y.K.; Cho, M.; Lee, J.W.; Kim, S.G.; Kang, D.H.; Bell, J.C.; et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci. Transl. Med. 2013, 5, 185ra63. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.C.; Umeki, S.; Kubo, M.; Hayashi, T.; Shimoda, H.; Mochizuki, M.; Maeda, K.; Baba, K.; Hiraoka, H.; Coffey, M.; et al. Oncolytic reovirus in canine mast cell tumor. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.C.; Umeki, S.; Igase, M.; Coffey, M.; Noguchi, S.; Okuda, M.; Mizuno, T. The effects of oncolytic reovirus in canine lymphoma cell lines. Vet. Comp. Oncol. 2015, 77, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Adelfinger, M.; Bessler, S.; Frentzen, A.; Cecil, A.; Langbein-Laugwitz, J.; Gentschev, I.; Szalay, A.A. Preclinical testing oncolytic vaccinia virus strain GLV-5b451 expressing an anti-VEGF single-chain antibody for canine cancer therapy. Viruses 2015, 7, 4075–4092. [Google Scholar] [CrossRef] [PubMed]
- Igase, M.; Hwang, C.C.; Kambayashi, S.; Kubo, M.; Coffey, M.; Miyama, T.S.; Baba, K.; Okuda, M.; Noguchi, S.; Mizuno, T. Oncolytic reovirus synergizes with chemotherapeutic agents to promote cell death in canine mammary gland tumor. Can. J. Vet. Res. 2016, 80, 21–31. [Google Scholar] [PubMed]
- Alcayaga-Miranda, F.; Cascallo, M.; Rojas, J.J.; Pastor, J.; Alemany, R. Osteosarcoma cells as carriers to allow antitumor activity of canine oncolytic adenovirus in the presence of neutralizing antibodies. Cancer Gene Ther. 2010, 17, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Gentschev, I.; Ehrig, K.; Donat, U.; Hess, M.; Rudolph, S.; Chen, N.; Yu, Y.A.; Zhang, Q.; Bullerdiek, J.; Nolte, I.; et al. Significant growth inhibition of canine mammary carcinoma xenografts following treatment with oncolytic vaccinia virus GLV-1h68. J. Oncol. 2010, 2010, 736907. [Google Scholar] [CrossRef] [PubMed]
- Porrello, A.; Cardelli, P.; Spugnini, E.P. Oncology of companion animals as a model for humans. an overview of tumor histotypes. J. Exp. Clin. Cancer Res. 2006, 25, 97–105. [Google Scholar] [PubMed]
- Le, L.P.; Rivera, A.A.; Glasgow, J.N.; Ternovoi, V.V.; Wu, H.; Wang, M.; Smith, B.F.; Siegal, G.P.; Curiel, D.T. Infectivity enhancement for adenoviral transduction of canine osteosarcoma cells. Gene Ther. 2006, 13, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, N.; Yazawa, M.; Goto-Koshino, Y.; Mochizuki, M.; Nishimura, R.; Ohno, K.; Sasaki, N.; Tsujimoto, H. Anti-tumor effect of adenoviral vector-mediated p53 gene transfer on the growth of canine osteosarcoma xenografts in nude mice. J. Vet. Med. Sci. 2011, 73, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Bull, L.B.; Dickinson, C.G. The specificity of the virus of rabbit myxomatosis. J. Counc. Sci. Ind. Res. 1937, 10, 291–294. [Google Scholar]
- Autio, K.P.M.; Ruotsalainen, J.J.; Anttila, M.O.; Niittykoski, M.; Waris, M.; Hemminki, A.; Vähä-Koskela, M.J.V.; Hinkkanen, A.E. Attenuated Semliki Forest virus for cancer treatment in dogs: Safety assessment in two laboratory Beagles. BMC Vet. Res. 2015, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.K.; Naik, S.; Galyon, G.D.; Jenks, N.; Steele, M.; Peng, K.W.; Federspiel, M.J.; Donnell, R.; Russell, S.J. Safety studies on intravenous administration of oncolytic recombinant vesicular stomatitis virus in purpose-bred beagle dogs. Hum. Gene Ther. Clin. Dev. 2013, 24, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.F.; Curiel, D.T.; Ternovoi, V.V.; Borovjagin, A.V.; Baker, H.J.; Cox, N.; Siegal, G.P. Administration of a conditionally replicative oncolytic canine adenovirus in normal dogs. Cancer Biother. Radiopharm. 2006, 21, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.C.; Igase, M.; Sakurai, M.; Haraguchi, T.; Tani, K.; Itamoto, K.; Shimokawa, T.; Nakaichi, M.; Nemoto, Y.; Noguchi, S.; et al. Oncolytic reovirus therapy: Pilot study in dogs with spontaneously occurring tumours. Vet. Comp. Oncol. 2018, 16, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Galyon, G.D.; Jenks, N.J.; Steele, M.B.; Miller, A.C.; Allstadt, S.D.; Suksanpaisan, L.; Peng, K.W.; Federspiel, M.J.; Russell, S.J.; et al. Comparative Oncology Evaluation of Intravenous Recombinant Oncolytic Vesicular Stomatitis Virus Therapy in Spontaneous Canine Cancer. Mol. Cancer Ther. 2018, 17, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Freytag, S.O.; Barton, K.N.; Brown, S.L.; Narra, V.; Zhang, Y.; Tyson, D.; Nall, C.; Lu, M.; Ajlouni, M.; Movsas, B.; Kim, J.H. Replication-competent adenovirus-mediated suicide gene therapy with radiation in a preclinical model of pancreatic cancer. Mol. Ther. 2007, 15, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Chang, G.; Zhang, J. Comparison of prostate-specific promoters and the use of PSP-Driven virotherapy for prostate cancer. BioMed. Res. Int. 2013, 2013, 624632. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.; Mizak, B.; Chrobocinska, M. Control of rabbit myxomatosis in Poland. Rev. Sci. Tech. 1994, 13, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Porrello, A.; Cardelli, P.; Spugnini, E.P. Pet models in cancer research: General principles 5. J. Exp. Clin. Cancer Res. 2004, 23, 181–193. [Google Scholar] [PubMed]
- Parato, K.A.; Senger, D.; Forsyth, P.A.; Bell, J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. 2005, 5, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Vanderplasschen, A.; Law, M. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 2002, 83, 2915–2931. [Google Scholar] [CrossRef] [PubMed]
- Thorne, S.H.; Bartlett, D.L.; Kirn, D.H. The use of oncolytic vaccinia viruses in the treatment of cancer: A new role for an old ally? Curr. Gene Ther. 2005, 5, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Flanagan, K.; Lee, C.S.; Perretta, D.J.; Horig, H. Insertion of interleukin-2 (IL-2) and interleukin-12 (IL-12) genes into vaccinia virus results in effective anti-tumor responses without toxicity. Vaccine 2002, 20, 1862–1869. [Google Scholar] [CrossRef]
- Karupiah, G.; Coupar, B.; Ramshaw, I.; Boyle, D.; Blanden, R.; Andrew, M. Vaccinia virus-mediated damage of murine ovaries and protection by virus-expressed interleukin-2. Immunol. Cell Biol. 1990, 68, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Selin, L.K.; Santolucito, P.A.; Pinto, A.K.; Szomolanyi-Tsuda, E.; Welsh, R.M. Innate immunity to viruses: Control of vaccinia virus infection by gamma delta T cells. J. Immunol. 2001, 166, 6784–6794. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, G.; Panchanathan, V.; Buller, R.M.L.; van den Eertwegh, A.J.M.; Claassen, E.; Zhou, J.; de Chazal, R.; Laman, J.D.; Karupiah, G. Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proc. Natl. Acad. Sci. USA 2004, 101, 9057–9062. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Johnson, A.J.; Liggitt, D.; Bevan, M.J. Cellular and humoral immunity against vaccinia virus infection of mice. J. Immunol. 2004, 172, 6265–6271. [Google Scholar] [CrossRef] [PubMed]
- Pol, J.; Bloy, N.; Obrist, F.; Eggermont, A.; Galon, J.; Cremer, I.; Erbs, P.; Limacher, J.M.; Preville, X.; Zitvogel, L.; et al. Trial Watch-Oncolytic viruses and cancer therapy. Oncoimmunology 2014, 3, e28694. [Google Scholar] [CrossRef] [PubMed]
- Andrewes, C.H.; Harisijades, S. Propagation of myxoma virus in one-day old mice. Br. J. Exp. Pathol. 1955, 36, 18–21. [Google Scholar] [PubMed]
- Fenner, F.; Woodroffe, G.M. The pathogenesis of infectious myxomatosis: The mechanism of infection and the immunological response in the European rabbit (Oryctolagus cuniculus). Br. J. Exp. Pathol. 1953, 34, 400–411. [Google Scholar] [PubMed]
- Fenner, F. Adventures with poxviruses of vertebrates. FEMS Microbiol. Rev. 2000, 24, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.W.; Dorn, C.R.; Saito, J.K.; McKercher, D.G. Absence of serological evidence of myxoma virus infection in humans exposed during an outbreak of myxomatosis. Nature 1966, 211, 313–314. [Google Scholar] [CrossRef] [PubMed]
- McCabe, V.J.; Spibey, N. Potential for broad-spectrum protection against feline calicivirus using an attenuated myxoma virus expressing a chimeric FCV capsid protein. Vaccine 2005, 23, 5380–5388. [Google Scholar] [CrossRef] [PubMed]
- McCabe, V.J.; Tarpey, I.; Spibey, N. Vaccination of cats with an attenuated recombinant myxoma virus expressing feline calicivirus capsid protein. Vaccine 2002, 20, 2454–2462. [Google Scholar] [CrossRef]
- Pignolet, B.; Boullier, S.; Gelfi, J.; Bozzetti, M.; Russo, P.; Foulon, E.; Meyer, G.; Delverdier, M.; Foucras, G.; Bertagnoli, S. Safety and immunogenicity of myxoma virus as a new viral vector for small ruminants. J. Gen. Virol. 2008, 89, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- MacNeill, A.L.; Moldenhauer, T.; Doty, R.; Mann, T. Myxoma virus induces apoptosis in cultured feline carcinoma cells. Res. Vet. Sci. 2012, 93, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, Y.; Barrett, J.W.; Gao, X.; Loh, J.; Barton, E.; Virgin, H.W.; McFadden, G. Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nat. Immunol. 2004, 5, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Kelly, K.J.; Stanford, M.M.; Galanis, C.; Chun, Y.S.; Fong, Y.; McFadden, G. Myxoma virus is oncolytic for human pancreatic adenocarcinoma cells. Ann. Surg. Oncol. 2008, 15, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.; Yang, W.; Alain, T.; Shi, Z.Q.; Muzik, H.; Barrett, J.W.; McFadden, G.; Bell, J.; Hamilton, M.G.; Senger, D.L.; et al. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005, 65, 9982–9990. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.A.; McFadden, G.; Roy, E.J.; MacNeill, A.L. Histological evaluation of intratumoral myxoma virus treatment in an immunocompetent mouse model of melanoma. Oncolytic Virotherapy 2013, 2, 1–17. [Google Scholar] [PubMed]
- Ogbomo, H.; Zemp, F.J.; Lun, X.; Zhang, J.; Stack, D.; Rahman, M.M.; McFadden, G.; Mody, C.H.; Forsyth, P.A. Myxoma virus infection promotes NK lysis of malignant gliomas in vitro and in vivo. PLoS ONE 2013, 8, e66825. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Rahman, M.M.; Cogle, C.R.; McFadden, G. Prevention of EBV lymphoma development by oncolytic myxoma virus in a murine xenograft model of post-transplant lymphoproliferative disease. Biochem. Biophys. Res. Commun. 2015, 462, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Zemp, F.J.; Mckenzie, B.A.; Lun, X.; Reilly, K.M.; Mcfadden, G.; Yong, V.W.; Forsyth, P.A. Cellular factors promoting resistance to effective treatment of glioma with oncolytic Myxoma virus. Cancer Res. 2014, 74, 7260–7273. [Google Scholar] [CrossRef] [PubMed]
- Wennier, S.T.; Liu, J.; Li, S.; Rahman, M.M.; Mona, M.; McFadden, G. Myxoma virus sensitizes cancer cells to gemcitabine and is an effective oncolytic virotherapeutic in models of disseminated pancreatic cancer. Mol. Ther. 2012, 20, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Kinn, V.G.; Hilgenberg, V.A.; MacNeill, A.L. Myxoma virus therapy for human embryonal rhabdomyosarcoma in a nude mouse model. Oncolytic Virotherapy 2016, 5, 59–71. [Google Scholar] [PubMed]
- Lun, X.; Alain, T.; Zemp, F.J.; Zhou, H.; Rahman, M.M.; Hamilton, M.G.; McFadden, G.; Bell, J.; Senger, D.L.; Forsyth, P.A. Myxoma virus virotherapy for glioma in immunocompetent animal models: Optimizing administration routes and synergy with rapamycin. Cancer Res. 2010, 70, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.Q.; Zhou, H.; Alain, T.; Sun, B.; Wang, L.; Barrett, J.W.; Stanford, M.M.; McFadden, G.; Bell, J.; Senger, D.L.; et al. Targeting human medulloblastoma: Oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 2007, 67, 8818–8827. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.M.; Shaban, M.; Barrett, J.W.; Werden, S.J.; Gilbert, P.A.; Bondy-Denomy, J.; Mackenzie, L.; Graham, K.C.; Chambers, A.F.; McFadden, G. Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo. Mol. Ther. 2008, 16, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.L.; Doty, R.; Tosic, V.; Liu, J.; Kranz, D.M.; McFadden, G.; Macneill, A.L.; Roy, E.J. Myxoma virus combined with rapamycin treatment enhances adoptive T cell therapy for murine melanoma brain tumors. Cancer Immunol. Immunother. 2011, 60, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Bartee, E.; Bartee, M.Y.; Bogen, B.; Yu, X. Systemic therapy with oncolytic myxoma virus cures established residual multiple myeloma in mice. Mol. Ther. Oncolytics 2016, 3, 16032. [Google Scholar] [CrossRef] [PubMed]
- Nounamo, B.; Liem, J.; Cannon, M.; Liu, J. Myxoma Virus Optimizes Cisplatin for the Treatment of Ovarian Cancer In Vitro and in a Syngeneic Murine Dissemination Model. Mol. Ther. Oncolytics. 2017, 6, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Mingzhe, W.; Mingdi, Z.; Yiyu, Q.; Wei, G.; Zhaohui, T.; Zhiwei, Q.; Kejin, W. Targeting gallbladder carcinoma: Bone marrow-derived stem cells as therapeutic delivery vehicles of myxoma virus. Chin. Med. J. (Engl). 2014, 127, 2350–2356. [Google Scholar] [CrossRef]
- Tosic, V.; Thomas, D.L.; Kranz, D.M.; Liu, J.; McFadden, G.; Shisler, J.L.; MacNeill, A.L.; Roy, E.J. Myxoma virus expressing a fusion protein of interleukin-15 (IL15) and IL15 receptor alpha has enhanced antitumor activity. PLoS ONE 2014, 9, e109801. [Google Scholar] [CrossRef] [PubMed]
- MacNeill, A.L.; Turner, P.C.; Moyer, R.W. Mutation of the Myxoma virus SERP2 P1-site to prevent proteinase inhibition causes apoptosis in cultured RK-13 cells and attenuates disease in rabbits, but mutation to alter specificity causes apoptosis without reducing virulence. Virology 2006, 356, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Nathaniel, R.; MacNeill, A.L.; Wang, Y.X.; Turner, P.C.; Moyer, R.W. Cowpox virus CrmA, Myxoma virus SERP2 and baculovirus P35 are not functionally interchangeable caspase inhibitors in poxvirus infections. J. Gen. Virol. 2004, 85, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumours in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Group, V.C.O. Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1. 0. Vet. Comp. Oncol. 2004, 2, 195–213. [Google Scholar]
- Marshall, J.L.; Hoyer, R.J.; Toomey, M.A.; Faraguna, K.; Chang, P.; Richmond, E.; Pedicano, J.E.; Gehan, E.; Peck, R.A.; Arlen, P.; et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J. Clin. Oncol. 2000, 18, 3964–3973. [Google Scholar] [CrossRef] [PubMed]
- Downs-Canner, S.; Guo, Z.S.; Ravindranathan, R.; Breitbach, C.J.; O’Malley, M.E.; Jones, H.L.; Moon, A.; McCart, J.A.; Shuai, Y.; Zeh, H.J.; et al. Phase 1 Study of Intravenous Oncolytic Poxvirus (vvDD) in Patients with Advanced Solid Cancers. Mol. Ther. 2016, 24, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.H.; Moon, A.; Burke, J.; Ribas, A.; Stephenson, J.; Breitbach, C.J.; Daneshmand, M.; De Silva, N.; Parato, K.; Diallo, J.-S.S.; et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol. Ther. 2011, 19, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kim-Schulze, S.; Manson, K.; DeRaffele, G.; Mitcham, J.; Seo, K.S.; Kim, D.W.; Marshall, J. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J. Transl. Med. 2007, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Conry, R.M.; Khazaeli, M.B.; Saleh, M.N.; Allen, K.O.; Barlow, D.L.; Moore, S.E.; Craig, D.; Arani, R.B.; Schlom, J.; LoBuglio, A.F. Phase I trial of a recombinant vaccinia virus encoding carcinoembryonic antigen in metastatic adenocarcinoma: Comparison of intradermal versus subcutaneous administration. Clin. Cancer Res. 1999, 5, 2330–2337. [Google Scholar] [PubMed]
- Meyer, R.; Britten, C.; Siepmann, U.; Petzold, B.; Sagban, T.; Lehr, H.; Weigle, B.; Schmitz, M.; Mateo, L.; Schmidt, B. A phase I vaccination study with tyrosinase in patients with stage II melanoma using recombinant modified vaccinia virus Ankara (MVA-hTyr). Cancer Immunol. Immunother. 2005, 54, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.T.; Gulley, J.L.; Oyelaran, O.; Hodge, J.W.; Schlom, J.; Gildersleeve, J.C. Humoral response to a viral glycan correlates with survival on PROSTVAC-VF. Proc. Natl. Acad. Sci. USA 2014, 111, E1749–E1758. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Dunbar, P.R.; Mirza, F.; Palmowski, M.J.; Shepherd, D.; Gilbert, S.C.; Coulie, P.; Schneider, J.; Hoffman, E.; Hawkins, R. Recombinant modified vaccinia Ankara primes functionally activated CTL specific for a melanoma tumor antigen epitope in melanoma patients with a high risk of disease recurrence. Int. J. Cancer 2005, 113, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Tysome, J.R.; Li, X.; Wang, S.; Wang, P.; Gao, D.; Du, P.; Chen, D.; Gangeswaran, R.; Chard, L.S.; Yuan, M. A Novel Therapeutic Regimen to Eradicate Established Solid Tumors with an Effective Induction of Tumor-Specific Immunity. Clin. Cancer Res. 2012, 18, 6679–6689. [Google Scholar] [CrossRef] [PubMed]
- Fend, L.; Remy-ziller, C.; Foloppe, J.; Kempf, J.; Cochin, S.; Barraud, L.; Accart, N.; Erbs, P.; Fournel, S.; Préville, X.; et al. Oncolytic virotherapy with an armed vaccinia virus in an orthotopic model of renal carcinoma is associated with modification of the tumor microenvironment. Oncoimmunology 2016, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.M.; Galivo, F.; Kottke, T.; Wongthida, P.; Qiao, J.; Thompson, J.; Valdes, M.; Barber, G.; Vile, R.G. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007, 67, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Workenhe, S.T.; Simmons, G.; Pol, J.G.; Lichty, B.D.; Halford, W.P.; Mossman, K.L. Immunogenic HSV-mediated Oncolysis shapes the antitumor immune response and contributes to therapeutic efficacy. Mol. Ther. 2014, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kim, D.W.; Deraffele, G.; Mitcham, J.; Coffin, R.S.; Kim-Schulze, S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010, 17, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Markert, J.M.; Razdan, S.N.; Kuo, H.C.; Cantor, A.; Knoll, A.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Agee, B.S.; Coleman, J.M.; et al. A phase 1 trial of oncolytic HSV-1, g207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol. Ther. 2014, 22, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Kicielinski, K.P.; Chiocca, E.A.; Yu, J.S.; Gill, G.M.; Coffey, M.; Markert, J.M. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol. Ther. 2014, 22, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Breitbach, C.J.; Lee, J.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Moon, A.; Mun, J.H.; Sommermann, E.M.; Maruri Avidal, L.; et al. Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer. Mol. Ther. 2015, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Reid, T.; Ruo, L.; Breitbach, C.J.; Rose, S.; Bloomston, M.; Cho, M.; Lim, H.Y.; Chung, H.C.; Kim, C.W.; et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 2013, 19, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Jourdier, T.M.; Moste, C.; Bonnet, M.C.; Delisle, F.; Tafani, J.P.; Devauchelle, P.; Tartaglia, J.; Moingeon, P. Local immunotherapy of spontaneous feline fibrosarcomas using recombinant poxviruses expressing interleukin 2 (IL2). Gene Ther. 2003, 10, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
| Target Gene Sequence | Technique | Forward Primer Sequence | Reverse Primer Sequence | Template Length (Base Pairs) | Annealing Temperature (°C) |
|---|---|---|---|---|---|
| Myxoma virus M135R-M136R | Standard PCR | 5′-CGA GAA TTC CAC CTG TGT ATG TT-3′ | 5′-CCA TGT ACA ATA ACA CAC AGT TCG G-3′ | 1164 | 52 |
| Myxoma virus M033R-M034L | Standard PCR | 5′-CAC CCT CTT TAG TAA AGT ATA CAC C-3′ | 5′-GAA ATG TTG TCG GAC GGG-3′ | 818 | 52 |
| Myxoma virus M033R | Droplet Digital PCR | 5′-CGC CAT CCT TTA CCT AAC GA-3′ | 5′-CGA CAA AAA TAA CAC CGG GT-3′ | 94 | 60 |
| Canine GAPDH | Droplet Digital PCR | 5′-GCC CTC AAT GAC CAC TTT GT-3′ | 5′-TCA GCT ACA GCA ACC AGG TG-3′ | 69 | 60 |
| Patient | Age (Years) | Breed | Sex | Pertinent Medical History Prior to Study Enrollment | Study Arm | Largest Tumor Diameter on Day 0 (cm) | Largest Tumor Diameter Median (Range) Day 0 to Day 28 (cm) | Tumor Location | Pre-Treatment Biopsy Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| Dog 1 | 10 | Mixed | Castrated male | Tumor excisions 1 and 2 years prior Regrowth~1 month | Intra-tumoral | 2.9 | 2.9 (2.6–3.5) | Right elbow | Grade 2 STS, PNST |
| Dog 2 | 12 | Miniature schnauzer | Castrated male | Chronic renal failure Tumor present~3 months | Intra-tumoral | 11.0 | 10.8 (10.2–11.3) | Left inguinal and perianal areas | Grade 1 STS, myxo-sarcoma |
| Dog 3 | 8 | Mixed | Castrated male | Tumor excision 1 year prior Regrowth~1 month | Intra-tumoral | 4.8 | 4.6 (4.0–5.0) | Left elbow | Grade 3 Undiffer-entiated sarcoma |
| Dog 4 | 11 | Italian grey-hound | Castrated male | Tumor present~1 month | Intra-tumoral | 7.5 | 7.6 (7.2–7.8) | Tail base | Grade 1 STS, PNST |
| Dog 5 | 10 | Shetland sheepdog | Castrated male | Tumor present~1 year | Intra-tumoral | 14.1 | 12.1 (10.0–15.3) | Right caudal brachium | Grade 2 STS, PNST |
| Dog 6 | 12 | Mixed | Spayed female | Tumor excision~4 months prior Regrowth~5 months | Post-operative | 5 (per lobule) | TE | Multi-lobulated mass dorsal thorax | Grade 3 Undiffer-entiated sarcoma |
| Dog 7 | 11 | Greyhound | Castrated male | Tumor excision 2 & 6 years prior Regrowth~1 month | Post-operative | 3.5 | TE | Left lateral meta-tarsus | Grade 2 STS, PNST |
| Dog 8 | 15 | Labrador retriever | Castrated male | Tumor present 2–3 years | Post-operative | 22 | TE | Ventral abdomen | Grade 2 STS, myxoid fibro-sarcoma |
| Dog 9 | 12 | Labrador retriever | Spayed female | Tumor excision 2 years prior Regrowth~1 month | Post-operative | 3.5 | TE | Right caudo-lateral thorax | Grade 2 STS, PNST |
| Dog 10 | 11 | Mixed | Spayed female | Tumor present~1 month | Post-operative | 6.5 | TE | Left flank | Grade 2 STS, PNST |
| Patient | Sample | Diagnosis and Comments | Mitotic Index Per 40× Field | Percent Necrosis | Inflammatory Infiltrate |
|---|---|---|---|---|---|
| Dog 1 | Pre-treatment biopsy | Grade 2 STS, PNST | 1 | 0 | Rare |
| Day 4 biopsy | No tumor | N/A | N/A | N/A | |
| Day 14 biopsy | Grade 2 STS, PNST | 1 | 0 | None | |
| Resected tumor | Grade 2 STS, PNST Slight edema | 1 | 0 | Rare | |
| Dog 2 | Pre-treatment biopsy | Grade 1 STS, myxosarcoma Edema | 1 | 0 | Rare |
| Day 4 biopsy | No tumor | N/A | N/A | N/A | |
| Day 14 biopsy | No tumor Granulation tissue | N/A | N/A | N/A | |
| Dog 3 | Pre-treatment biopsy | No tumor | N/A | N/A | N/A |
| Day 4 biopsy | Grade 3 undifferentiated sarcoma | 6 | 0 | Rare | |
| Day 14 biopsy | No tumor | N/A | N/A | N/A | |
| Resected tumor | Grade 3 undifferentiated sarcoma Slight apoptosis | 2 | 10 | Rare | |
| Dog 4 | Pre-treatment biopsy | Grade 1 STS, PNST | < 1 | 0 | Occasional |
| Day 4 biopsy | Grade 1 STS, PNST Fibrosis and hemorrhage | <1 | 0 | Mild | |
| Day 14 biopsy | Grade 1 STS, PNST | <1 | 35% | Occasional | |
| Resected tumor | Grade 1 STS, PNST | <1 | 10% | Occasional | |
| Dog 5 | Pre-treatment biopsy | Grade 2 STS, PNST | 1 | 5% | Mild |
| Day 4 biopsy | Grade 2 STS, PNST | 1 | 10% | Occasional | |
| Day 14 biopsy | Grade 2 STS, PNST | 1 | 5% | Mild | |
| Resected tumor | Grade 2 STS, PNST | 1 | 50% | Moderate |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacNeill, A.L.; Weishaar, K.M.; Séguin, B.; Powers, B.E. Safety of an Oncolytic Myxoma Virus in Dogs with Soft Tissue Sarcoma. Viruses 2018, 10, 398. https://doi.org/10.3390/v10080398
MacNeill AL, Weishaar KM, Séguin B, Powers BE. Safety of an Oncolytic Myxoma Virus in Dogs with Soft Tissue Sarcoma. Viruses. 2018; 10(8):398. https://doi.org/10.3390/v10080398
Chicago/Turabian StyleMacNeill, Amy L., Kristen M. Weishaar, Bernard Séguin, and Barbara E. Powers. 2018. "Safety of an Oncolytic Myxoma Virus in Dogs with Soft Tissue Sarcoma" Viruses 10, no. 8: 398. https://doi.org/10.3390/v10080398
APA StyleMacNeill, A. L., Weishaar, K. M., Séguin, B., & Powers, B. E. (2018). Safety of an Oncolytic Myxoma Virus in Dogs with Soft Tissue Sarcoma. Viruses, 10(8), 398. https://doi.org/10.3390/v10080398





